Abstract
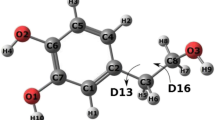
SAMPL challenges (Mobley et al. in J Comput Aided Mol Des 28:135–150, 2014; Skillman in J Comput Aided Mol Des 26:473–474, 2012; Geballe in J Comput Aided Mol Des 24:259–279, 2010; Guthrie in J Phys Chem B 113:4501–4507, 2009) provide excellent opportunities to assess theoretical approaches on new data sets with a goal of gaining greater insight towards protein and ligand modeling. In the SAMPL5 experiment, cyclohexane–water partition coefficients were determined using a vertical solvation scheme in conjunction with the SMD continuum solvent model. Several DFT functionals partnered with correlation consistent basis sets were evaluated for the prediction of the partition coefficients. The approach chosen for the competition, a B3PW91 vertical solvation scheme, yields a mean absolute deviation of 1.9 logP units and performs well at estimating the correct hydrophilicity and hydrophobicity for the full SAMPL5 molecule set.


Similar content being viewed by others
References
Mobley DL, Wymer KL, Lim NM, Guthrie JP (2014) Blind prediction of solvation free energies from the SAMPL4 challenge. J Comput Aided Mol Des 28:135–150. doi:10.1007/s10822-014-9718-2
Skillman AG (2012) SAMPL3: blinded prediction of host–guest binding affinities, hydration free energies, and trypsin inhibitors. J Comput Aided Mol Des 26:473–474. doi:10.1007/s10822-012-9580-z
Geballe MT, Skillman AG, Nicholls A et al (2010) The SAMPL2 blind prediction challenge: introduction and overview. J Comput Aided Mol Des 24:259–279. doi:10.1007/s10822-010-9350-8
Guthrie JP (2009) A blind challenge for computational solvation free energies: introduction and overview. J Phys Chem B 113:4501–4507. doi:10.1021/jp806724u
Nieto-Draghi C, Fayet G, Creton B et al (2015) A general guidebook for the theoretical prediction of physicochemical properties of chemicals for regulatory purposes. Chem Rev 115:13093–13164. doi:10.1021/acs.chemrev.5b00215
Le T, Epa VC, Burden FR, Winkler DA (2012) Quantitative structure–property relationship modeling of diverse materials properties. Chem Rev 112:2889–2919. doi:10.1021/cr200066h
Martin YC (2009) Let’s not forget tautomers. J Comput Aided Mol Des 23:693–704. doi:10.1007/s10822-009-9303-2
Cherkasov A, Muratov EN, Fourches D et al (2014) QSAR modeling: where have you been? Where are you going to? J Med Chem 57:4977–5010. doi:10.1021/jm4004285
Gramatica P, Sangion A (2016) A historical excursus on the statistical validation parameters for QSAR models: a clarification concerning metrics and terminology. J Chem Inf Model. doi:10.1021/acs.jcim.6b00088
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001. doi:10.1021/jp9716997
Klamt A, Schüürmann G (1993) COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J Chem Soc, Perkin Trans 2:799–805. doi:10.1039/P29930000799
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. doi:10.1021/jp810292n
Riojas AG, Wilson AK (2014) Solv-ccCA: implicit solvation and the correlation consistent composite approach for the determination of pKa. J Chem Theory Comput 10:1500–1510. doi:10.1021/ct400908z
König G, Pickard FC IV, Mei Y, Brooks BR (2014) Predicting hydration free energies with a hybrid QM/MM approach: an evaluation of implicit and explicit solvation models in SAMPL4. J Comput Aided Mol Des 28:245–257. doi:10.1007/s10822-014-9708-4
König G, Hudson PS, Boresch S, Woodcock HL (2014) Multiscale free energy simulations: an efficient method for connecting classical MD simulations to QM or QM/MM free energies using non-Boltzmann Bennett reweighting schemes. J Chem Theory Comput 10:1406–1419. doi:10.1021/ct401118k
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100. doi:10.1103/PhysRevA.38.3098
Lee C, Yang W, Parr RGR (1998) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627. doi:10.1021/j100096a001
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007. doi:10.1063/1.456153
Woon DE, Dunning TH Jr (1994) Gaussian basis sets for use in correlated molecular calculations. IV. Calculation of static electrical response properties. J Chem Phys 100:2975. doi:10.1063/1.466439
Woon DE, Dunning TH Jr (1993) Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J Chem Phys 98:1358. doi:10.1063/1.464303
Woon DE, Dunning TH Jr (1995) Gaussian basis sets for use in correlated molecular calculations. V. Core-valence basis sets for boron through neon. J Chem Phys 103:4572. doi:10.1063/1.470645
Dunning TH Jr, Peterson KA, Wilson AK (2001) Gaussian basis sets for use in correlated molecular calculations. X. The atoms aluminum through argon revisited. J Chem Phys 114:9244. doi:10.1063/1.1367373
Wang N, Wilson A (2003) Effects of basis set choice upon the atomization energy of the second-row compounds SO2, CCl, and ClO2 for B3LYP and B3PW91. J Phys Chem A 107:6720–6724
Wang NX, Wilson AK (2005) Density functional theory and the correlation consistent basis sets: the tight d effect on HSO and HOS. J Phys Chem A 109:7187–7196. doi:10.1021/jp045622b
Prascher BP, Wilson AK (2007) The behaviour of density functionals with respect to basis set. V. Recontraction of correlation consistent basis sets. Mol Phys 105:2899–2917. doi:10.1080/00268970701749278
Wang NX, Wilson AK (2004) The behavior of density functionals with respect to basis set. I. The correlation consistent basis sets. J Chem Phys 121:7632–7646. doi:10.1063/1.1792071
Laury ML, Boesch SE, Haken I et al (2011) Harmonic vibrational frequencies: scale factors for pure, hybrid, hybrid meta, and double-hybrid functionals in conjunction with correlation consistent basis sets. J Comput Chem 32:2339–2347. doi:10.1002/jcc.21811
Prascher BP, Wilson BR, Wilson AK (2007) Behavior of density functionals with respect to basis set. VI. Truncation of the correlation consistent basis sets. J Chem Phys 127:124110. doi:10.1063/1.2768602
Wang NX, Venkatesh K, Wilson AK (2006) Behavior of density functionals with respect to basis set. 3. Basis set superposition error. J Phys Chem A 110:779–784. doi:10.1021/jp0541664
Wang NX, Wilson AK (2005) Behaviour of density functionals with respect to basis set: II. Polarization consistent basis sets. Mol Phys 103:345–358. doi:10.1080/00268970512331317264
Jiang W, Laury ML, Powell M, Wilson AK (2012) Comparative study of single and double hybrid density functionals for the prediction of 3d transition metal thermochemistry. J Chem Theory Comput 8:4102–4111. doi:10.1021/ct300455e
Bryantsev VS, Diallo MS, van Duin ACT, Goddard WA (2009) Evaluation of B3LYP, X3LYP, and M06-class density functionals for predicting the binding energies of neutral, protonated, and deprotonated water clusters. J Chem Theory Comput 5:1016–1026. doi:10.1021/ct800549f
Rayne S, Rayne S, Forest K (2010) Accuracy of computational solvation free energies for neutral and ionic compounds: dependence on level of theory and solvent model. Nat Preced. doi:10.1038/npre.2010.4864.1
Kelly CP, Cramer CJ, Truhlar DG (2005) SM6: a density functional theory continuum solvation model for calculating aqueous solvation free energies of neutrals, ions, and solute–water clusters. J Chem Theory Comput 1:1133–1152. doi:10.1021/ct050164b
Kelly CP, Cramer CJ, Truhlar DG (2006) Aqueous solvation free energies of ions and ion–water clusters based on an accurate value for the absolute aqueous solvation free energy of the proton. J Phys Chem B 110:16066–16081. doi:10.1021/jp063552y
Takano Y, Houk KN (2005) Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous solvation free energies of neutral and ionic organic molecules. J Chem Theory Comput 1:70–77. doi:10.1021/ct049977a
Guthrie JP, Povar I (2009) A test of various computational solvation models on a set of “difficult” organic compounds. Can J Chem 87:1154–1162. doi:10.1139/V09-071
Tekarli SM, Drummond ML, Williams TG et al (2009) Performance of density functional theory for 3d transition metal-containing complexes: utilization of the correlation consistent basis sets. J Phys Chem A 113:8607–8614. doi:10.1021/jp811503v
Riley KE, Op’t Holt BT, Merz KM (2007) Critical assessment of the performance of density functional methods for several atomic and molecular properties. J Chem Theory Comput 3:407–433. doi:10.1021/ct600185a
Martell JM, Goddard JD, Eriksson LA (1997) Assessment of basis set and functional dependencies in density functional theory: studies of atomization and reaction energies. J Phys Chem A 101:1927–1934. doi:10.1021/jp962783+
Cohen AJ, Mori-Sanchez P, Yang W, Mori-Sánchez P (2012) Challenges for density functional theory. Chem Rev 112:289–320. doi:10.1021/cr200107z
Sousa SF, Fernandes PA, Ramos MJ (2007) General performance of density functionals. J Phys Chem A 111:10439–10452. doi:10.1021/jp0734474
Hamprecht FA, Cohen AJ, Tozer DJ, Handy NC (1998) Development and assessment of new exchange-correlation functionals. J Chem Phys 109:6264–6271. doi:10.1063/1.477267
Schmider HL, Becke AD (1998) Optimized density functionals from the extended G2 test set. J Chem Phys 108:9624–9631. doi:10.1063/1.476438
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Zhao Y, Schultz NE, Truhlar DG (2005) Exchange-correlation functional with broad accuracy for metallic and nonmetallic compounds, kinetics, and noncovalent interactions. J Chem Phys 123:161103. doi:10.1063/1.2126975
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382. doi:10.1021/ct0502763
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor Chem Acc 120:215–241. doi:10.1007/s00214-007-0310-x
Zhao Y, Truhlar DG (2006) Density functional for spectroscopy: no long-range self-interaction error, good performance for Rydberg and charge-transfer states, and better performance on average than B3LYP for ground states. J Phys Chem A 110:13126–13130. doi:10.1021/jp066479k
Chai JD, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128:1–15. doi:10.1063/1.2834918
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615. doi:10.1039/b810189b
Wheeler SE, Houk KN (2010) Integration grid errors for meta-GGA-predicted reaction energies: origin of grid errors for the M06 suite of functionals. J Chem Theory Comput 6:395–404. doi:10.1021/ct900639j
Frisch MJ, Trucks GW, Schlegel HB, et al (2009) Gaussian 09, Revision A.1
Palmer DS, Mitchell JBO (2014) Is experimental data quality the limiting factor in predicting the aqueous solubility of druglike molecules? Mol Pharm 11:2962–2972. doi:10.1021/mp500103r
Denis PA, Ventura ON (2000) Density functional investigation of atmospheric sulfur chemistry. I. Enthalpy of formation of HSO and related molecules. Int J Quantum Chem 80:439–453. doi:10.1002/1097-461X(2000)80:3<439:AID-QUA14>3.0.CO;2-O
Kah M, Brown CD (2008) LogD: lipophilicity for ionisable compounds. Chemosphere 72:1401–1408. doi:10.1016/j.chemosphere.2008.04.074
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, NHLBI. During the beginning of the SAMPL5 competition, AKW and MRJ were at the University of North Texas, where the calculations were done. Thus, the authors gratefully acknowledge Research Computing Services at the University of North Texas for computational resources. The authors thank Frank C. Pickard IV and Yihan Shao for their comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jones, M.R., Brooks, B.R. & Wilson, A.K. Partition coefficients for the SAMPL5 challenge using transfer free energies. J Comput Aided Mol Des 30, 1129–1138 (2016). https://doi.org/10.1007/s10822-016-9964-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-016-9964-6