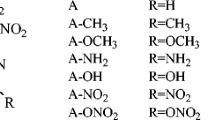Abstract
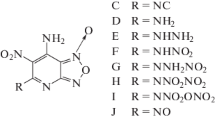
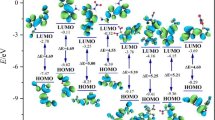
In this work, six (A–F) nitramino (–NHNO2)-substituted ditetrazole 2-N-oxides with different bridging groups (–CH2–, –CH2–CH2–, –NH–, –N=N–, and –NH–NH–) were designed. The six compounds were based on the parent compound tetrazole 2-N-oxide, which possesses a high oxygen balance and high density. The structure, heat of formation, density, detonation properties (detonation velocity D and detonation pressure P), and the sensitivity of each compound was investigated systematically via density functional theory, by studying the electrostatic potential, and using molecular mechanics. The results showed that compounds A–F all have outstanding energetic properties (D: 9.1–10.0 km/s; P: 38.0–46.7 GPa) and acceptable sensitivities (h 50: 28–37 cm). The bridging group present was found to greatly affect the detonation performance of each ditetrazole 2-N-oxide, and the compound with the –NH–NH– bridging group yielded the best results. Indeed, this compound (F) was calculated to have comparable sensitivity to the famous and widely used high explosive 1,3,5,7-tetranitro-1,3,5,7-tetrazocane (HMX), but with values of D and P that were about 8.7% and 19.4% higher than those for HMX, respectively. The present study shows that tetrazole 2-N-oxide is a useful parent compound which could potentially be used in the design of new and improved high-energy compounds to replace existing energetic compounds such as HMX.




Similar content being viewed by others
References
Zhang MX, Eaton PE, Gilardi R (2000) Angew Chem Int Ed 39:401–404
Wu Q, Zhu WH, Xiao HM (2014) J Mater Chem A 2:13006–13015
Göbel M, Karaghiosoff K, Klapötke TM, Piercey DG, Stierstorfer J (2010) J Am Chem Soc 132:17216–17226
Klapötke TM, Piercey DG, Stierstorfer J (2011) Chem Eur J 17:13068–13077
Harel T, Rozen S (2010) J Org Chem 75:3141–3143
Fischer N, Gao L, Klapçtke TM, Stierstorfer J (2013) Polyhedron 51:201–210
Song X, Li J, Hou H, Wang B (2009) J Comput Chem 30:1816–1820
He P, Zhang JG, Wang K, Yin X, Zhang TL (2015) J Org Chem 80:5643–5651
Jia Y, Xin PL, Chao YZ (2016) J Phys Chem A 120:9446–9457
Wei PL, Peng L, Zhong XG, Ying ZL, Tao Y, Jian L (2016) J Mol Model 22:83
Politzer P, Murray JS (2017) Struct Chem 1–19
Trzciński WA, Cudziło S, Chyłek Z, Szymańczyk L (2008) J Hazard Mater 157:605–612
Simpson RL, Urtiew PA, Ornellas DL, Moody GL, Scribner KJ, Hoffman DM (1997) Pyrotech 22:249–255
Kamlet MJ, Jacobs SJ (1968) J Chem Phys 48:23–35
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) Mol Phys 107:2095–2101
Atkins PW (1982) Physical chemistry. Oxford University Press, Oxford
Byrd EFC, Rice BM (2006) J Phys Chem A 199:1005–1013
PospĺŠil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) J Mol Model 16:895–901
PospĺŠil M, Vávra P, Concha MC, Murray JS, Politzer P (2011) J Mol Model 17:2569–2574
Politzer P, Murray JS (2014) J Mol Model 20:2223–2230
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko, A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2009) Gaussian 09, revision A.01. Gaussian, Inc., Wallingford
Zhao Y, Truhlar DG (2008) Theor Chem Accounts 120:215–241
Politzer P, Murray JS (2015) J Mol Model 21:262
Singh HJ, Upadhyaya MK (2013) J Energ Mater 31:301
Wang GX, Shi CH, Gong XD, Zhu WH, Xiao HM (2009) J Hazard Mater 169:813–818
Wang F, Du HC, Zhang JY (2011) J Phys Chem A 115:11788–11795
Acknowledgements
The present work was supported by the Natural Science Foundation of the Nanjing Institute of Technology (YKJ201507, CKJA201603), the National Natural Science Foundation of China (NSFC21603102) and the Outstanding Scientific and Technological Innovation Team in Colleges and Universities of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Wu, Q., Kou, B., Hang, Z. et al. Comparative theoretical studies of differently bridged nitramino-substituted ditetrazole 2-N-oxides with high detonation performance and an oxygen balance of around zero. J Mol Model 23, 186 (2017). https://doi.org/10.1007/s00894-017-3359-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3359-z