Abstract
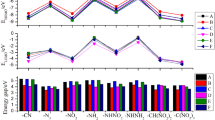
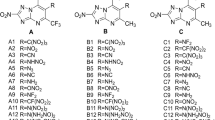
In this study, 32 energetic compounds were designed using oxadiazoles (1,2,5-oxadiazole, 1,3,4-oxadiazole) as the parent by inserting different groups as well as changing the bridge between the parent. These compounds had high density and excellent detonation properties. The electrostatic potentials of the designed compounds were analyzed using density functional theory (DFT). The structure, heat of formation (HOF), density, detonation performances (detonation pressure P, detonation velocity D, detonation heat Q), and thermal stability of each compound were systematically studied based on molecular dynamics. The results showed that the -N3 group has the greatest improvement in HOF. For the detonation performances, the directly linked -N=N- and -NH-NH- were beneficial when used as a bridge between 1,2,5-oxadiazole and 1,3,4-oxadiazole, and it can also be found that bridge changing had little effect on the trend of detonation performance, while energetic groups changing influenced differently. In general, the introduction of nitro groups contributes to the improvement of the detonation performance of the compounds. In this study, the compounds containing the highest amount of nitro groups were found to have better detonation performance than their counterparts and were not significantly different from RDX and HMX.







Similar content being viewed by others
References
Thottempudi V, Gao H, Shreeve JN (2011) Trinitromethyl-substituted 5-nitro-or 3-azo-1, 2, 4-triazoles: synthesis, characterization, and energetic properties. J Am Chem Soc 133(16):6464–6471. https://doi.org/10.1021/ja2013455
Wu Q, Zhu W, Xiao H (2014) A new design strategy for high-energy low-sensitivity explosives: combining oxygen balance equal to zero, a combination of nitro and amino groups, and N-oxide in one molecule of 1-amino-5-nitrotetrazole-3 N-oxide. J Mater Chem A 2(32):13006–13015. https://doi.org/10.1039/C4TA01879F
Sikder AK, Sikder N (2004) A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J Hazard Mater 112(1–2):1–15. https://doi.org/10.1016/j.jhazmat.2004.04.003
Ma P, Wang J, Zhai D, Hao L, Ma C, Pan Y, Jiang J, Zhu S (2019) Structural transformation and absorption properties of 2, 4, 6-trinitro-2, 4, 6-triazacyclohexanone under high pressures. J Mol Struct 1196:691–698. https://doi.org/10.1016/j.molstruc.2019.07.020
Ma P, Pan Y, Jiang JC, Zhu SG (2018) Molecular dynamic simulation and density functional theory insight into the nitrogen rich explosive 1, 5-diaminotetrazole (DAT). Procedia Eng 211:546–554. https://doi.org/10.1016/j.proeng.2017.12.047
Sheremetev AB (1995) Chemistry of furazans fused to five-membered rings. J Heterocyclic Chem 32(2):371–385. https://doi.org/10.1002/jhet.5570320201
Pivina TS, Sukhachev DV, Evtushenko AV, Khmelnitskii LI (1995) Comparative characteristic of energy content calculating methods for the furazan series as an example of energetic materials. Propellants Explos Pyrotech 20(1):5–10. https://doi.org/10.1002/prep.19950200103
Sheremetev AB, Mantseva EV (1996) One-pot synthesis of 4, 4′-diamino-3, 3′-bifurazan. Mendeleev Commun 6(6):246–247. https://doi.org/10.1070/MC1996v006n06ABEH000745
Zhang C (2006) Computational investigation of the detonation properties of furazans and furoxans. THEOCHEM J Mol Struct 765(1–3):77–83. https://doi.org/10.1016/j.theochem.2006.03.007
Tang Y, He C, Mitchell LA, Parrish DA, Jean’Ne MS (2015) Energetic compounds consisting of 1, 2, 5-and 1, 3, 4-oxadiazole rings. J Mater Chem A 3.46 (2015): 23143–23148. https://doi.org/10.1039/C5TA06898C
Wang Q, Shao Y, Lu M (2019) Azo1,3,4-oxadiazole as a novel building block to design high-performance energetic materials. Cryst Growth Des 19(2):839–844. https://doi.org/10.1021/acs.cgd.8b01404
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X (2016). Gaussian:16
Fan XW, Ju XH (2008) Theoretical studies on four-membered ring compounds with NF2, ONO2, N3, and NO2 groups. J Comput Chem 29(4):505–513. https://doi.org/10.1002/jcc.20809
Wei T, Zhu W, Zhang X, Li YF, Xiao H (2009) Molecular design of 1, 2, 4, 5-tetrazine-based high-energy density materials. J Phys Chem A 113(33):9404–9412. https://doi.org/10.1021/jp902295v
Pan Y, Li J, Cheng B, Zhu W, Xiao H (2012) Computational studies on the heats of formation, energetic properties, and thermal stability of energetic nitrogen-rich furazano [3, 4-b] pyrazine-based derivatives. Comput Theor Chem 992:110–119. https://doi.org/10.1016/j.comptc.2012.05.013
O’Malley R (1983) Physical chemistry. J Chem Educ 60(2):A63. https://doi.org/10.1021/ed060pA63.2
Politzer P, Ma Y, Lane P, Concha MC (2005) Computational prediction of standard gas, liquid, and solid-phase heats of formation and heats of vaporization and sublimation. Int J Quantum Chem 105(4):341–347. https://doi.org/10.1002/qua.20709
Byrd EF, Rice BM (2006) Improved prediction of heats of formation of energetic materials using quantum mechanical calculations. J Phys Chem A 110(3):1005–1013. https://doi.org/10.1021/jp0536192
Lu T, Chen F (2012) Multiwfn: a multifunctional wave function analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) An electrostatic interaction correction for improved crystal density prediction. Mol Phys 107(19):2095–2101. https://doi.org/10.1080/00268970903156306
Kamlet MJ, Jacobs SJ (1968) Chemistry of detonations. I. A simple method for calculating detonation properties of C–H–N–O explosives. J Chem Phys 48(1):23–35. https://doi.org/10.1063/1.1667908
Lin H, Zhu Q, Huang C, Yang DD, Lou N, Zhu SG, Li HZ (2019) Dinitromethyl, fluorodinitromethyl derivatives of RDX and HMX as high energy density materials: a computational study. Struct Chem 30(6):2401–2408. https://doi.org/10.1007/s11224-019-01366-1
Stobiecka A, Sikora M, Bonikowski R, Kula J (2016) An exploratory study on the peroxyl-radical-scavenging activity of 2, 6-dimethyl-5-hepten-2-ol and its heterocyclic analogues. J Mol Struct 1107:82–90. https://doi.org/10.1016/j.molstruc.2015.11.043
Zhai D, Ma C, Ma P, Pan Y, Hao L, Liu X, Jiang J (2021) Theoretical insight into different energetic groups on the performance of energetic materials featuring RDX ring. Fuel 294:120497. https://doi.org/10.1016/j.fuel.2021.120497
Politzer P, Murray JS (2017) High performance, low sensitivity: the impossible (or possible) dream? Energetic materials. Springer, Cham, pp 1–22
Qu Y, Babailov SP (2018) Azo-linked high-nitrogen energetic materials. J Mater Chem A 6(5):1915–1940. https://doi.org/10.1039/C7TA09593G
Gutowski KE, Rogers RD, Dixon DA (2007) Accurate thermochemical properties for energetic materials applications. II. Heats of formation of imidazolium-, 1, 2, 4-triazolium-, and tetrazolium-based energetic salts from isodesmic and lattice energy calculations. J Phys Chem B 111(18):4788–4800. https://doi.org/10.1021/jp066420d
Miroshnichenko EA, Kon’kova TS, Matyushin YN (2003) Thermochemistry of primary nitramines. Dokl Phys Chem 392:253–255. https://doi.org/10.1023/A:1026182227885
Zhai D, Wang J, Hao L, Ma C, Ma P, Pan Y, Jiang J (2019) Molecular design and properties of bridged energetic pyridines derivatives. RSC Adv 9(65):37747–37758. https://doi.org/10.1039/C9RA07087G
Furka Á (2009) Relative energy of organic compounds II. Halides, nitrogen, and sulfur compounds. Struct Chem 20(4):605–616. https://doi.org/10.1007/s11224-009-9450-z
Mader CL (2007) Numerical modeling of explosives and propellants. CRC press
Wu Q, Pan Y, Xia X, Shao Y, Zhu W, Xiao H (2013) Theoretic design of 1, 2, 3, 4-tetrazine-1, 3-dioxide-based high-energy density compounds with oxygen balance close to zero. Struct Chem 24(5):1579–1590. https://doi.org/10.1007/s11224-012-0190-0
Yu Q, Wang Z, Wu B, Yang H, Ju X, Lu C, Cheng G (2015) A study of N-trinitroethyl-substituted aminofurazans: high detonation performance energetic compounds with good oxygen balance. J Mater Chem A 3(15):8156–8164. https://doi.org/10.1039/C4TA06974A
Hao L, Liu X, Zhai D, Qiu L, Ma C, Ma P, Jiang J (2020) Theoretical studies on the performance of HMX with different energetic groups. ACS Omega 5(46):29922–29934. https://doi.org/10.1021/acsomega.0c04237
Henry DJ, Parkinson CJ, Mayer PM, Radom L (2001) Radom, Bond dissociation energies and radical stabilization energies associated with substituted methyl radicals. J Phys Chem A 105.27: 6750–6756. https://doi.org/10.1021/jp010442c
Rice BM, Sahu S, Owens FJ (2002) Density functional calculations of bond dissociation energies for NO2 scission in some nitroaromatic molecules. THEOCHEM J Mol Struct 583(1–3):69–72. https://doi.org/10.1016/S0166-1280(01)00782-5
Harris NJ, Lammertsma K (1997) Ab initio density functional computations of conformations and bond dissociation energies for hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine. J Am Chem Soc 119(28):6583–6589. https://doi.org/10.1021/ja970392i
Lu T, Chen F (2013) Bond order analysis based on the Laplacian of electron density in fuzzy overlap space. J Phys Chem A 117(14):3100–3108. https://doi.org/10.1021/jp4010345
Chung G, Schmidt MW, Gordon MS (2000) An ab initio study of potential energy surfaces for N8 isomers. J Phys Chem A 104(23):5647–5650. https://doi.org/10.1021/jp0004361
Murray JS, Concha MC, Politzer P Links between surface electrostatic potentials of energetic molecules, impact sensitivities and C–NO2/N–NO2 bond dissociation energies. Mol Phys 107.1: 89–97. https://doi.org/10.1080/00268970902744375
Peter JS (1998) Effects of strongly electron-attracting components on molecular surface electrostatic potentials: application to predicting impact sensitivities of energetic molecules. Mol Phys 93(2):187–194. https://doi.org/10.1080/002689798169203
Rice BM, Hare JJ (2002) A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J Phys Chem A 106(9):1770–1783. https://doi.org/10.1021/jp012602q
Availability of data and material
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Funding
This study was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 20KJB620001) and the National Natural Science Foundation of China (Grant No. 11702129).
Author information
Authors and Affiliations
Contributions
Wenxin Xia: software, data curation, writing—original draft preparation, formal analysis; Congming Ma: conceptualization, methodology, Peng Ma: resources, project administration; Renfa Zhang: investigation, formal analysis; Xiaosong Xu: investigation, validation; Yong Pan: resources; Juncheng Jiang: resources, supervision
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1742 kb)
Rights and permissions
About this article
Cite this article
Xia, W., Zhang, R., Xu, X. et al. Theoretical studies of novel high energy density materials based on oxadiazoles. J Mol Model 27, 204 (2021). https://doi.org/10.1007/s00894-021-04805-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04805-1