Abstract
Hydration reactions of two anticancer Pt(IV) complexes JM149 and JM216 (Satraplatin) were studied computationally together with the hydration of the Pt(II) complex JM118, which is a product of the Satraplatin reduction. Thermodynamic and kinetic parameters of the reactions were determined at the B3LYP/6-311++G(2df.2pd)//B3LYP/6-31 + G(d)) level of theory. The water solution was modeled using the COSMO implicit solvation model, with cavities constructed using Klamt’s atomic radii. It was found that hydration of the Pt(IV) complexes is an endergonic/endothermic reaction. It follows the (pseudo)associative mechanism is substantially slower (k ≈ 10-11 s−1) than the corresponding reaction of Pt(II) analogues ((k ≈ 10-5 s−1). Such a low value of the reaction constant signifies that the hydration of JM149 and Satraplatin is with high probability a kinetically forbidden reaction. Similarly to JM149 and Satraplatin, the hydration of JM118 is an endothermic/endoergic reaction. On the other hand, the kinetic parameters are similar to those of cisplatin Zimmermann et al. (J Mol Model 17:2385–2393, 2011), allowing the hydration reaction to occur at physiological conditions. These results suggest that in order to become active Satraplatin has to be first reduced to JM118, which may be subsequently hydrated to yield the active species.

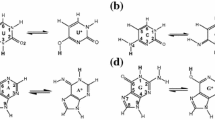
Comparison of the reaction profiles of JM216, JM149, JM118, and cisplatin









Similar content being viewed by others
References
Zimmermann T, Leszczynski J, Burda JV (2011) J Mol Model 17:2385–2393
Alderden RA, Hall MD, Hambley TW (2006) J Chem Educ 83:728–724
Wheate NJ, Walker S, Craig GE, Oun R (2010) Dalton Trans 39:8113–8127
Ehrsson H, Wallin I, Yachnin J (2002) Med Oncol 19:261–265
Lippert B (ed) (1999) Cisplatin: chemistry and biochemistry of a leading anticancer drug. Wiley-VCH Verlag GmbH, Wienheim, 1999
Kasparkova J, Marini V, Najajreh Y, Gibson D, Brabec V (2003) Biochem 42:6321–6332
Bloemink MJ, Heetebrij RJ, Ireland J, Deacon GB, Reedijk J (1996) J Biol Inorg Chem 1:278–283
Farrell N, Kelland LR, Roberts JD, Van Beusichem M (1992) Cancer Res 52:5065–5072
Kasparkova J, Novakova O, Farrell N, Brabec V (2003) Biochemistry 42:792–800
Deubel DV (2006) J Am Chem Soc 128:1654–1663
Lau JKC, Deubel DV (2006) J Chem Theory Comput 2:103–106
Chval Z, Šíp M (2000) J Mol Struct (THEOCHEM) 532:59–68
Chval Z, Sip M, Burda JV (2008) J Comput Chem 29:2370–2381
Raber J, Zhu C, Eriksson LA (2005) J Phys Chem 109:11006–11015
Zhu C, Raber J, Eriksson LA (2005) J Phys Chem B 109:12195–12205
Costa LA, Hambley TW, Rocha WR, Almeida WB, Dos Santos HF (2006) Int J Quantum Chem 106:2129–2144
Costa LAS, Rocha WR, De Almeida WB, Dos Santos HF (2003) J Chem Phys 118:10584–10592
Costa LAS, Rocha WR, De Almeida WB, Dos Santos HF (2005) J Inorg Biochem 99:575–583
Dos Santos HF, Marcial BL, De Miranda CF, Costa LAS, De Almeida WB (2006) J Inorg Biochem 100:1594–1605
Lopes JF, Menezes VSD, Duarte HA, Rocha WR, De Almeida WB, Dos Santos HF (2006) J Phys Chem B 110:12047–12054
Lopes JF, Rocha WR, Dos Santos HF, De Almeida WB (2008) J Chem Phys 128:165103
Lopes JF, Rocha WR, dos Santos HF, de Almeida WB (2010) J Braz Chem Soc 21:887–896
Carloni P, Sprik M, Andreoni W (2000) J Phys Chem B 104:823–835
Spiegel K, Rothlisberger U, Carloni P (2004) J Phys Chem B 108:2699–2707
Burda JV, Zeizinger M, Šponer J, Leszczynski J (2000) J Chem Phys 113:2224–2232
Burda JV, Zeizinger M, Leszczynski J (2005) J Comput Chem 26:907–914
Burda JV, Šponer J, Hrabáková J, Zeizinger M, Leszczynski J (2003) J Phys Chem B 107:5349–5356
Burda JV, Šponer J, Leszczynski J (2000) J Biol Inorg Chem 5:178–188
Burda JV, Leszczynski J (2003) Inorg Chem 42:7162–7172
Zeizinger M, Burda JV, Leszczynski J (2004) Phys Chem Chem Phys 6:3585–3590
Zimmermann T, Zeizinger M, Burda JV (2005) J Inorg Biochem 99:2184–2196
Zimmermann T, Burda JV (2009) J Chem Phys 131:135101
Zimmermann T, Burda JV (2010) Dalton Trans 39:1295–1301
Fokkema E, Groen HJM, Helder MN, de Vries EGE, Meijer C (2002) Biochem Pharmacol 63:1989–1996
Galettis P, Carr JL, Paxton JW, McKeage MJ (1999) J Anal At Spectrom 14:953–956
Kelland LR, Abel G, McKeage MJ, Jones M, Goddard PM, Valenti M, Murrer BA, Harrap KR (1993) Cancer Res 53:2581–2586
Goddard PM, Orr RM, Valenti MR, Barnard CF, Murrer BA, Kelland LR, Harrap KR (1996) Anticancer Res 16:33–38
Poon GK, Mistry P, Raynaud FI, Harrap KR, Murrer BA, Barnard CFJ (1995) J Pharm Biomed Anal 13:1493–1498
Kelland LR, Sharp SY, O’Neill CF, Raynaud FI, Beale PJ, Judson IR (1999) J Inorg Biochem 77:111–115
Raynaud FI, Mistry P, Donaghue A, Poon GK, Kelland LR, Barnard CFJ, Murrer BA, Harrap KR (1996) Cancer Chemother Pharmacol 38:155–162
Farrell N, Povirk LF, Dange Y, DeMasters G, Gupta MS, Kohlhagen G, Khan QA, Pommier Y, Gewirtz DA (2004) Biochem Pharmacol 68:857–866
Andzelm J, Kolmel C, Klamt A (1995) J Chem Phys 103:9312–9320
Klamt A, Schuurmann G (1993) J Chem Soc Perkin Trans:2799–805.
Andrae D, Haussermann U, Dolg M, Stoll H, Preuss H (1990) Theor Chim Acta 77:123–141
Bergner A, Dolg M, Kuechle W, Stoll H, Preuss H (1993) Mol Phys 80:1431–1439
Chval Z, Futera Z, Burda JV (2011) J Chem Phys 134:024520
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Urban M, Hobza P (1975) Theor Chim Acta 36:207–214
Urban M, Hobza P (1975) Theor Chim Acta 36:215–220
Weinhold F (2001) NBO 5.0 Program University of Wisconsin, Madison, Wisconsin 53706, Wisconsin.
Hammond GS (1955) J Am Chem Soc 77:334–338
Acknowledgments
Authors are grateful to grant projects of Ministerium of Education ME-10149 and Grant Agency of the Czech Republic No. P208/12/0622 for financial support of this study. Part of the calculations was performed in Meta-supercomputational centers in Prague and Brno.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bradáč, O., Zimmermann, T. & Burda, J.V. Can Satraplatin be hydrated before the reduction process occurs? The DFT computational study. J Mol Model 19, 4669–4680 (2013). https://doi.org/10.1007/s00894-012-1442-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1442-z