Abstract
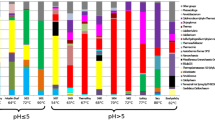
Los Azufres spa consists of a hydrothermal spring system in the Mexican Volcanic Axis. Five samples (two microbial mats, two mud pools and one cenote water), characterized by high acidity (pH between 1 and 3) and temperatures varying from 27 to 87 °C, were investigated for their microbial diversity by Terminal-Restriction Fragment Length Polymorphism (T-RFLP) and 16S rRNA gene library analyses. These data are the first to describe microbial diversity from Los Azufres geothermal belt. The data obtained from both approaches suggested a low bacterial diversity in all five samples. Despite their proximity, the sampling points differed by their physico-chemical conditions (mainly temperature and matrix type) and thus exhibited different dominant bacterial populations: anoxygenic phototrophs related to the genus Rhodobacter in the biomats, colorless sulfur oxidizers Acidithiobacillus sp. in the warm mud and water samples, and Lyzobacter sp.-related populations in the hot mud sample (87 °C). Molecular data also allowed the detection of sulfate and sulfur reducers related to Thermodesulfobium and Desulfurella genera. Several strains affiliated to both genera were enriched or isolated from the mesophilic mud sample. A feature common to all samples was the dominance of bacteria involved in sulfur and iron biogeochemical cycles (Rhodobacter, Acidithiobacillus, Thiomonas, Desulfurella and Thermodesulfobium genera).







Similar content being viewed by others
Abbreviations
- AM1 and AM2:
-
Los Azufres mud samples (AM1, 87 °C and AM2, 37 °C)
- AB1 and AB2:
-
Los Azufres microbial mat samples (27 and 35 °C, respectively)
- AW:
-
Los Azufres water sample (36 °C at the surface)
- AZLFE3:
-
Isolated strain from AM2 sample, related to Desulfurella kamchatkensis
References
Aguilar Y, Vargas VH, Verma SP (1987) Composición química (elementos mayores) de los magmas en el cinturón volcánico mexicano. Geofis Int 26:195–272
Anderson A (1979) Mercury in soils. In: Nriagu JO (ed) The biogeochemistry of mercury in the environment. Elsevier, Amsterdam, pp 79–112
Bagnato E, Aiuppa A, Parello F, D’Alessandro W, Allard PS (2009a) Calabrese mercury concentration, speciation and budget in volcanic aquifers: Italy and Guadeloupe (Lesser Antilles). J Volcanol Geotherm Res 179:96–106
Bagnato E, Parello F, Valenza M, Caliro S (2009b) Mercury content and speciation in the Phlegrean Fields volcanic complex: evidence from hydrothermal system and fumaroles. J Volcanol Geotherm Res 187:250–260
Bagnato E, Allard P, Parello F, Aiuppa A, Calabrese S, Hammouya G (2009c) Mercury gas emissions from La Soufrière volcano, Guadeloupe island (Lesser Antilles). Chem Geol 266:267–273
Barns SM, Fundyga RE, Jeffries MW, Pace NR (1994) Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc Natl Acad Sci USA 91:1609–1613
Bastos WR, Malm O, Pfeiffer WC, Cleary D (1998) Establishment and analytical quality control of laboratories for Hg determination in biological and geological samples in the Amazon, Brazil. Ciencia e Cultura 50:255–260
Birkle P, Merkel B (2001) Mineralogical–chemical composition and environmental risk potential of pond sediments at the geothermal field of Los Azufres, Mexico. Environ Geol 41:583–592
Birkle P, Merkle B (2000) Environmental impact by spill of geothermal fluids at the geothermal field of Los Azufres, Michoacán, Mexico. Water Air Soil Pollut 124:371–410
Blank CE, Cady SL, Pace NR (2002) Microbial composition of near-boiling silica-depositing thermal springs throughout Yellowstone National Park. Appl Environ Microbiol 68:5123–5135
Brito EMS, Guyoneaud R, Goñi-Urriza M, Ranchou-Peyrouse A, Verbaere A, Crapez MAC, Wasserman JCA, Duran R (2006) Characterization of hydrocarbonoclastic bacterial communities from mangrove sediments of Guanabara Bay, Brazil. Res Microbiol 157:752–762
Brito EMS, Andrade LH, Caretta CA, Duran R (2007) Microorganisms bioprospection: a new tendency in microbial ecology. In: Pawley LE (ed) Leading-edge environmental biodegradation research, 1st edn. Nova Sc Publ, New York, pp 199–222
Brock TD, Freeze H (1969) Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol 98:289–297
Brito EMS, Piñón-Castillo H, Guyoneaud R, Caretta CA, Gutiérrez-Corona, JF, Duran R, Reyna-López G, Nevárez-Moorillón VG, Fahy A, Goñi-Urriza M (2013) Bacterial biodiversity from anthropogenic extreme environments: a hyper-alkaline and hyper-saline industrial residue contaminated by chromium and iron. Appl Microbiol Biotechnol 97:369–378
Brock TD, Brock KM, Belly RT, Weiss RL (1972) Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol 84:54–68
Burton NP, Norris PR (2000) Microbiology of acidic, geothermal springs of Montserrat: environmental rDNA analysis. Extremophiles 4:315–320
Camerlenghi A, Cita MB, Della Vedova B, Fusi N, Mirabile L, Pellis G (1995) Geophysical evidence of mud diapirism on the mediterranean ridge accretionary complex. Mar Geophys Res 17:115–141
Caretta CA, Brito EMS (2011) In silico restriction for identifying microbial communities in T-RFLP fingerprints. J Comp Interdiscip Sci 2:123–129
Castorena G, Mugica V, Le Borgne S, Acun ME, Bustos-Jaimes I, Aburto J (2006) Carbazole biodegradation in gas oil/water biphasic media by a new isolated bacterium Burkholderia sp. strain IMP5GC. J Appl Microbiol 100:739–745
Chao A, Lee SM (1992) Estimating the number of classes via sample coverage. J Am Stat Assoc 87:210–217
Christensen P, Cook FD (1978) Lysobacter, a new genus of nonfruiting, gliding bacteria with a high base ratio [soil and water organisms]. Int J Syst Bacteriol 28:367–393
Christenson BW, Mroczek EK (2003) Potential reaction pathways of Hg in some New Zealand hydrothermal environments. Soc Econ Geol 10:111–132
Clingenpeel S, Macur RE, Kan J, Inskeep WP, Lovalvo D, Varley J, Mathur E, Nealson K, Gorby Y, Jiang H, LaFracois T, McDermott TR (2011) Yellowstone Lake: high-energy geochemistry and rich bacterial diversity. Environ Microbiol 13:2172–2185
Fliermans CB, Brock TD (1972) Ecology of sulfur-oxidizing bacteria in hot acid soils. J Bacteriol 111:343–350
Folman LB, Postma J, van Veen JA (2003) Characterisation of Lysobacter enzymogenes (Christensen and Cook 1978) strain 3.1T8, a powerful antagonist of fungal diseases of cucumber. Microbiol Res 158:107–115
Giggenbach WF (1988) Geothermal solute equilibria derivation of Na–K–Mg–Ca geoindicators. Geochim Cosmochim Acta 52:2749–2763
Gomez-Alvarez V, King GM, Nusslein K (2007) Comparative bacterial diversity in recent Hawaiian volcanic deposits of different ages. FEMS microbial Ecol 60:60–73
Good IJ (1953) On the population frequencies of species and the estimation of population parameters. Biometrika 40:237–264
Grüning B, Hills G, Veit T, Weitemeyer C, Frave-Bulle O, Nguyen H-K, Ravot G (2009) Thermostable esterases from thermophilic bacteria. US Paten 7,595,181 B2
Guidry SA, Chafetz HS (2002) Factors governing subaqueous siliceous sinter precipitation in hot springs: examples from Yellowstone National Park, USA. Sedimentology 49:1253–1267
Gumerov VM, Mardanov AV, Beletsky AV, Bonch-Osmolovskaya EA, Ravin NV (2011) Molecular analysis of microbial diversity in the Zavarzin spring, Uzon Caldera, Kamchatka. Microbiol 80:244–251
Guyoneaud R, Matheron R, Baulaigue R, Podeur K, Hirschler A, Caumette P (1996) Anoxygenic phototrophic bacteria in eutrophic coastal lagoons of the French Mediterranean and Atlantic coasts (Prévost Lagoon, Arcachon Bay, Certes Fishponds). Hydrobiologia 329:33–43
Guyoneaud R, Mouné S, Eatock C, Bothorel V, Hirschler-Réa A, Willison J, Duran R, Liesack W, Herbert R, Matheron R, Caumette P (2002) Characterization of three spiral-shaped purple nonsulfur bacteria isolated from coastal lagoon sediments, saline sulfur springs, and microbial mats: emended description of the genus Roseospira and description of Roseospira marina sp. nov., Roseospira navarrensis sp. nov., and Roseospira thiosulfatophila sp. nov. Arch Microbiol 178:315–324
Hall JR, Mitchell KR, Jackson-Weaver O, Kooser AS, Cron BR, Crossey LJ, Takacs-Vesbach CD (2008) Molecular characterization of the diversity and distribution of a thermal spring microbial community by using rRNA and metabolic genes. Appl Environ Microbiol 74:4910–4922
Hamilton-Brehm SD, Mosher JJ, Vishnivetskaya T, Podar M, Carrol S, Allman S, Phelps TJ, Keller M, Elkins JG (2010) Caldicellulosiruptor obsidiansis sp. nov., an anaerobic, extremely thermophilic, cellulolytic bacterium isolated from Obsidian Pool, Yellowstone National Park. Appl Environ Microbiol 76:1014–1020
Hedrich S, Schlömann M, Johnson DB (2011) The iron-oxidizing proteobacteria. Microbiol 157:1551–1564
Hetzer A, Morgan HW, McDonald IR, Daughney CJ (2007) Microbial life in Champagne Pool, a geothermal spring in Waiotapu, New Zealand. Extremophiles 11:605–614
Hewson I, Fuhrman JA (2006) Improved strategy for comparing microbial assemblage fingerprints. Microb Ecol 51:147–153
Hovland M, Hill A, Stokes D (1997) The structure and geomorphology of the Dashgil mud volcano, Azerbaijan. Geomorphology 21:1–15
Huang Q, Dong CZ, Dong RM, Jiang H, Wang S, Wang G, Fang B, Ding X, Niu L, Li X, Zhang C, Dong H (2011) Archaeal and bacterial diversity in hot springs on the Tibetan Plateau, China. Extremophiles 15:549–563
Huber R, Eder W, Heldwein S, Wanner G, Huber H, Rachel R, Stetter KO (1998) Thermocrinisruber gen. nov., sp. nov., a pink-filament-forming hyperthermophilic bacterium isolated from Yellowstone National Park. Appl Environ Microbiol 64:3576–3583
Hugenholtz P, Pitulle C, Hershberger KL, Pace NR (1998) Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol 180:366–376
Imhoff JF (2001) Transfer of Rhodopseudomonas acidophila to the new genus Rhodoblastus as Rhodoblastus acidophilus gen. nov., comb. nov. Int J Syst Evol Microbiol 51:1863–1866
Inagaki F, Hayashi S, Doi K, Motomura Y, Izawa E, Ogata S (1997) Microbial participation in the formation of siliceous deposits from geothermal water and analysis of the extremely thermophilic bacterial community. FEMS Microbiol Ecol 24:41–48
Jenne EA (1970) Atmospheric and fluvial transport of mercury. Mercury in the environment. Geol Surv Prof 713:40–45
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Tot Environ 338:3–14
Jones B, Renaut RW (1996) Influence of thermophilic bacteria on calcite and silica precipitation in hot springs with water temperatures above 90 °C: evidence from Kenya and New Zealand. Can J Earth Sci 33:72–83
Jones B, Renaut RW (1997) Formation of silica oncoids around geysers and hot springs at El Tatio, northern Chile. Sedimentology 44:287–304
Jones B, Renaut RW, Rosen MR (2000) Stromatolites forming in acidic hot-spring waters, North Island, New Zealand. Palaios 15:450–475
Kaksonen AH, Puhakka JA (2007) Sulfate reduction based bioprocess for the treatment of acid mine drainage and recovery of metals. Eng Life Sci 7:541–564
Kimura H, Sugihara M, Yamamoto H, Patel BK, Kato K, Hanada S (2005) Microbial community in a geothermal aquifer associated with the subsurface of the Great Artesian Basin, Australia. Extremophiles 9:407–414
Lane DJ (1991) rRNA sequencing. In: Stackebrandt GME (ed) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 115–175
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analysis. Proc Natl Acad Sci USA 82:6955–6959
Lee JW, Im W-T, Kim MK, Yang D-C (2006) Lysobacter koreensis sp. nov., isolated from a ginseng field. Int J Syst Evol Microbiol 56:231–235
Liu M, Liu Y, Wang Y, Luo X, Dai J, Fang C (2011) Lysobacter xinjiangensis sp. nov., a moderately thermotolerant and alkalitolerant bacterium isolated from a gamma-irradiated sand soil sample. Int J Syst Evol Microbiol 61:433–437
Malm O, Pfeiffer WC, Fiszman M, Azcue JMP (1989) Heavy metal concentrations and availability in the bottom sediments of the Paraiba do Sul-Guandu river system, RJ, Brazil. Environ Technol Lett 10:675–680
Miroshnichenko ML, Rainey FA, Hippe H, Chernyh NA, Kostrikina NA, Bonch-Osmolovskaya EA (1998) Desulfurella karnchatkensis sp. nov. and Desulfurella propionica sp. nov., new sulfur-respiring thermophilic bacteria from Kamchatka thermal environments. Int J Syst Bacteriol 48:475–479
Newton RS, Cunningham RC, Schubert CE (1980) Mud volcanoes and pockmarks: seafloor engineering hazards or geological curiosities? Proc Offshore Technol Conf Pap OTC 3729:425–435
Ogram A, Sayler GS, Barkay T (1987) The extraction and purification of microbial DNA from sediments. J Microbiol Methods 7:57–66
Paissé S, Coulon F, Goñi-Urriza M, Peperzak L, McGenity TJ, Duran R (2008) Structure of bacterial communities along a hydrocarbon contamination gradient in a coastal sediment. FEMS Microbiol Ecol 66:295–305
Park JH, Kim R, Aslam Z, Jeon CO, Chung YR (2008) Lysobacter capsici sp. nov., with antimicrobial activity, isolated from the rhizosphere of pepper, and emended description of the genus Lysobacter. Int J Syst Evol Microbiol 58:387–392
Romanenko LA, Uchino M, Tanaka N, Frolova GM, Mikhailov VV (2008) Lysobacter spongiicola sp. nov., isolated from a deep-sea sponge. Int J Syst Evol Microbiol 58:370–374
Santos R, Fernandes J, Fernandes N, Oliveira F, Cadete M (2007) Mycobacterium parascrofulaceum in acidic hot springs in Yellowstone National Park. Appl Environ Microbiol 73:5071–5073
Sayeh R, Birrien JL, Alain K, Barbier G, Hamdi M, Prieur D (2010) Microbial diversity in Tunisian geothermal springs as detected by molecular and culture-based approaches. Extremophiles 14:501–514
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506
Schultze-Lam S, Ferris FG, Konhauser KO, Wiese RG (1995) In situ silicification of an Icelandic hot spring microbial mat: implications for microfossil formation. Can J Earth Sci 32:2021–2026
Silva AP, Alves MCC (2006) Como iniciar a validação de métodos analíticos. ENQUALAB-2006-Congresso e Feira da Qualidade em Metrologia: 8–15
Tobler DJ, Benning LG (2011) Bacterial diversity in five Icelandic geothermal waters: temperature and sinter growth rate effects. Extremophiles 15:473–485
Tobler DJ, Stefansson A, Benning LG (2008) In situ grown silica sinters in Icelandic geothermal areas. Geobiology 6:481–502
Tomova I, Stoilova-Disheva M, Lyutskanova D, Pascual J, Petrov P, Kambourova M (2010) Phylogenetic analysis of the bacterial community in a geothermal spring, Rupi Basin, Bulgaria. World J Microbiol Biotechnol 26:2019–2028
Torres-Sanchez R, Magaña-Vazquez A, Sanchez-Yañez JM, Martinez-Gomez L (1996) High temperature microbial corrosion in the condenser of a geothermal electric power unit. CORROSION96 The NACE Int Ann Conf Exp, No. 293, pp 1–14
Tsai YL, Olson BH (1991) Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol 57:1070–1074
Tsubokura A, Yoneda H, Mizuta H (1999) Paracoccus carotinifaciens sp. nov., a new aerobic gram-negative astaxanthin-producing bacterium. Int J Syst Bacteriol 49:277–282
US EPA (1990) National oil and hazardous substances pollution contingency Plan, 40 CRF Part 300. US Environmental Protection Agency, Washington, DC
Varekamp JC, Buseck PR (1984) The speciation of mercury in hydrothermal systems, with applications for ore deposition. Geochim Cosmochim Acta 48:177–186
Walter MR, Bauld J, Brock TD (1972) Siliceous algal and bacterial stromatolites in hot spring and geyser effluents of Yellowstone National Park. Science 178:402–405
WHO (1993) WHO guidelines for drinking water quality. http://www.who.int/water_sanitation_health/GDWQ/index.htlm. Accessed 12 October 2011
Yakubov AA, Ali-Zade AA, Zeinalov MM (1971) Gryazevye vulkany Azerbaidzhanskoi SSR: Atlas (mud volcanoes of the Azerbaijan SSR: Atlas). Azerbaijan Academy of Sciences, Baku
Yang H-M, Lou K, Sun J, Zhang T, Ma XL (2012) Prokaryotic diversity of an active mud volcano in the Usu city of Xinjiang, China. J Basic Microbiol 52:79–85
Yassin AF, Chen WM, Hupfer H, Siering C, Kroppenstedt RM, Arun AB, Lai W-A, Shen F-T, Rekha PD, Young CC (2007) Lysobacter defluvii sp. nov., isolated from municipal solid waste. Int J Syst Evol Microbiol 57:1131–1136
Acknowledgments
This work was supported by grants from ECOS-NORD-SEP-CONACyT-ANUIES (M07A01), SEP-PROMEP (UGTO-NPTC-164), CNPq-CONACyT (491022/2008-5), ANR-CONACyT (C0011-FR12-01, No. 188775) and Universidad de Guanajuato-DAIP (0115/2011 and 0195/2013). We acknowledge financial support from the Aquitaine Regional Government Council (France). N. Villegas-Negrete and I.A. Sotelo-González received a fellowship from UG-DAIP, Mexico.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Oren.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Brito, E.M.S., Villegas-Negrete, N., Sotelo-González, I.A. et al. Microbial diversity in Los Azufres geothermal field (Michoacán, Mexico) and isolation of representative sulfate and sulfur reducers. Extremophiles 18, 385–398 (2014). https://doi.org/10.1007/s00792-013-0624-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0624-7