Abstract
Children, adolescents and adults with attention-deficit/hyperactivity disorder (ADHD) experience functional impairment and poor health-related quality of life (HRQoL) in addition to symptoms of inattention/hyperactivity–impulsivity. To synthesize qualitatively the published evidence from randomized, double-blind, placebo-controlled trials of the effectiveness of pharmacotherapy on functional impairment or HRQoL in patients with ADHD, a systematic PubMed searching and screening strategy was designed to identify journal articles meeting pre-specified criteria. Post hoc analyses and meta-analyses were excluded. HRQoL outcomes, functional outcomes and the principal ADHD symptom-based outcome were extracted from included studies. An effect size of 0.5 versus placebo was used as a threshold for potential clinical relevance (unreported effect sizes were calculated when possible). Of 291 records screened, 35 articles describing 34 studies were included. HRQoL/functioning was usually self-rated in adults and proxy-rated in children/adolescents. Baseline data indicated substantial HRQoL deficits in children/adolescents. Placebo-adjusted effects of medication on ADHD symptoms, HRQoL and functioning, respectively, were statistically or nominally significant in 18/18, 10/12 and 7/9 studies in children/adolescents and 14/16, 9/11 and 9/10 studies in adults. Effect sizes were ≥0.5 versus placebo for symptoms, HRQoL and functioning, respectively, in 14/16, 7/9 and 4/8 studies in children/adolescents; and 6/12, 1/6 and 1/8 studies in adults. Effect sizes were typically larger for stimulants than for non-stimulants, for symptoms than for HRQoL/functioning, and for children/adolescents than for adults. The efficacy of ADHD medication extends beyond symptom control and may help reduce the related but distinct functional impairments and HRQoL deficits in patients with ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) affects approximately 6.8% of children, 2.8% of adolescents and 2.5% of adults worldwide [75, 76, 83]. The disorder is defined as persistent and developmentally inappropriate symptoms of inattention and/or hyperactivity–impulsivity that interfere with a patient’s social, academic and/or occupational functioning [8, 9]. The nature of functional impairment varies from patient to patient and with age and may encompass diverse outcomes such as underperformance at school or at work, unemployment or low income, substance abuse, smoking, teenage pregnancy, arrest, divorce or acquiring sexually transmitted disease [61]. Health-related quality of life (HRQoL) is generally acknowledged to represent the impact of ill-health on an individual’s ‘perception of their position in life, in the context of culture and value systems in which they live, and in relation to their goals, expectations, standards and concerns’ [2, 104]. It has become clear that ADHD has a negative impact on patients’ HRQoL and that this may be further exacerbated by, or may increase the risk of, other psychiatric conditions such as anxiety and depression [31, 34, 54, 93]. Impaired day-to-day functioning in domains such as educational achievement and interpersonal relations is the reason that most often underlies a patient’s or parent’s decision to seek medical advice [72].
Pharmacotherapies for ADHD include the psychostimulants methylphenidate (MPH) and amphetamines [including the prodrug lisdexamfetamine (LDX)], and the non-stimulants atomoxetine (ATX) and guanfacine. Guidelines recommend that drug therapy is used as part of a multi-modal treatment plan, which should include psychoeducation or other non-pharmacological interventions such as parent training and cognitive behavioural therapy [13, 102]. Randomized controlled trials of pharmacotherapies in children, adolescents and adults with ADHD have typically used a clinician-rated symptom scale as the primary efficacy outcome measure, and meta-analyses of these studies have confirmed that ADHD medications are very effective in relieving patients’ symptoms [33, 77, 88, 89]. As recognized in the recent European Medicines Agency guidance on inclusion of functional outcomes in clinical studies of ADHD medications [44], it is now widely acknowledged that treatment of ADHD should aim not only to relieve patients’ symptoms, but also to improve their functioning and HRQoL.
Assessing functional impairment or HRQoL in a randomized controlled trial typically relies on completion of one or more of the many available ADHD-specific or generic questionnaires by patients themselves or by proxy raters (physicians, parents, teachers or family members) [30]. Use of these measures in clinical trials of ADHD medications has grown rapidly in recent years [30]. Because ADHD is a behavioural disorder that affects multiple aspects of patients’ lives, the 18 well-defined symptoms of ADHD interact and partially intersect with the constructs of functional impairment and HRQoL (Fig. 1). Although measures of functional impairment may share many similarities with measures of HRQoL, functional impairment is usually considered to be objective and ideally assessed by unbiased methods, whereas HRQoL is usually considered to be subjective and ideally assessed by the patients themselves. What precisely is being measured by a patient-rated functional impairment instrument or a proxy-rated HRQoL instrument may therefore be open to debate and discussion [2]. Another important aspect of measuring functional impairment and HRQoL is that the instruments should sample domains of impairment that are commonly affected by patients’ symptoms, but should not merely serve as surrogate measures of symptoms. Moderate, but not strong, correlations between measures of symptoms, functional impairment and HRQoL suggest that related, but distinct, constructs are being assessed [18, 19, 21, 32, 47, 81, 92, 95].
These factors present challenges for the development and selection of instruments to measure HRQoL and functional impairment. Generic instruments have the advantage of capturing a broad picture of health status, but the potential disadvantage of poor sensitivity to the deficits characteristic of ADHD. Disorder-specific instruments may offer improved sensitivity by specifying particular impairments, but may miss others. When any single patient typically reports impairment in only a small proportion of the specified items within a domain, the instrument may suffer from poor sensitivity if the scoring system averages out this potentially severe and clinically relevant impairment across all the specified items within that domain. US Food and Drug Administration guidance on patient-reported outcome measures states that instruments should be psychometrically validated and standardized to a reference population [46]. Standardization may present problems for ADHD-specific instruments if they are not designed for use in people who do not have ADHD. An advantage of generic instruments that are standardized to a reference population is that they enable direct comparison of the burden of disease in patients with ADHD with that of community norms and patients with other physical or mental health problems.
Results from clinical trials and observational studies using questionnaire-based instruments indicate that patients with ADHD experience severely compromised HRQoL [34]. In a 2010 systematic review of clinical trials, open-label studies and post hoc analyses, evidence supporting a positive short-term effect of medication on quality of life was limited mainly to studies of ATX in children and adolescents [29]. Recent evidence from observational and registry studies indicates that pharmacological treatment of ADHD is associated with increased achievement and decreased absenteeism at school [16], a reduced risk of trauma-related emergency hospital visits [58], reduced risks of suicide and attempted suicide [28], and decreased rates of substance abuse [27] and criminality [56].
This systematic review focuses on the effect of pharmacological treatment of ADHD on functional impairment and HRQoL outcomes in randomized placebo-controlled studies. We aim to: survey the instruments used in these studies; assess the severity of baseline impairment relative to controls, when possible; collate the most robust evidence about the impact of medications on HRQoL and functional impairment outcomes; and assess the relationship of treatment-related changes in these measures with changes in ADHD symptom scales.
Methods
A systematic literature searching and screening strategy was designed to identify articles published in peer-reviewed journals that reported findings of randomized placebo-controlled clinical trials of pharmacotherapy for ADHD using measures of functional impairment or HRQoL as outcomes. We extracted these outcomes for qualitative review and analysis, together with the principal ADHD symptom-based outcomes from the same studies.
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was registered with the PROSPERO international prospective register of systematic reviews (identifier: CRD42015027595).
Search and selection
PubMed (http://www.ncbi.nlm.nih.gov/pubmed) was searched on 29 June 2016 using a search string (see Supplementary Table 1) designed to identify journal articles in English that reported the results of randomized, placebo-controlled, human clinical trials of pharmacological medications for ADHD that included assessments of functional impairment or HRQoL as study outcomes. Two individuals screened records independently and resolved all disagreements by discussion. In a first screen based on title and abstract, articles were excluded if they clearly failed to meet the inclusion criteria or met any of the exclusion criteria. In a second screen based on full text, articles were included only if they met all inclusion criteria and no exclusion criterion. References cited in all included articles were then screened in the same way.
Inclusion and exclusion criteria
Studies were included only if they enrolled participants with a primary diagnosis of ADHD. Participants could be children, adolescents and/or adults. ADHD diagnosis had to be based on the Diagnostic and Statistical Manual of Mental Disorders (DSM) III, IIIR, IV, IV-TR or 5 criteria [8,9,10,11,12]; the International Statistical Classification of Diseases 10th Revision (ICD-10) criteria [103]; or on a diagnostic instrument that uses these criteria (e.g. ADHD Rating Scale IV [ADHD-RS-IV] [39], Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) [53], or Conners’ Adult ADHD Diagnostic Interview for DSM-IV [CAADID] [41]). Studies in patients with a primary diagnosis of another mental disorder and secondary comorbid ADHD were excluded.
Studies were included only if they assessed the effects of medication alone or in combination with non-pharmacological interventions. Medications did not need to have regulatory approval for the treatment of ADHD. Folic acid, omega-3 fatty acids and other dietary supplements were not considered to be medications.
Only randomized placebo-controlled studies with prospectively defined comparisons between medication(s) and placebo were included. Studies could have parallel-group, crossover, treatment initiation or treatment withdrawal designs. Studies with an active control were included only if they also had a placebo control or placebo reference arm. Reviews and articles reporting post hoc analyses and meta-analyses were excluded.
Only studies that assessed patients’ functional impairment (or functioning) and/or HRQoL [or quality of life (QoL)] during randomized placebo-controlled treatment (not in a separate uncontrolled study period) were included. Instruments used for assessing these outcomes could be generic or disease-specific; investigator-rated, proxy-rated (e.g. by parents or teachers) or patient-rated; and did not need to be psychometrically validated. Assessments of any of the following were not eligible as measures of functional impairment: executive function, emotional dysregulation, emotional lability, mood, behaviour, (neuro)cognition, (neuro)cognitive function, (neuro)development, neuropsychiatric function, memory, reaction time, alertness, psychomotor function, response inhibition, pre-pulse inhibition, lexical function, intelligence, sleep, creativity, anxiety and depression. ‘Global functioning’ measured using the Clinical Global Impressions (CGI) scale was also not considered to be an eligible functional measure.
Data extraction and analysis
The following study details were extracted from each included article: duration of the randomized assessment period, age range of participants, active treatment(s) and doses, number of participants and randomization ratio. For each study, effect sizes and p values were extracted for functional impairment outcomes, HRQoL outcomes and the principal symptom-based outcome (e.g. ADHD-RS-IV). When necessary, data for symptom-based outcomes of a study were extracted from references cited in the included article. If relevant results from a single study were published in more than one article, the articles were treated as a single entity. When effect sizes were not reported, they were calculated from published data if possible (using ‘n’, mean change and standard deviation or standard error of the mean; or ‘n’ and F-statistic). An effect size threshold of 0.5 was used as an indicator of minimum clinically important differences in the qualitative analysis [71].
The registered protocol for this systematic review (PROSPERO identifier: CRD42015027595) stated that meta-analysis would not be conducted unless sufficient studies of the same medication using the same functional/(HR)QoL outcome measure over approximately the same treatment period were considered to have been identified in the searches.
Risk of bias
This review includes only study outcomes published in peer-reviewed journals. Functional impairment and HRQoL are usually secondary efficacy outcomes in ADHD studies and may be less likely to be reported than primary efficacy outcomes (e.g. ADHD symptoms). This could lead to bias if studies or study outcomes that indicate favourable effects of medication are more likely to be published that those that do not. We did not formally assess the risk of bias in the studies or outcomes included in this review.
Post hoc analyses were excluded because they may be more likely than pre-specified analyses to report favourable effects of medication and are not typically controlled for multiple statistical comparisons. Pre-specified analyses of secondary efficacy outcomes, however, may also not have been controlled for multiplicity of comparison and may be more likely to yield significant p values than primary analyses or secondary analyses that were included in a study-wide algorithm to control type I error. The p values quoted from some included studies may therefore represent nominal rather than strict statistical significance; both nominally significant and statistically significant p values are described as ‘significant’ in this review. To mitigate the associated risk of bias in favour of medication, we focus, when possible, on effect sizes of active medication versus placebo rather than p values to assess the efficacy of ADHD pharmacotherapy. This approach could lead to bias if studies with published or calculable effect sizes are more likely than those without available effect sizes to report favourable effects of medication.
Results
Study selection
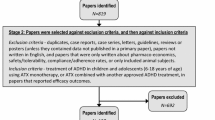
Of 288 articles identified by the search, 244 were excluded during screening of the title and abstract and a further ten were excluded during full-text screening. Articles excluded at full-text screening reported studies that: lacked a comparison of medication with placebo [20, 60, 87], lacked an eligible HRQoL or functional impairment outcome [23, 24, 26, 63, 74] or were post hoc analyses [62, 69]. The references cited in the remaining 34 included articles and in previous systematic reviews [29, 34, 88] were inspected, yielding three additional articles for screening based on title and abstract. Of these, two were excluded and one was screened based on full text and included [68], bringing the total number of included articles to 35. Two of these reported HRQoL data from the same study [84, 85], meaning that 34 studies were included for qualitative analysis (Fig. 2). Study data were extracted from all of the included articles and from eight additional articles that reported the symptom-based outcomes for the included studies (Tables 1, 2). A meta-analysis was not conducted because the included articles were not considered to report enough comparable data from studies of the same medications in similar populations and of similar duration for quantitative synthesis.
Patients, medications and outcomes assessed
Of the 34 included studies, 18 were conducted in children aged 3–12 years and/or adolescents aged 13–18 years (Table 1), and 16 were conducted in adults aged 18 years or older (Table 2). None of the studies selected patients based on measures of HRQoL or functional impairment at baseline, but nearly all recruited patients with at least moderately severe ADHD symptoms (e.g. ADHD-RS-IV total score ≥28). Fourteen studies investigated stimulant medications (amphetamines, including LDX, and various formulations of MPH), and 21 studies investigated non-stimulant medications (ATX, guanfacine extended release [GXR], metadoxine and reboxetine). Three studies investigated more than one medication. With the exception of metadoxine and reboxetine, all of the medications investigated in the studies are approved for treatment of ADHD in one or more countries.
Twenty-nine studies were short-term double-blind studies (≤20 weeks); two were long-term (≥6 months) studies in children and adolescents (both double-blind, randomized withdrawal studies); and three were long-term double-blind studies in adults (Tables 1, 2). Of the 18 studies in children and adolescents, 12 assessed HRQoL and 9 assessed functional impairment; of the 16 adult studies, 12 assessed HRQoL and 11 assessed functional impairment (Table 3).
HRQoL in children and adolescents was always assessed using generic instruments, but HRQoL in adults was mostly assessed using ADHD-specific instruments. Conversely, functional impairment was mostly assessed using ADHD-specific instruments in children and adolescents, but generic instruments in adults. HRQoL in adults was self-rated in all studies, but proxy-rated (by parents) in all but three studies in children and adolescents (two in adolescents [42, 45] and one in children/adolescents [91]) (Table 3).
Baseline impairment
Some of the studies in children and adolescents used generic HRQoL instruments that have been standardized to community norms or for which reference population data are available. These can provide an indication of the burden of untreated ADHD when patients are assessed at baseline. The domains with the greatest deficits may reflect impairments characteristic of the disorder and may also be considered as potential targets for treatment.
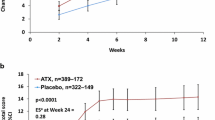
The Child Health and Illness Profile-Child Edition: Parent Report Form (CHIP-CE:PRF) is a generic, parent-rated measure of children’s HRQoL in five domains (Achievement, Risk Avoidance, Resilience, Satisfaction and Comfort). Baseline CHIP-CE:PRF T-scores from five included studies (two of LDX and three of ATX) were strikingly consistent in indicating that children and adolescents with ADHD have substantially impoverished HRQoL before treatment, especially in the Achievement and Risk Avoidance domains [14, 15, 37, 42, 91]. Impairment was less marked in the domains of Resilience and Satisfaction, and scores in Comfort were close to the community norm [14, 15, 37, 42, 91]. These findings are also consistent with the results of two observational population studies (not included in this review) that assessed the impact of ADHD on HRQoL using the CHIP-CE:PRF (Fig. 3) [32, 80].
Pre-treatment baseline CHIP-CE:PRF T-scores in children and adolescents with ADHD. T-scores have a mean of 50 and a standard deviation of 10. Circle diameter is proportional to T-score, with a diameter of zero corresponding to a T-score of 20. Rings indicate the mean in the reference population. aStudy SPD489-326 [15] (included in this review) involved mainly the same patients as the short-term LDX study SPD489-325 [14] (also included in this review), so only the former is shown. bPooled analysis of five ATX studies [43]: three randomized placebo-controlled trials (included in this review) [37, 42, 91], and two open-label studies (not included in this review). cObservational study (not included in this review), shown for comparison [80]. dObservational study with non-ADHD control groups (not included in this review), shown for comparison [32]. ADHD attention-deficit/hyperactivity disorder, ADORE Attention-Deficit/Hyperactivity Disorder Observational Research in Europe, ATX atomoxetine, CHIP-CE:PRF Child Health and Illness Profile-Child Edition: Parent Report Form, LDX lisdexamfetamine, T1DM type 1 diabetes mellitus
The German, parent-rated Revidierter Fragebogen für Kinder und Jugendliche zur Erfassung der gesundheitsbezogenen Lebensqualität (KINDL-R; revised questionnaire to assess health-related quality of life in children and adolescents) also assesses children’s HRQoL overall and in six domains, namely Physical Well-Being, Emotional Well-Being, Self-Esteem, Friends, Family and School. In one included study [94], overall mean pre-treatment KINDL-R scores in children and adolescents with ADHD were substantially lower than the mean in the German reference population [22] [62.9 (95% confidence interval: 61.0–64.8) vs 78.2 (77.8–78.7) on a scale of 0–100], with the largest deficits in the domains of Family and Friends, and no deficit in the Physical Well-Being domain. The parent-rated Child Health Questionnaire-50-item Parent Form (CHQ-PF50) provides Psychosocial and Physical summary scores. In one included study, the mean baseline Psychosocial T-score in children and adolescents with ADHD was almost two standard deviations below the normative mean (31.3–35.4 vs 50) [66].
Considered together, baseline parent-rated HRQoL data from the included child/adolescent studies indicate that parent-rated HRQoL of children with ADHD is approximately 1.5–2.0 standard deviations lower than that of control populations in domains reflecting achievement and risk-taking, as was also found in a previous systematic review [34]. HRQoL deficits may be less marked with self-rated than with parent-rated instruments in children and adolescents. In one included study, mean T-scores on the self-rated Child Health and Illness Profile-Child Edition: Self-Report Form or Adolescent Edition (CHIP-CE:SRF/AE) was not as far below the normative mean as those on the CHIP-CE:PRF [42]. This agrees with an observational study (not included in this review) in which self-rated CHIP-CE:SRF scores correlated only moderately with parent-rated CHIP-CE:PRF T-scores [32]. In another included study [45], mean scores on the self-rated Youth Quality of Life Instrument-Research Version (YQOL-R) were not far below the mean for community norms [73] (79.2–79.5 vs 82.2 on a 0–100 scale).
The other multi-domain instruments used in the studies included in this review do not have reference population data. However, some domains consistently had worse pre-treatment scores than others. Weiss Functional Impairment Rating Scale-Parent (WFIRS-P) baseline scores were generally worst in the Learning and School domain and in the Family domain in children and adolescents. In adults, ADHD Impact Module-Adult (AIM-A) scores were generally worst in the Performance and Daily Functioning domain, and Adult ADHD Quality of Life (AAQoL) scores were generally worst in the Productivity domain.
Effectiveness of pharmacological treatment in children and adolescents
ADHD symptoms in children and adolescents
All of the 18 studies in children and/or adolescents demonstrated significant beneficial effects of medication on ADHD symptoms compared with placebo (Table 1). Effect sizes of active treatment versus placebo were reported for 12 studies and were calculated using published data from four studies. Effect sizes of at least 0.5 (an approximate indicator of minimum clinically important difference) [71] for ADHD symptom outcomes in at least one active treatment group were observed in all of these 16 studies except the long-term ATX withdrawal study [65] and the adjunctive GXR study [100]. Effect sizes for symptom-based outcomes were generally lower for non-stimulants (range 0.32–1.20) than for stimulants (range 0.80–1.80) (Table 4).
HRQoL in children and adolescents
Of the 12 studies that assessed HRQoL, 10 reported significant placebo-adjusted effects of medication in at least one domain or cross-domain summary statistic, all using patient proxy ratings (Table 1). Effect sizes of active treatment versus placebo were available for all but one of these studies [50] and were 0.5 or above in at least one domain in seven of the remaining nine [14, 15, 42, 66, 68, 91, 94].
Seven studies used multi-domain instruments (e.g. CHIP-CE:PRF, KINDL-R, CHQ-PF50) to assess changes in HRQoL with medication. Effect sizes varied across domains and were generally larger in domains relating to risk-taking and achievement than in domains relating to physical components of HRQoL in patients treated with ADHD medications (Table 4). In the most responsive domains, effect sizes observed for HRQoL were generally larger for stimulants (range 0.54–1.28) than for non-stimulants (0.29–0.87).
The CHIP-CE:PRF was used in five studies: three short-term studies of ATX [37, 42, 91], a short-term study of LDX with an osmotic-release oral system MPH (OROS-MPH) reference arm [15], and a long-term, randomized withdrawal study of LDX (Table 4) [14]. In all of the short-term studies, the largest effect sizes for active treatment versus placebo were in the Risk Avoidance and Achievement domains. The improvements in these domains with short-term LDX treatment [15] were maintained with continued treatment in the subsequent randomized withdrawal study [14]. Results in the Resilience and Satisfaction domains differed across studies, with significant effects and small or moderate effect sizes in the LDX studies but not in the ATX studies. In the Comfort domain, no significant effects of treatment were reported in any study [14, 15, 37, 42, 91]. In the study of ATX using the KINDL-R, significant beneficial effects of ATX were reported in all domains except School and Physical Well-Being, with a significant effect in favour of placebo in the latter [94]. This suggests that the KINDL-R Physical Well-Being domain may be more sensitive to side effects of medication than other instruments [94].
Three of the five studies that used the CHQ-PF50 reported significant placebo-adjusted improvements in Psychosocial summary score, with effect sizes of 0.29–0.87 for ATX and 0.54 for OROS-MPH (the reference arm in one ATX study, in which the ATX effect size was 0.37) (Table 4) [65, 66, 68]. Effect sizes were not available for one [50]. In the remaining study, the improvement in Psychosocial summary score with ATX treatment was not significant, but a significantly greater proportion of patients receiving ATX were classified as CHQ-PF50 responders, compared with placebo (43.8 vs 22.2%; p < 0.04) [17]. The effect size of 0.32 and relatively small number of patients (N = 153) suggests that this study may have been statistically underpowered. Physical summary score was reported in only one of the studies to use the CHQ-PF50 [66], which showed negative and non-significant effect sizes of ATX versus placebo (Table 4).
Functional impairment in children and adolescents
Five of the nine studies that assessed functional impairment in children and adolescents used the parent-rated WFIRS-P. Four of these five studies (three short-term studies and one randomized withdrawal study) reported significant placebo-adjusted effects of treatment on WFIRS-P total score (Table 4) [14, 15, 51, 86]. Scores in the Family domain and the Learning and School domain were most consistently improved relative to placebo in these studies. Effects were not consistent in other domains, although scores in the Life Skills and Child’s Self-Concept domains were the least responsive to treatment (Table 4). In the most responsive domains, WFIRS-P effect sizes were larger for stimulants (range 0.86–1.25) than for non-stimulants (0.32–0.58). Three studies reported significant effects of medications on single-domain functional impairment measures (Table 1) [1, 99, 100], with an effect size above 0.5 in a study of transdermal MPH (TD-MPH) using the Before-School Functioning Questionnaire (BSFQ) [99].
Patient self-rating and proxy ratings in children and adolescents
Results from several studies suggest that observed treatment effects may be larger when questionnaires are completed by proxies than by patients, both for instruments described as HRQoL measures and those described as functional impairment measures. This mirrors the greater baseline HRQoL deficits observed with parent-rated than with self-rated HRQoL instruments.
The self-rated CHIP-CE:SRF/AE and parent-rated CHIP-CE:PRF were used together in one study of ATX [42]. The pattern of changes observed across the five domains was similar with the CHIP-CE:SRF/AE and the CHIP-CE:PRF, but effect sizes were smaller with the self-rated than with the parent-rated instrument in all domains except Satisfaction (Table 4). An effect size above 0.5 for ATX versus placebo was seen only in the CHIP-CE:PRF Risk Avoidance domain in this study. In another of the ATX studies that used the CHIP-CE:PRF, the significant effects of ATX versus placebo in the Achievement domain and the Risk Avoidance domain (effect sizes, 0.53 and 0.41, respectively; Table 4) were not reflected in a one-dimensional self-rated Swedish HRQoL scale (effect size 0.10). It is possible that this scale, the Jag Tycker Jag Är [I think I am] (JTJA), may not be sensitive to HRQoL deficits in patients with ADHD [91]. Similarly, self-rated YQOL-R scores did not significantly improve from baseline to endpoint in either the LDX or placebo groups (and did not differ between groups) of a study in adolescents, despite an effect size for LDX 70 mg of 0.72 (p ≤ 0.0056) on ADHD symptoms in the same study [45].
Two studies used the BSFQ, an ADHD-specific instrument with a proxy-rated section (completed by investigators or parents; Table 4) and a self-rated section (completed by patients in collaboration with their parents). In one study, significantly greater improvement with TD-MPH than with placebo was observed using the investigator-rated BSFQ, with an effect size of 1.08 (p < 0.01), but not using the self-reported BSFQ [99]. Similarly, significantly greater improvement with GXR than with placebo as an adjunct to stimulant medication was observed using the parent-rated BSFQ (effect size 0.38–0.39; p < 0.001), but not using the self-rated BSFQ [100].
Effectiveness of pharmacological treatment on HRQoL and functional impairment in adults
ADHD symptoms in adults
Of the 16 included studies conducted in adults, 14 demonstrated significant beneficial effects of medication on ADHD symptoms compared with placebo (Table 2). Effect sizes were available for ADHD symptom outcomes in 12 of these 14 studies and were 0.5 or above in three of the five studies of stimulants and three of the seven studies of non-stimulants (Table 5). Effect sizes could not be calculated for two studies that reported significant effects of medications on ADHD symptoms [52, 85]. Two studies did not find significant effects of medication on ADHD symptoms: a parallel-group study of reboxetine (reboxetine, n = 23; placebo, n = 17) [79], and a parallel-group study of dextroamphetamine in patients receiving cognitive behavioural therapy (dextroamphetamine, n = 23; placebo, n = 25) [96].
HRQoL in adults
Of the 11 studies that assessed HRQoL in adults, significant improvement versus placebo in at least one measure or domain was reported in nine (Table 2). Of these nine, effect sizes were available for six and were 0.5 or above only in a 10-week study of LDX that used the AAQoL [3]. In the studies that used the AAQoL, placebo-adjusted effects varied across the four domains, with the largest effect sizes usually in the Life Productivity domain (0.21–0.91) (Table 5) [3, 5,6,7, 40, 48, 55]. The other HRQoL measures used in adults were the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) [82] and the Overall Quality of Life (QoL) section of the AIM-A. Significant effects of stimulants versus placebo were reported using the latter measure in two studies, with an effect size of 0.44 for LDX [3, 84, 85].
Functional impairment in adults
Of the 10 studies that assessed functional impairment in adults, significant improvements versus placebo in at least one measure were reported in nine (Table 2). Of these nine studies, effect sizes were available for eight and were 0.5 or above in at least one measure or domain in only one study (Table 5) [3]. This revealed significant effects of LDX in all six domains of the AIM-A, with effect sizes of 0.93 in the Performance and Daily Functioning domain and 0.57–0.79 in four of the remaining five domains (p < 0.05) (Table 5) [3]. Effect sizes were not calculable for the other two studies that used the AIM-A, but significant placebo-adjusted effects of MAS-XR3 (triple-bead mixed amphetamine salts extended release) were reported in all domains in one study [85] and of OROS-MPH in three to four domains, depending on dose, in the other [25]. Five studies used the self-rated Sheehan Disability Scale (SDS), a global measure of functional impairment across domains of Work/School, Social Life and Family Life. Significant effect sizes of long-acting MPH versus placebo were about 0.4 in three of these studies (Table 5) [25, 78, 82]. Functional impairment was assessed in only three studies of non-stimulants, with significant effects of medication versus placebo in measures of global functioning, marital/family functioning and driving behaviour; effect sizes were about 0.3, when available [7, 79, 97].
Relationship between ADHD symptom measures and HRQoL/functional measures
To assess the relationship between improvements in ADHD symptoms and improvements in HRQoL or functioning, we collated effect sizes for active treatment versus placebo in all studies, when reported or calculable. Data were available for 16 studies (19 active treatment groups) in children and adolescents and 14 studies in adults (Tables 4, 5).
Children and adolescents
Effect sizes were greater for ADHD symptom outcomes than for HRQoL or functional outcomes in all but two studies of children and adolescents; the exceptions were a 2-week study of TD-MPH (effect sizes 0.90 for ADHD-RS-IV and 1.08 for investigator-rated BSFQ) and an 8-week study of ATX (effect sizes 0.33, 0.62 and 0.60 for ADHD-RS-IV total score and 0.47, 0.66 and 0.87 for CHQ-PF50 Psychosocial summary score [low, medium and high dose, respectively]) [66, 99]. Effect sizes were 0.5 or above for both ADHD symptoms and at least one aspect of HRQoL or functioning in five out of 13 non-stimulant treatment groups (ATX or GXR) [42, 66, 86, 91, 94] and five out of six stimulant treatment groups (LDX, OROS-MPH or TD-MPH) [14, 15, 99]. Effect sizes of active medications versus placebo were 0.5 or above for ADHD symptoms but below 0.5 for HRQoL or functioning in five out of 13 non-stimulant treatment groups (ATX or GXR) [17, 37, 51, 68, 101] and one out of six stimulant treatment groups (MPH) [1]. Effect sizes were below 0.5 for both ADHD symptoms and HRQoL or functioning in the remaining three non-stimulant treatment groups (ATX or adjunctive GXR) [51, 65, 100].
Some studies investigated the relationship between ADHD symptom measures and HRQoL or functional measures and consistently identified significant associations. Escobar et al. [42] reported that CHIP-CE:PRF Risk Avoidance domain T-scores correlated moderately with ADHD-RS-IV total scores at baseline (Pearson’s R, −0.46; p < 0.05), with weak but significant correlations in the other four domains of the CHIP-CE:PRF and in the Risk Avoidance and Comfort domains of the self-rated CHIP-CE:SRF/AE. In an 8-week GXR study, mean changes from baseline in WFIRS-P total score were significantly greater in patients who responded symptomatically (defined as a CGI-Improvement score of 1 or 2 with ≥30% reduction in ADHD-RS-IV total score) than in those who did not (p < 0.001) [86]. Finally, improvement in ADHD-RS-IV total score was significantly correlated with improvement in CHQ-PF-50 scores in a post hoc analysis [62] of an 8-week study of ATX [66].
Adults
Effect sizes were greater for ADHD symptom outcomes than for HRQoL or functional outcomes in all but one of the 12 studies (seven of non-stimulants and five of stimulants) that reported significant effects of medication on ADHD symptoms in adults (Table 5). Effect sizes were at least 0.5 for both ADHD symptoms and at least one aspect of HRQoL or functioning in two out of these 12 studies: a 10-week study of LDX that used the AAQoL and AIM-A [3, 4] and a 10-week study of ATX that used the AAQoL (although effects in the latter were not significant) [55]. Effect sizes were at least 0.5 for ADHD symptoms but below 0.5 for HRQoL or functioning in four out of these 12 studies: two of long-acting MPH and two of ATX [48, 52, 78, 97]. Effect sizes were below 0.5 for both ADHD symptoms and HRQoL or functioning in six out of these 12 studies: three of ATX, two of OROS-MPH and one of metadoxine [25, 40, 59, 79, 82, 96].
Discussion
This systematic review aimed to evaluate HRQoL and functional outcomes in published randomized placebo-controlled clinical trials of medications for ADHD. Tandem extraction of primary or principal symptom-based efficacy outcomes alongside HRQoL and functional outcomes allowed the results of interest to be considered in the context of the effect of study drugs on ADHD symptoms.
Baseline data from the included studies were consistent with published observational studies in showing that children and adolescents with ADHD have substantially impoverished parent-rated HRQoL compared with population norms [32, 80]. Adult HRQoL instruments used in the studies were nearly all ADHD specific, and baseline values are therefore not comparable with reference populations. Baseline deficits on all multi-domain HRQoL and functional impairment instruments, whether generic or ADHD specific, or self- or proxy-rated, were more pronounced in domains relating to achievement/productivity, risk-taking and interpersonal relations than in other psychosocial domains. Little or no impairment was evident in domains relating to physical functioning on the generic instruments. Participants in the included studies were not selected based on HRQoL assessments, but were usually required to have moderate to severe ADHD symptoms (e.g. ADHD-RS-IV total score ≥28), indicating that the poor HRQoL observed at baseline is a reflection of the patients’ characteristics rather than the study inclusion criteria. Overall, these findings support the notion that day-to-day functional impairments and HRQoL deficits are typical in patients with ADHD.
Pharmacotherapy relieved ADHD symptoms to a significantly greater extent than did placebo in all but two of the included studies, as would be expected from the large body of published clinical trials and recent meta-analyses [33, 77, 88, 89]. The two studies in which no significant benefit over placebo was found were both small studies in adults (N ≤ 50), one of reboxetine and one of dextroamphetamine. Effect sizes of medication versus placebo for ADHD symptom-based outcomes (when available) were above 0.5 in nearly all of the included studies in children and adolescents, and in most of the studies in adults. We used an effect size of 0.5 as an approximate but universally applicable threshold for a clinically meaningful difference. This is supported by a study showing that nearly all estimates of minimum important differences for HRQoL instruments were close to 0.5 standard deviations and that this may correspond to the limit of people’s ability to discriminate differences over a range of criteria [71].
Functional impairment and poor HRQoL can be viewed as constructs that relate to, but are distinct from, ADHD symptoms and that reflect the impact of ADHD on patients’ day-to-day lives (Fig. 1). In support of this conceptualization, severity of or improvement in ADHD symptoms correlated moderately to strongly (but not perfectly) with severity of or improvement in functional impairment and HRQoL deficits, both in the included studies for which this was reported [42, 62, 66, 86] and elsewhere in the literature [18, 19, 21, 32, 47, 81, 92, 95]. Furthermore, effect sizes (when available) of active medications versus placebo were smaller for functional or HRQoL outcomes than for symptom-based outcomes in nearly all of the included studies (Tables 4, 5). None of this evidence, however, reveals whether medications affect functioning and HRQoL directly, or only through the medium of symptom relief, or both.
In children and adolescents, large effect sizes (≥0.8) were observed for HRQoL and/or functional outcomes in short-term studies of long-acting stimulant medications (LDX, OROS-MPH and TD-MPH). Large effect sizes were not observed in any included studies of non-stimulants (ATX and GXR) and were below 0.5 in many of these. Treatment effect sizes in the included studies suggest that placebo-adjusted improvements in some measures of functioning and of HRQoL are likely to be of sufficient magnitude to be considered potentially clinically relevant, especially with stimulant treatment. Large effect sizes in an individual domain of an instrument may not necessarily reflect large overall effects on HRQoL if some domains have less impact on overall HRQoL than others according to the instrument’s factor structure. Nevertheless, effect sizes on multi-domain instruments were largest in domains relating to achievement/school, risk-taking and interpersonal relations, reflecting the domains with the greatest deficits observed at baseline. In adults, stimulant and non-stimulant effect sizes for functional or HRQoL outcomes were below 0.5 in all included studies except the single adult LDX study. Before drawing any conclusions about the effectiveness of medications or the nature of impairments and deficits in different age groups, however, it should be noted that almost all HRQoL and functional outcomes were parent-rated in child/adolescent studies and self-rated in adult studies. If HRQoL is a subjective experience that can only be judged by patients themselves, then it is questionable whether HRQoL can really be rated by parents, teachers or investigators acting as proxies. Proxy-rated HRQoL assessments may therefore be more appropriately regarded as measures of functional impairment than of HRQoL.
The diversity of instruments used to assess HRQoL and functional impairment in the clinical trials of ADHD medications included in this review suggests a continuing lack of consensus in the field, and perhaps more generally, about which non-symptom-based outcomes should be included and how they should best be assessed. For example, the only HRQoL or functional impairment data identified in this systematic review for patients receiving OROS-MPH were from studies of LDX or ATX in which OROS-MPH was a reference treatment; and the only functional impairment data for patients receiving ATX were from a study of GXR in which ATX was a reference treatment. While some of the differences in choice of HRQoL or functional impairment outcome measure may reflect the changing demands of regulators, it is also the case that the design of a clinical trial involves balancing inclusion of additional outcomes of interest with the feasibility of conducting the study and analysing the results. For example, the use of parent-rated instruments in child/adolescent studies may reflect concerns that some young patients would be unable to complete questionnaires [31, 34], while the use of ADHD-specific HRQoL instruments in adults may reflect concerns that generic instruments would not be sensitive to the impairments characteristic of ADHD in adults. The use of these instruments may also reflect a lack of suitable alternatives: this systematic review focussed only on instruments that have been used in published randomized placebo-controlled studies in patients with ADHD. These complexities reflect the difficulty of sampling the key domains of impairment in individual patients from different perspectives, without merely sampling symptoms. It will be interesting to see whether recent work to develop a standardized nomenclature and toolkit to describe and code functional impairment in people with ADHD based on the World Health Organization’s International Classification of Functioning, Disability and Health will result in a more cohesive and structured approach to assessing HRQoL and functional impairment in clinical trials than at present [35, 36].
This systematic review was limited to randomized, double-blind, placebo-controlled studies published in peer-reviewed journals. The limitations of this approach should be considered when interpreting the results. One potential source of bias is that secondary outcomes or entire studies that do not favour the ADHD medication being tested may be less likely to be published than those that indicate beneficial effects. To limit this bias, we excluded post hoc analyses because they may be more likely than pre-specified analyses to report results favouring the investigational product. Another potential source of bias is that the review did not aim to assess the safety of ADHD medications. Side effects, adverse events or poor tolerability may themselves negatively affect present or future functioning and HRQoL, in addition to other potentially undesirable effects. Finally, the review included studies with different enrolled populations: although all studies enrolled patients with diagnosed ADHD, recruitment criteria differed among studies. For example, some studies included patients with specific psychiatric comorbidities or inadequate responses to previous ADHD medication. This could have affected the qualitative synthesis if study population factors are responsible for differential effects of medication across studies. Despite these limitations, and the limitations of the included studies, the results presented here comprise the most robust available evidence to date that ADHD medications not only provide effective relief of symptoms, but may also reduce functional impairments and improve HRQoL in children, adolescents and adults with ADHD. Furthermore, real-world evidence from registry studies suggests that this may be the case for patients in clinical practice [16, 56, 57], as well as for those enrolled in clinical trials.
Whether pharmacological therapy is appropriate, and if so which of the treatment options and which accompanying behavioural or psychological intervention is most suitable for each patient, are decisions for healthcare professionals, parents and patients. The evidence presented here should encourage everyone involved in a patient’s treatment to aim for reduced functional impairment and improved HRQoL, as well as relief of symptoms, for all patients with ADHD.
References
Abikoff HB, Vitiello B, Riddle MA, Cunningham C, Greenhill LL, Swanson JM, Chuang SZ, Davies M, Kastelic E, Wigal SB, Evans L, Ghuman JK, Kollins SH, McCracken JT, McGough JJ, Murray DW, Posner K, Skrobala AM, Wigal T (2007) Methylphenidate effects on functional outcomes in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS). J Child Adolesc Psychopharmacol 17:581–592
Adamo N, Seth S, Coghill D (2015) Pharmacological treatment of attention-deficit/hyperactivity disorder: assessing outcomes. Expert Rev Clin Pharmacol 8:383–397
Adler LA, Dirks B, Deas P, Raychaudhuri A, Dauphin M, Saylor K, Weisler R (2013) Self-reported quality of life in adults with attention-deficit/hyperactivity disorder and executive function impairment treated with lisdexamfetamine dimesylate: a randomized, double-blind, multicenter, placebo-controlled, parallel-group study. BMC Psychiatry 13:253
Adler LA, Dirks B, Deas PF, Raychaudhuri A, Dauphin MR, Lasser RA, Weisler RH (2013) Lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder who report clinically significant impairment in executive function: results from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 74:694–702
Adler LA, Liebowitz M, Kronenberger W, Qiao M, Rubin R, Hollandbeck M, Deldar A, Schuh K, Durell T (2009) Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety 26:212–221
Adler LA, Spencer T, Brown TE, Holdnack J, Saylor K, Schuh K, Trzepacz PT, Williams DW, Kelsey D (2009) Once-daily atomoxetine for adult attention-deficit/hyperactivity disorder: a 6-month, double-blind trial. J Clin Psychopharmacol 29:44–50
Adler LA, Spencer TJ, Levine LR, Ramsey JL, Tamura R, Kelsey D, Ball SG, Allen AJ, Biederman J (2008) Functional outcomes in the treatment of adults with ADHD. J Atten Disord 11:720–727
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Washington, DC
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders (fourth edition, text revision). American Psychiatric Association, Washington, DC
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Publishing, Washington DC
American Psychiatric Association (1987) Diagnostic and statistical manual of mental disorders (revised, 3rd edn. American Psychiatric Publishing, Washington DC
American Psychiatric Association (1980) Diagnostic and statistical manual of mental disorders, 3rd edn. American Psychiatric Assocation, Washington DC
Atkinson M, Hollis C (2010) NICE guideline: attention deficit hyperactivity disorder. Arch Dis Child Educ Pract Ed 95:24–27
Banaschewski T, Johnson M, Lecendreux M, Zuddas A, Adeyi B, Hodgkins P, Squires LA, Coghill DR (2014) Health-related quality of life and functional outcomes from a randomized-withdrawal study of long-term lisdexamfetamine dimesylate treatment in children and adolescents with attention-deficit/hyperactivity disorder. CNS Drugs 28:1191–1203
Banaschewski T, Soutullo C, Lecendreux M, Johnson M, Zuddas A, Hodgkins P, Adeyi B, Squires LA, Coghill D (2013) Health-related quality of life and functional outcomes from a randomized, controlled study of lisdexamfetamine dimesylate in children and adolescents with attention deficit hyperactivity disorder. CNS Drugs 27:829–840
Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ (2007) Modifiers of long-term school outcomes for children with attention-deficit/hyperactivity disorder: does treatment with stimulant medication make a difference? Results from a population-based study. J Dev Behav Pediatr 28:274–287
Brown RT, Perwien A, Faries DE, Kratochvil CJ, Vaughan BS (2006) Atomoxetine in the management of children with ADHD: effects on quality of life and school functioning. Clin Pediatr (Phila) 45:819–827
Brown TE, Landgraf JM (2010) Improvements in executive function correlate with enhanced performance and functioning and health-related quality of life: evidence from two large, double-blind, randomized, placebo-controlled trials in ADHD. Postgrad Med 122:42–51
Buitelaar JK, Casas M, Philipsen A, Kooij JJ, Ramos-Quiroga JA, Dejonckheere J, van Oene JC, Schauble B (2012) Functional improvement and correlations with symptomatic improvement in adults with attention deficit hyperactivity disorder receiving long-acting methylphenidate. Psychol Med 42:195–204
Buitelaar JK, Trott GE, Hofecker M, Waechter S, Berwaerts J, Dejonkheere J, Schauble B (2012) Long-term efficacy and safety outcomes with OROS-MPH in adults with ADHD. Int J Neuropsychopharmacol 15:1–13
Buitelaar JK, Wilens TE, Zhang S, Ning Y, Feldman PD (2009) Comparison of symptomatic versus functional changes in children and adolescents with ADHD during randomized, double-blind treatment with psychostimulants, atomoxetine, or placebo. J Child Psychol Psychiatry 50:335–342
Bullinger M, Brutt AL, Erhart M, Ravens-Sieberer U, Group BS (2008) Psychometric properties of the KINDL-R questionnaire: results of the BELLA study. Eur Child Adolesc Psychiatry 17(Suppl 1):125–132
Butterfield ME, Saal J, Young B, Young JL (2016) Supplementary guanfacine hydrochloride as a treatment of attention deficit hyperactivity disorder in adults: a double blind, placebo-controlled study. Psychiatry Res 236:136–141
Cannon M, Pelham WH, Sallee FR, Palumbo DR, Bukstein O, Daviss WB (2009) Effects of clonidine and methylphenidate on family quality of life in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19:511–517
Casas M, Rosler M, Sandra Kooij JJ, Ginsberg Y, Ramos-Quiroga JA, Heger S, Berwaerts J, Dejonckheere J, van der Vorst E, Schauble B (2013) Efficacy and safety of prolonged-release OROS methylphenidate in adults with attention deficit/hyperactivity disorder: a 13-week, randomized, double-blind, placebo-controlled, fixed-dose study. World J Biol Psychiatry 14:268–281
Chacko A, Pelham WE, Gnagy EM, Greiner A, Vallano G, Bukstein O, Rancurello M (2005) Stimulant medication effects in a summer treatment program among young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 44:249–257
Chang Z, Lichtenstein P, Halldner L, D’Onofrio B, Serlachius E, Fazel S, Langstrom N, Larsson H (2014) Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry 55:878–885
Chen Q, Sjolander A, Runeson B, D’Onofrio BM, Lichtenstein P, Larsson H (2014) Drug treatment for attention-deficit/hyperactivity disorder and suicidal behaviour: register based study. BMJ 348:g3769
Coghill D (2010) The impact of medications on quality of life in attention-deficit hyperactivity disorder: a systematic review. CNS Drugs 24:843–866
Coghill D (2011) Pragmatic measures in paediatric psychopharmacology—are we getting it right? Eur Neuropsychopharmacol 21:571–583
Coghill D, Danckaerts M, Sonuga-Barke E, Sergeant J, Group AEG (2009) Practitioner review: quality of life in child mental health—conceptual challenges and practical choices. J Child Psychol Psychiatry 50:544–561
Coghill D, Hodgkins P (2016) Health-related quality of life of children with attention-deficit/hyperactivity disorder versus children with diabetes and healthy controls. Eur Child Adolesc Psychiatry 25:261–271
Cunill R, Castells X, Tobias A, Capella D (2016) Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology 233:187–197
Danckaerts M, Sonuga-Barke EJ, Banaschewski T, Buitelaar J, Dopfner M, Hollis C, Santosh P, Rothenberger A, Sergeant J, Steinhausen HC, Taylor E, Zuddas A, Coghill D (2010) The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry 19:83–105
de Schipper E, Lundequist A, Wilteus AL, Coghill D, de Vries PJ, Granlund M, Holtmann M, Jonsson U, Karande S, Levy F, Al-Modayfer O, Rohde L, Tannock R, Tonge B, Bolte S (2015) A comprehensive scoping review of ability and disability in ADHD using the International Classification of Functioning, Disability and Health-Children and Youth Version (ICF-CY). Eur Child Adolesc Psychiatry 24:859–872
de Schipper E, Mahdi S, Coghill D, de Vries PJ, Gau SS, Granlund M, Holtmann M, Karande S, Levy F, Almodayfer O, Rohde L, Tannock R, Bolte S (2015) Towards an ICF core set for ADHD: a worldwide expert survey on ability and disability. Eur Child Adolesc Psychiatry 24:1509–1521
Dell’Agnello G, Maschietto D, Bravaccio C, Calamoneri F, Masi G, Curatolo P, Besana D, Mancini F, Rossi A, Poole L, Escobar R, Zuddas A (2009) Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a placebo-controlled Italian study. Eur Neuropsychopharmacol 19:822–834
Dittmann RW, Schacht A, Helsberg K, Schneider-Fresenius C, Lehmann M, Lehmkuhl G, Wehmeier PM (2011) Atomoxetine versus placebo in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a double-blind, randomized, multicenter trial in Germany. J Child Adolesc Psychopharmacol 21:97–110
DuPaul GJ, Power TJ, Anastopoulos AD, Reid R (1998) ADHD Rating Scale IV: checklists, norms, and clinical interpretation. Guildford, New York
Durell TM, Adler LA, Williams DW, Deldar A, McGough JJ, Glaser PE, Rubin RL, Pigott TA, Sarkis EH, Fox BK (2013) Atomoxetine treatment of attention-deficit/hyperactivity disorder in young adults with assessment of functional outcomes: a randomized, double-blind, placebo-controlled clinical trial. J Clin Psychopharmacol 33:45–54
Epstein J, Johnson DE, Conners CK (2004) Conners Adult ADHD Diagnostic Interview for DSM-IV. Multi Health Systems Inc, North Tonawanda
Escobar R, Montoya A, Polavieja P, Cardo E, Artigas J, Hervas A, Fuentes J (2009) Evaluation of patients’ and parents’ quality of life in a randomized placebo-controlled atomoxetine study in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19:253–263
Escobar R, Schacht A, Wehmeier PM, Wagner T (2010) Quality of life and attention-deficit/hyperactivity disorder core symptoms: a pooled analysis of 5 non-US atomoxetine clinical trials. J Clin Psychopharmacol 30:145–151
European Medicines Agency (2010) Guideline on the clinical investigation of medicinal products for the treatment of attention-deficit/hyperactivity disorder (ADHD) http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/08/WC500095686.pdf. Accessed 26 Oct 2015
Findling RL, Childress AC, Cutler AJ, Gasior M, Hamdani M, Ferreira-Cornwell MC, Squires L (2011) Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50:395–405
Food and Drug Administration (2009) Patient-reported outcome measures: use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM193282.pdf. Accessed 28 Oct 2015
Gajria K, Kosinski M, Sikirica V, Huss M, Livote E, Reilly K, Dittmann RW, Erder MH (2015) Psychometric validation of the Weiss Functional Impairment Rating Scale-Parent Report Form in children and adolescents with attention-deficit/hyperactivity disorder. Health Qual Life Outcomes 13:184
Goto T, Hirata Y, Takita Y, Trzepacz PT, Allen AJ, Song DH, Gau SS, Ichikawa H, Takahashi M (2013) Efficacy and safety of atomoxetine hydrochloride in Asian adults with ADHD: a multinational 10-week randomized double-blind placebo-controlled Asian study. J Atten Disord. doi:10.1177/1087054713510352
Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, McGough J, Wigal S, Wigal T, Vitiello B, Skrobala A, Posner K, Ghuman J, Cunningham C, Davies M, Chuang S, Cooper T (2006) Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry 45:1284–1293
Greenhill LL, Muniz R, Ball RR, Levine A, Pestreich L, Jiang H (2006) Efficacy and safety of dexmethylphenidate extended-release capsules in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 45:817–823
Hervas A, Huss M, Johnson M, McNicholas F, van Stralen J, Sreckovic S, Lyne A, Bloomfield R, Sikirica V, Robertson B (2014) Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: a randomized, controlled, phase III trial. Eur Neuropsychopharmacol 24:1861–1872
Huss M, Ginsberg Y, Tvedten T, Arngrim T, Philipsen A, Carter K, Chen CW, Kumar V (2014) Methylphenidate hydrochloride modified-release in adults with attention deficit hyperactivity disorder: a randomized double-blind placebo-controlled trial. Adv Ther 31:44–65
Kaufman J, Birmaher B, Brent D, Rao U, Ryan N (1996) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL). Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh
Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM (2006) The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 163:716–723
Lee SI, Song DH, Shin DW, Kim JH, Lee YS, Hwang JW, Park TW, Yook KH, Lee JI, Bahn GH, Hirata Y, Goto T, Takita Y, Takahashi M, Lee S, Treuer T (2014) Efficacy and safety of atomoxetine hydrochloride in Korean adults with attention-deficit hyperactivity disorder. Asia Pac Psychiatry 6:386–396
Lichtenstein P, Halldner L, Zetterqvist J, Sjolander A, Serlachius E, Fazel S, Langstrom N, Larsson H (2012) Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med 367:2006–2014
Ljung T, Chen Q, Lichtenstein P, Larsson H (2014) Common etiological factors of attention-deficit/hyperactivity disorder and suicidal behavior: a population-based study in Sweden. JAMA Psychiatry 71:958–964
Man KK, Chan EW, Coghill D, Douglas I, Ip P, Leung LP, Tsui MS, Wong WH, Wong IC (2015) Methylphenidate and the risk of trauma. Pediatrics 135:40–48
Manor I, Ben-Hayun R, Aharon-Peretz J, Salomy D, Weizman A, Daniely Y, Megiddo D, Newcorn JH, Biederman J, Adler LA (2012) A randomized, double-blind, placebo-controlled, multicenter study evaluating the efficacy, safety, and tolerability of extended-release metadoxine in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 73:1517–1523
Manos M, Frazier TW, Landgraf JM, Weiss M, Hodgkins P (2009) HRQL and medication satisfaction in children with ADHD treated with the methylphenidate transdermal system. Curr Med Res Opin 25:3001–3010
Mattingley GW, Adler LA, Montano CB, Newcorn JH (2011) Optimizing clinical outcomes across domains of life in adolescents and adults with ADHD. J Clin Psychiatry 72:1008–1014
Matza LS, Johnston JA, Faries DE, Malley KG, Brod M (2007) Responsiveness of the adult attention-deficit/hyperactivity disorder quality of life scale (AAQoL). Qual Life Res 16:1511–1520
McBride MC (1988) An individual double-blind crossover trial for assessing methylphenidate response in children with attention deficit disorder. J Pediatr 113:137–145
Medori R, Ramos-Quiroga JA, Casas M, Kooij JJ, Niemela A, Trott GE, Lee E, Buitelaar JK (2008) A randomized, placebo-controlled trial of three fixed dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 63:981–989
Michelson D, Buitelaar JK, Danckaerts M, Gillberg C, Spencer TJ, Zuddas A, Faries DE, Zhang S, Biederman J (2004) Relapse prevention in pediatric patients with ADHD treated with atomoxetine: a randomized, double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry 43:896–904
Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee FR, Spencer T (2001) Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 108:E83
Montoya A, Hervas A, Cardo E, Artigas J, Mardomingo MJ, Alda JA, Gastaminza X, Garcia-Polavieja MJ, Gilaberte I, Escobar R (2009) Evaluation of atomoxetine for first-line treatment of newly diagnosed, treatment-naive children and adolescents with attention deficit/hyperactivity disorder. Curr Med Res Opin 25:2745–2754
Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, Michelson D, Atomoxetine/Methylphenidate Comparative Study Group (2008) Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry 165:721–730
Newcorn JH, Spencer TJ, Biederman J, Milton DR, Michelson D (2005) Atomoxetine treatment in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry 44:240–248
Newcorn JH, Stein MA, Childress AC, Youcha S, White C, Enright G, Rubin J (2013) Randomized, double-blind trial of guanfacine extended release in children with attention-deficit/hyperactivity disorder: morning or evening administration. J Am Acad Child Adolesc Psychiatry 52:921–930
Norman GR, Sloan JA, Wyrwich KW (2003) Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 41:582–592
Parens E, Johnston J (2009) Facts, values, and attention-deficit hyperactivity disorder (ADHD): an update on the controversies. Child Adolesc Psychiatry Ment Health 3:1
Patrick DL, Edwards TC, Topolski TD (2002) Adolescent quality of life, part II: initial validation of a new instrument. J Adolesc 25:287–300
Pelham WE Jr, Manos MJ, Ezzell CE, Tresco KE, Gnagy EM, Hoffman MT, Onyango AN, Fabiano GA, Lopez-Williams A, Wymbs BT, Caserta D, Chronis AM, Burrows-Maclean L, Morse G (2005) A dose-ranging study of a methylphenidate transdermal system in children with ADHD. J Am Acad Child Adolesc Psychiatry 44:522–529
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164:942–948
Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA (2014) ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 43:434–442
Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, Vohra S (2016) Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev 2:CD009996
Retz W, Rosler M, Ose C, Scherag A, Alm B, Philipsen A, Fischer R, Ammer R (2012) Multiscale assessment of treatment efficacy in adults with ADHD: a randomized placebo-controlled, multi-centre study with extended-release methylphenidate. World J Biol Psychiatry 13:48–59
Riahi F, Tehrani-Doost M, Shahrivar Z, Alaghband-Rad J (2010) Efficacy of reboxetine in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled clinical trial. Hum Psychopharmacol 25:570–576
Riley AW, Coghill D, Forrest CB, Lorenzo MJ, Ralston SJ, Spiel G, ADORE Study Group (2006) Validity of the health-related quality of life assessment in the ADORE study: Parent Report Form of the CHIP-Child Edition. Eur Child Adolesc Psychiatry 15(Suppl 1):i63–i71
Riley AW, Spiel G, Coghill D, Dopfner M, Falissard B, Lorenzo MJ, Preuss U, Ralston SJ, Group AS (2006) Factors related to health-related quality of life (HRQoL) among children with ADHD in Europe at entry into treatment. Eur Child Adolesc Psychiatry 15(Suppl 1):i38–i45
Rosler M, Ginsberg Y, Arngrim T, Adamou M, Niemela A, Dejonkheere J, van Oene J, Schauble B (2013) Correlation of symptomatic improvements with functional improvements and patient-reported outcomes in adults with attention-deficit/hyperactivity disorder treated with OROS methylphenidate. World J Biol Psychiatry 14:282–290
Simon V, Czobor P, Balint S, Meszaros A, Bitter I (2009) Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry 194:204–211
Spencer TJ, Adler LA, Weisler RH, Youcha SH (2008) Triple-bead mixed amphetamine salts (SPD465), a novel, enhanced extended-release amphetamine formulation for the treatment of adults with ADHD: a randomized, double-blind, multicenter, placebo-controlled study. J Clin Psychiatry 69:1437–1448
Spencer TJ, Landgraf JM, Adler LA, Weisler RH, Anderson CS, Youcha SH (2008) Attention-deficit/hyperactivity disorder-specific quality of life with triple-bead mixed amphetamine salts (SPD465) in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 69:1766–1775
Stein MA, Sikirica V, Weiss MD, Robertson B, Lyne A, Newcorn JH (2015) Does guanfacine extended release impact functional impairment in children with attention-deficit/hyperactivity disorder? Results from a randomized controlled trial. CNS Drugs 29:953–962
Stein MA, Waldman ID, Charney E, Aryal S, Sable C, Gruber R, Newcorn JH (2011) Dose effects and comparative effectiveness of extended release dexmethylphenidate and mixed amphetamine salts. J Child Adolesc Psychopharmacol 21:581–588
Storebo OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, Nilausen TD, Magnusson FL, Zwi M, Gillies D, Rosendal S, Groth C, Rasmussen KB, Gauci D, Kirubakaran R, Forsbol B, Simonsen E, Gluud C (2015) Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. BMJ 351:h5203
Stuhec M, Munda B, Svab V, Locatelli I (2015) Comparative efficacy and acceptability of atomoxetine, lisdexamfetamine, bupropion and methylphenidate in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis with focus on bupropion. J Affect Disord 178:149–159
Svanborg P, Thernlund G, Gustafsson PA, Hagglof B, Poole L, Kadesjo B (2009) Efficacy and safety of atomoxetine as add-on to psychoeducation in the treatment of attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in stimulant-naive Swedish children and adolescents. Eur Child Adolesc Psychiatry 18:240–249
Svanborg P, Thernlund G, Gustafsson PA, Hagglof B, Schacht A, Kadesjo B (2009) Atomoxetine improves patient and family coping in attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in Swedish children and adolescents. Eur Child Adolesc Psychiatry 18:725–735
Tarakcioglu MC, Memik NC, Olgun NN, Aydemir O, Weiss MD (2015) Turkish validity and reliability study of the Weiss Functional Impairment Rating Scale-Parent Report. Atten Defic Hyperact Disord 7:129–139
Velo S, Kereszteny A, Szentivanyi D, Balazs J (2013) Quality of life of patients with attention-deficit/hyperactivity disorder: systematic review of the past 5 years. Neuropsychopharmacol Hung 15:73–82
Wehmeier PM, Schacht A, Dittmann RW, Helsberg K, Schneider-Fresenius C, Lehmann M, Bullinger M, Ravens-Sieberer U (2011) Effect of atomoxetine on quality of life and family burden: results from a randomized, placebo-controlled, double-blind study in children and adolescents with ADHD and comorbid oppositional defiant or conduct disorder. Qual Life Res 20:691–702
Wehmeier PM, Schacht A, Escobar R, Savill N, Harpin V (2010) Differences between children and adolescents in treatment response to atomoxetine and the correlation between health-related quality of life and attention deficit/hyperactivity disorder core symptoms: meta-analysis of five atomoxetine trials. Child Adolesc Psychiatry Ment Health 4:30
Weiss M, Murray C, Wasdell M, Greenfield B, Giles L, Hechtman L (2012) A randomized controlled trial of CBT therapy for adults with ADHD with and without medication. BMC Psychiatry 12:30
Wietecha L, Young J, Ruff D, Dunn D, Findling RL, Saylor K (2012) Atomoxetine once daily for 24 weeks in adults with attention-deficit/hyperactivity disorder (ADHD): impact of treatment on family functioning. Clin Neuropharmacol 35:125–133
Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, Lyne A, Grannis K, Youcha S (2012) A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 51(74–85):e72
Wilens TE, Hammerness P, Martelon M, Brodziak K, Utzinger L, Wong P (2010) A controlled trial of the methylphenidate transdermal system on before-school functioning in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry 71:548–556
Wilens TE, McBurnett K, Turnbow J, Rugino T, White C, Youcha S (2013) Morning and evening effects of guanfacine extended release adjunctive to psychostimulants in pediatric ADHD: results from a phase III multicenter trial. J Atten Disord. doi:10.1177/1087054713500144
Wilens TE, Robertson B, Sikirica V, Harper L, Young JL, Bloomfield R, Lyne A, Rynkowski G, Cutler AJ (2015) A randomized, placebo-controlled trial of guanfacine extended release in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 54(916–925):e912
Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S (2011) ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022
World Health Organization (2010) International statistical classification of diseases and related health problems, 10th edn. World Health Organization, Geneva
World Health Organization (1995) The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med 41:1403–1409
Acknowledgements
This systematic review was funded by Shire International GmbH. Shire develops and markets medications for ADHD. The following authors have received compensation for serving as consultants or speakers for, or they or the institutions they work for have received research support or royalties from, the companies or organizations indicated: T Banaschewski (Bristol-Myers Squibb, Develco Pharma, Janssen McNeil, Lilly, Medice, Novartis, Shire and Vifor Pharma); DR Coghill (Eli Lilly, Flynn Pharma, Janssen-Cilag, Medice, Novartis, Otsuka, Oxford University Press, Pfizer, Schering-Plough, Shire, UCB and Vifor Pharma); C Soutullo (Alicia Koplowitz Foundation, Editorial Médica Panamericana, Eli Lilly, EUNSA [University of Navarra Press], Fundación Caja Navarra, Janssen, Lundbeck, Mayo Eds, Medice/Juste, Rubiò, Shire, University of Navarra Research Projects [PIUNA] and Wolters Kluwer); A Zuddas (AstraZeneca, Giunti OS, Lilly, Lundbeck, Otsuka, Oxford University Press, Roche, Shire and Vifor Pharma). MG Cottingham is an employee of Oxford PharmaGenesis. Oxford PharmaGenesis received funding from Shire International GmbH for this systematic review, including assistance in screening, formatting, proofreading, copy-editing and fact checking. A Panayi from Shire International GmbH and P Sarocco from Shire Development LLC reviewed the manuscript for scientific accuracy. The authors independently determined the content of this manuscript, interpreted the data and made the decision to submit the manuscript for publication in this journal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Coghill, D.R., Banaschewski, T., Soutullo, C. et al. Systematic review of quality of life and functional outcomes in randomized placebo-controlled studies of medications for attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry 26, 1283–1307 (2017). https://doi.org/10.1007/s00787-017-0986-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-017-0986-y