Abstract
Objectives
To explore the influence of age on treatment responses to atomoxetine and to assess the relationship between core symptoms of attention deficit/hyperactivity disorder (ADHD) and health-related quality of life (HR-QoL) outcomes.
Data Sources
Data from five similar clinical trials of atomoxetine in the treatment of children and adolescents with ADHD were included in this meta-analysis.
Study Selection
Atomoxetine studies that used the ADHD Rating Scale (ADHD-RS) and the Child Health and Illness Profile Child Edition (CHIP-CE) as outcome measures were selected.
Interventions
Treatment with atomoxetine.
Main Outcome Measures
Treatment group differences (atomoxetine vs placebo) in terms of total score, domains, and subdomains of the CHIP-CE were compared across age groups, and correlations between ADHD-RS scores and CHIP-CE scores were calculated by age.
Results
Data of 794 subjects (611 children, 183 adolescents) were pooled. At baseline, adolescents showed significantly (p < 0.05) greater impairment compared with children in the Family Involvement, Satisfaction with Self, and Academic Performance subdomains of the CHIP-CE. Treatment effect of atomoxetine was significant in both age groups for the Risk Avoidance domain and its subdomains. There was a significant age-treatment interaction with greater efficacy seen in adolescents in both the Risk Avoidance domain and the Threats to Achievement subdomain. Correlations between ADHD-RS and CHIP-CE scores were generally low at baseline and moderate in change from baseline and were overall similar in adolescents and children.
Conclusions
Atomoxetine was effective in improving some aspects of HR-QoL in both age groups. Correlations between core symptoms of ADHD and HR-QoL were low to moderate.
Similar content being viewed by others
1. Introduction
Attention deficit/hyperactivity disorder (ADHD) is one of the most frequently diagnosed psychiatric disorders in childhood, characterized by 3 core symptoms: inattentiveness, hyperactivity, and impulsivity. According to a recent meta-analysis[1], ADHD affects 5.29% of school-aged children worldwide. ADHD was consistently associated with complex short-term and long-term impairments and negative outcomes regarding educational achievement, social and emotional impairment, behavioral disturbances, problems with interpersonal relations, and psychiatric comorbidity [2–5].
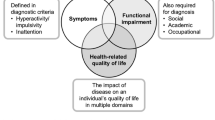
The impact of ADHD goes beyond the direct effects of core symptoms on the individual's everyday functions and represents a serious burden on the patient's and the family's life, seriously impairing the emotional, social, and physical well-being of patients and hence their health-related quality of life (HR-QoL)[6].
HR-QoL has received increasing attention in children and adolescents with ADHD, both from clinicians and investigators [7–10]. HR-QoL is a multidimensional concept that reflects the subjective physical, social, and psychological aspects of health, and goes beyond symptoms of the disorder and objective functional outcomes [11].
Based on consistent findings in the literature, effective treatments exist for the management of ADHD with both pharmacotherapy and psychosocial interventions. The treatment options for ADHD include psychostimulants (e.g. methylphenidate, mixed amphetamine salts) or atomoxetine, which is a non-stimulant treatment option for ADHD [12], both in combination with behavioral therapy [13]. Atomoxetine is a selective norepinephrine reuptake inhibitor, and its efficacy and tolerability were demonstrated in a number of randomized, placebo-controlled trials among children and adolescents [14–17]. In addition, several studies have shown improvement of emotional well-being and HR-QoL in children and adolescents treated with atomoxetine [15, 18–25]. As a non-controlled substance with no abuse liability, atomoxetine can be of value in certain populations such as patients with ADHD and co-morbid substance abuse disorder [26].
Although it has previously been thought that ADHD is essentially a disorder of childhood, a growing body of literature suggests that the disorder persists through adolescence and into adulthood with some core features and associated impairments still evident [2, 7, 27–29].
The clinical symptoms of ADHD change over time [3, 28–32]. Specifically, hyperactive/impulsive symptoms generally decline, while inattentive symptoms might persist, or even become relatively more pronounced, taking into consideration the increased complexity of those cognitive tasks that a child or an adolescent is exposed to [3, 30]. This is not surprising, as transition from childhood to adolescence involves a number changes that touch upon many areas of the adolescent's daily life. These changes include an increase in physical size and maturation, the desire to individuate from parents, resulting in more time spent away from home, an increase in the number of life activities to which the adolescent must adapt. Most of these changes are adversely affected by the delay in self-regulation that is usually associated with ADHD. Impaired self-esteem and sociability in adolescents is often the result. In adolescence, symptoms of inattention and impaired executive function (EF) generally have a greater impact on school functioning than the symptoms of hyperactivity and impulsivity. Impulsivity, in turn, is more related to functional impairment in non-academic domains and may be associated with the development of oppositional defiant disorder (ODD), drug experimentation, speeding while driving, engaging in risky sexual behavior, impulsive verbal behavior, and reactive aggression [5].
Thus, it is important to understand the implications for the individual as they get older and to evaluate medication effects with respect to age.
We therefore conducted a meta-analysis all atomoxetine clinical trials measuring HR-QoL using the Child Health and Illness Profile, Child Edition (CHIP-CE) Parent Edition that were in the Lilly data base to investigate the possible age effect on baseline impairments with regard to HR-QoL [8, 33–35], and to explore the influence of age on treatment effects of atomoxetine regarding HR-QoL outcomes, in children (6-11 years) and adolescents (12-17 years) with ADHD. Additionally, we analyzed the correlation between ADHD core symptoms and HR-QoL at baseline, at endpoint, and for change from baseline in order to evaluate the association between the improvement of the core symptoms and the improvement of HR-QoL. Treatment effects were assessed based on the 3 placebo-controlled trials and correlations were examined leveraging all 5 studies found in the Lilly data base.
2. Methods
2.1 Studies included in the meta-analysis
Data from 5 atomoxetine clinical trials (4 from Europe, 1 from Canada) with similar inclusion and exclusion criteria and similar duration of treatment (8-12 weeks follow-up) were included in the meta-analysis [23, 36–39]. The total number of patients was 794. Design, sample size, and duration of the respective studies are described in Table 1.
All included patients met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) [40] diagnostic criteria for ADHD and had a symptom severity of at least 1.5 standard deviations (SD) above the normative values of the Attention Deficit/Hyperactivity Disorder Rating Scale-IV, (ADHD-RS) Parent Version [41] except for Study 3, where the ADHD subscale of the SNAP (Swanson, Nolan, and Pelham-IV) [42] was applied. In all studies, except in Study 5, the diagnosis was confirmed using the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version (K-SADS-PL) [43], a semistructured diagnostic interview that includes a supplement for ADHD. In studies 2 and 3, baseline Clinical Global Impression of Severity (CGI-S) [44] scores for ADHD were at least 4 or higher.
Studies 1 and 2 recruited only stimulant-naïve patients. Study 3, which was carried out in Italy, did not explicitly require medication-naïve patients, but at the time of recruitment, there were no ADHD drugs approved by authorities in that country.
2.2 Measures
2.2.1 CHIP-CE
The primary scale on which this meta-analysis was based is the CHIP-CE Parent Report Form [33, 34], a 76-item generic HR-QoL questionnaire, covering a total of 5 domains (Satisfaction, Comfort, Risk Avoidance, Resilience, and Achievement) and 12 subdomains (Satisfaction with Health [SH], Satisfaction with Self [SS], Physical Comfort [PC], Emotional Comfort [EC], Restricted Activity [RA], Individual Risk Avoidance [IRA], Threats to Achievement [TA], Family Involvement [FI], Physical Activity [PA], Social Problem Solving [SPS], Academic Performance [AP], and Peer Relations [PR]). Table 2 explains which aspects of HR-QoL are assessed by each domain of the CHIP-CE. More recently, a CHIP-CE total score has been developed, which can be used as a global measure of HR-QoL [35].
The structure of the CHIP-CE was developed in non-ADHD samples. The CHIP-CE scores are standardized to t scores with a mean (± SD) of 50 (± 10), with higher scores indicating better health. Normative data were derived from a sample of 1049 school-aged children from the United States [33, 34].
2.2.2 ADHD-RS
The evaluation of the treatment effect of atomoxetine on core ADHD symptoms was based on the ADHD-RS [41], which evaluates all 18 symptoms of ADHD according to the DSM-IV diagnostic criteria. Improvement is indicated by a decrease in the score. The ADHD-RS comprises a total score, an inattentive sub-score, and a hyperactive/impulsive sub-score.
2.3 Statistical analysis
The demographic and baseline data were summarized by descriptive statistics unadjusted for study. Group comparisons at baseline were based on two-way analysis of variance (ANOVA) using the terms age and study for continuous variables and based on the Cochran-Mantel-Haenszel test controlling for study in the case of categorical variables.
Treatment efficacy over time was analyzed on an intent-to-treat basis. The intent-to-treat population included patients who had been randomized, had a baseline observation, and at least one postbaseline observation. The last observation was the one reported for change from baseline. Treatment-group differences were compared using a fixed effect analysis of covariance (ANCOVA) model including the terms treatment, study, age group, baseline ADHD-RS score, and the respective baseline CHIP-CE score. The model was run for a second time with the treatment-by-age subgroup interaction term added. Effect size (Cohen's d) was calculated for treatment overall and within age subgroups. Effect size was calculated as the ratio of the difference between atomoxetine and placebo at endpoint divided by the standard deviation of the residuals.
A consistent treatment effect in the groups is stated, if the overall treatment effect is significant and the effect sizes are clinically similar in both age groups.
Correlations between ADHD-RS scores (total score, inattentive, and hyperactive/impulsive sub-scores) and CHIP-CE scores (total, domain, and sub-domain scores) at baseline, at endpoint, and for the change from baseline to endpoint, are shown by age subgroup using Pearson's correlation coefficient and the corresponding 95% confidence interval.
All tests of hypotheses were considered statistically significant if the two-sided p-value was < 0.05. An alpha level of 0.10 was used to judge the statistical significance of an interaction. No correction was done for multiple testing as this is a post hoc analysis on existing data. The Statistical Analysis System (version 9; SAS Institute, Cary NC) was used for all analyses.
3. Results
3.1 Patient disposition
Data from a total of 794 patients were included in the analysis. The age range was 6 to 15 years. The mean age was 9.7 years (SD 2.30 years). Most of the patients of the pooled sample were children (< 12 years): 611 (77.0%), and male 658 (82.9%). For the evaluation of the effect of atomoxetine on HR-QoL, as measured by the CHIP-CE, samples from only the placebo-controlled trials were included. In total, data of n = 183 and n = 92 children (6-11 years) and n = 72 and n = 40 adolescents (12-17 years) were analyzed in the atomoxetine and placebo groups, respectively. For the comparison of the correlations between core ADHD symptoms and HR-QoL, across age groups, we included the data of all studies in the analyses. Demographic data of the pooled sample are summarized in Table 3.
3.2 Baseline differences across age groups
In the population of the five studies, gender distribution was similar across age groups. The proportion of ADHD combined subtype according to DSM-IV was significantly higher and, accordingly, the proportion of the inattentive subtype was significantly lower in children compared with adolescents. This difference was also reflected in the ADHD-RS scores, where the hyperactive/impulsive subscore was significantly higher in children, leading to a significantly higher total score (Table 3).
Impaired HR-QoL was observed at baseline as the CHIP-CE total score and four of the five domain scores (Table 4) had means of less than 40. Impairments in the following sub-domains were observed (mean <40 for at least one group - all studies): Satisfaction with Self, Emotional Comfort, Individual Risk Avoidance, Threats to Achievement, Family Involvement, Social Problem Solving, Academic Performance, and Peer Relations. Adolescents were significantly more impaired at baseline in the Satisfaction with Self and the Family Involvement sub-domains as well as in the Achievement domain and the Academic Performance sub-domain. On the other hand, children were significantly more impaired at baseline in the Emotional Comfort sub-domain. The Restricted Activity sub-domain showed a significant difference between children and adolescents; however, mean and SD in this sub-domain were within the normal range, indicating relevant impairment neither in children nor in adolescents. Although the Individual Risk Avoidance sub-domain score showed a statistically significant difference between adolescents and children in the analysis adjusting for study (p = 0.004), unadjusted descriptive scores did not indicate a clinically relevant difference (mean = 35.6, SD = 15.71 for children; mean = 35.8, SD = 15.28 for adolescents).
3.3 Treatment effect of atomoxetine
The treatment effect of atomoxetine as reflected by the CHIP-CE was significant overall and consistent within both age groups for the total score, the Emotional Comfort sub-domain, and for the Achievement domain with its two sub-domains Academic Performance and Peer Relations. In the Risk Avoidance domain, there was a significant age interaction with the therapeutic effect of atomoxetine (p < 0.10). This interaction was due to the significant interaction found in the Threats to Achievement sub-domain (p < 0.10). Specifically, in the Risk Avoidance domain and in its two sub-domains (IRA, TA), effect sizes indicated a more pronounced therapeutic effect of atomoxetine for adolescents compared with children (see Table 5, 6 and Figure 1).
3.4 Correlations between ADHD-RS and CHIP-CE scores
The correlation values with the 95% CI are summarized in Table 7, 8, 9 and Figure 2 and 3, by age groups. The CHIP-CE scores and the ADHD-RS scores showed consistent negative correlations at baseline, endpoint, and in change from baseline. Negative correlations indicate that patients with high ADHD-RS scores have low CHIP-CE scores and vice versa. Overall, correlations were in the small to medium range, showing a consistent trend toward stronger correlations at endpoint and in change from baseline, compared with the baseline correlations. In general, correlations were consistently the strongest for the Risk Avoidance and Achievement domains and their sub-domains, while correlations were consistently the weakest for the Satisfaction domain and sub-domains. The relatively strong correlation between the Risk Avoidance domain and ADHD-RS total score was predominantly influenced by the correlation with the hyperactive/impulsive ADHD-RS sub-score, while the inattentive sub-score exerted greater influence on the correlations between the Achievement domain and the ADHD-RS scores.
Figure 3 shows the Pearson's correlation coefficients between the CHIP-CE change from baseline and ADHD-RS total score, by age groups based on data of all 5 trials. Colored text and dots are used for illustrational purposes in case of those subdomains where remarkable change can be detected compared with baseline values.
3.5 Differences in correlations between ADHD-RS and CHIP-CE scores, across age groups
No substantial age differences with respect to the correlations between ADHD-RS and CHIP-CE scores were found. However, in some cases, a trend for age differences in the correlations was observed. Figure 2 and 3 show the correlation between ADHD-RS total score and CHIP-CE, by age group.
4. Discussion
This meta-analysis must be seen in the broader context of previous research on Health-Related Quality of Life (HR-QoL) in children and adolescents with ADHD [45]. Several studies have investigated HR-QoL in these patients. These studies have shown robust negative effects on HR-QoL as reported both by parents and in patient self-reports. However, children with ADHD tend to rate their own HR-QoL less negatively than their parents and do not always see themselves as functioning less well than healthy controls [6]. More severe symptoms and greater impairment predict poorer HR-QoL. Evidence is increasing that HR-QoL improves with effective treatment, both with psychostimulants and with atomoxetine, but most treatment studies have had relatively short follow-up periods [6].
In comparing children and adolescents with ADHD, this meta-analysis investigated three different aspects: the evaluation of HR-QoL at baseline, the association between HR-QoL and ADHD core symptoms, and the treatment effect of atomoxetine on HR-QoL. The first two aspects were based on all 5 studies, whilst the treatment effect could only be evaluated in the 3 placebo-controlled trials.
In the population of the five studies, gender distribution was similar across age groups. As the studies were not designed to include the same proportion of boys across different age-groups, this finding is surprising. Usually one would assume that there would be a larger proportion of boys in a sample of children compared to a sample of adolescents. This could be due to the composition of samples in clinical trials as opposed to epidemiological samples.
Analyzing the ADHD-RS in the present post-hoc analysis, children had significantly higher hyperactive/impulsive sub-scores and total scores compared with adolescents at baseline. This finding is in line with previous literature regarding the differences in symptom patterns across age groups. Specifically, hyperactive/impulsive symptoms show a definite decline over time, while inattentive symptoms may become even more pronounced during adolescence [3, 29–32]. In our sample, adolescents showed numerically higher inattentive sub-scores, although the difference in scores did not reach statistical significance and are unlikely to be clinically relevant. However, as the hyperactivity/impulsivity issues decrease, the relative importance of the inattention problems may increase. These findings need to put into perspective. Goodman et al 2010 [46] showed that ADHD-RS total score of 38.7 corresponded to moderately ill patients and 45.5 corresponded to markedly ill patients as measured by the CGI-S. Unfortunately, such data is lacking for the sub-scores of the ADHD-RS.
Baseline impairments in HR-QoL as measured by the CHIP-CE were seen on several dimensions (e.g., Satisfaction with Self, Threats to Achievement, and Academic Performance) in both age groups. Previous studies consistently reported on remarkable impairments in HR-QoL among children and adolescents with ADHD, especially in the emotional, behavioral, and achievement aspects [6]. Similarly, in our meta-analysis clinically relevant impairments were found in the Risk Avoidance and Achievement domains (and in their sub-domains), in the Emotional Comfort and in the Satisfaction with Self sub-domains as well as Family involvement and Social Problem Solving. Adolescents were generally more impaired, compared with children, in the Satisfaction with Self sub-domain, the Family Involvement sub-domain and in the Achievement domain, while children were more impaired on the Emotional Comfort sub-domain. It may be that inter-family relationships, cooperation with family members, self-satisfaction, and academic performance are more sensitive areas of life in an adolescent compared to a child (especially in the lower age-range, 6-7 years), and that ADHD symptoms might have a more pronounced effect on these domains among adolescents relative to children.
The baseline correlations between the CHIP-CE and ADHD-RS scores indicated a consistent, small-to-moderate negative correlation between the core symptoms of ADHD and HR-QoL in both age groups without substantial age differences. This finding provides additional insight into the broad effect of ADHD symptoms. However, it should be noted that these correlations do not fully explain the background of the impaired HR-QoL in children and adolescents with ADHD. Besides the core symptoms (as measured by the ADHD-RS), other factors might play a role in the observed HR-QoL impairments. For example, comorbidities such as oppositional defiant disorder (ODD), conduct disorder (CD), anxiety, and depression were found to increase impairment and decrease HR-QoL in children and adolescents with ADHD as measured by the CHIP-CE in a cross-sectional analysis of observational data [47]. This may explain the low to moderate correlation between ADHD core symptoms and HR-QoL in this meta-analysis. However, in order to analyze differential effects between children and adolescents in terms of factors influencing the impairment of HR-QoL, an even larger sample size would be required.
Based on our analysis, atomoxetine was effective in improving certain HR-QoL dimensions in both age groups. This finding is in line with several previous studies [15, 18–25, 48]. Our results indicate that adolescents might benefit more from atomoxetine treatment than children with regard to improvement in the Risk Avoidance domain and Threats to Achievement sub-domain. It must be taken into account that the sample size of adolescents in these studies was rather low, which may have prevented some of the observed therapeutic effects from reaching statistical significance (e.g. in the Achievement domain).
In both age groups, correlations between the ADHD core symptoms and the HR-QoL were small to moderate at endpoint and with regard to the change from baseline. There was no substantial age effect on the correlations, except for a clear trend in the Risk Avoidance domain and sub-domains. Specifically, the correlations between the ADHD-RS scores (both sub-scores and total score) and the Risk Avoidance domain and sub-domains were smaller in adolescents with regard to change from baseline. This reduction in the strength of the correlation between changes in core symptoms and HR-QoL may indicate a slight detachment from the primary therapeutic effect of atomoxetine on core symptoms, especially when taking into account that atomoxetine showed the highest effect sizes in improving HR-QoL in the Risk Avoidance domain and sub-domains. Our findings regarding the low and moderate correlations between core symptoms and HR-QoL (and the small correlations found in several instances regarding change from baseline after treatment), warrant further investigation to determine more precisely which additional factors contribute to the overall impairment in ADHD beyond core symptoms, and which particular factors have an adverse impact on the HR-QoL of the individuals and their family.
4.1 Limitations
The results of this meta-analysis need to be interpreted in light of a number of limitations. First, the samples of the five clinical trials showed heterogeneity in terms of cultural diversity, history of stimulant medication, and comorbidity. For example, the patients were from five different countries, where both public opinion on ADHD and approaches to treatment by physicians vary considerably. Such differences in terms of cultural diversity could have had an impact on the evaluation both of core symptoms as measured with the ADHD-RS, and health-related quality of life as measured with the CHIP-CE. Moreover, the pooled sample size of the adolescent treatment group from the three placebo-controlled trials was rather small, and thus, effect size estimations have to be interpreted with caution.
Second, drug history of the patients was mostly unknown (with the exception of Studies 2 and 4, where one of the inclusion criteria was that the patients had to be treatment-naïve): this could have introduced some variability in the evaluation of treatment efficacy. It has been already suggested in the literature that medication-naïve patients show better improvement [19]. However, in Study 4, authors reported a lack of interaction between the treatment group (atomoxetine or standard care) and whether patients had been previously treated with medication for their ADHD, indicating that the treatment effect was similar for both groups of patients (treatment-naïve or not) in terms of improving CHIP-CE total score [23]. Unfortunately, power to detect such interactions is generally low and further research is needed to obtain more information on treatment effect modifiers to ultimately tailor the medication to the individual patient.
Third, 8-12 weeks of follow-up might have been too brief for the evaluation of the improvement of HR-QoL. Though the findings of Perwien et al. [19] indicate that the treatment effect of atomoxetine with regard to the improvement in HR-QoL can be detected after 7 to 8 weeks of treatment with atomoxetine, long-term studies are warranted in this regard: primary symptoms might change significantly within 3 months, but the consequences, at least in part, might need a longer period for improvement and/or stabilization. This needs to be taken into account when evaluating the clinical impact of the differences. The developers of the CHIP-CE have proposed that a threshold of 0.6 standard deviations is clinically meaningful [49].
Fourth, in all studies, parents were the source of information on both core symptoms of ADHD and HR-QoL. This might have influenced the results in the sense that the parents and patients might have provided different responses, especially when evaluating adolescents. The views of the young people themselves, however, need to be sought in addition to parent reports, as the patient perspective reflects the subjective well-being of these children and adolescents and takes into account their autonomy as individuals [45].
An additional limitation that introduces a difficulty in interpreting our results is that HR-QoL is a construct that, to date has not yet been well-defined. Hence, measuring this construct is still a challenge, as are all measurements of subjectively perceived psychological constructs [6]. Although the CHIP-CE was validated and standardized on a large community sample of children and adolescents, it cannot be assured that CHIP-CE really reflects and captures all the relevant aspects of HR-QoL with regard to the evaluation of the broad impact of ADHD on the individual's life.
4.2 Strengths
This meta-analysis also had several strengths. Most importantly, the sample size was large. Secondly, three of the five studies were placebo-controlled. Thirdly, the analysis was based on individual patient data rather than publication-based meta-analysis. Fourthly, the inclusion and exclusion criteria of the five studies included in the meta-analysis [47] were very similar, resulting in a fairly homogeneous sample in terms of patient characteristics. Finally, the meta-analysis included patient reported HR-QoL outcomes as a secondary endpoint. Thus, the analysis can be considered an important contribution to the body of data on the relationship between outcomes in terms of ADHD core symptoms and HR-QoL outcomes based on closely monitored clinical trials rather than cross-sectional (or observational) studies.
5 Conclusion
Overall, this meta-analysis found that, compared with children, adolescents with ADHD were somewhat more impaired at baseline, in regard to some domains of HR-QoL as measured by the CHIP-CE. Impairments were seen in the Risk Avoidance and Achievement domains and their sub-domains as well as in the sub-domains Emotional Comfort, Satisfaction with Self, Family Involvement, and Social Problem Solving, both in children and adolescents. Atomoxetine was generally shown to be effective in improving certain aspects of HR-QoL as reflected by the CHIP-CE. In the Risk Avoidance domain and Threats to Achievement sub-domain, there was a significant age effect with better efficacy seen in adolescents. Correlations between ADHD core symptoms and HR-QoL at baseline and for change from baseline to endpoint were small to moderate, suggesting that next to the effect of core symptoms, other factors might play a role in the background of the observed impairments in HR-QoL. Further studies are needed to investigate the long-term effects of atomoxetine on HR-QoL, as well as to develop more specific tools in the assessment of the effect of ADHD treatments on HR-QoL in children and adolescents.
References
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA: The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007, 164 (6): 942-948. 10.1176/appi.ajp.164.6.942.
Biederman J, Faraone SV, Spencer TJ, Mick E, Monuteaux MC, Aleardi M: Functional impairments in adults with self-reports of diagnosed ADHD: A controlled study of 1001 adults in the community. J Clin Psychiatry. 2006, 67 (4): 524-540. 10.4088/JCP.v67n0403.
Brassett-Harknett A, Butler N: Attention-deficit/hyperactivity disorder: an overview of the etiology and a review of the literature relating to the correlates and lifecourse outcomes for men and women. Clin Psychol Rev. 2007, 27 (2): 188-210. 10.1016/j.cpr.2005.06.001.
Escobar R, Hervas A, Soutullo CA, Mardomingo MJ, Uruñuela A, Gilaberte I: Attention-deficit/hyperactivity disorder: burdens of the disease according to subtypes in recently diagnosed children. Actas Esp Psiquiatr. 2008, 36 (5): 285-294.
Wehmeier PM, Schacht A, Barkley RA: Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010, 46: 209-217. 10.1016/j.jadohealth.2009.09.009.
Danckaerts M, Sonuga-Barke EJ, Banaschewski T, Buitelaar J, Döpfner M, Hollis C, Santosh P, Rothenberger A, Sergeant J, Steinhausen HC, Taylor E, Zuddas A, Coghill D: The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010, 19 (2): 83-105. 10.1007/s00787-009-0046-3.
Harpin VA: The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Arch Dis Child. 2005, 90 (1): i2-i7. 10.1136/adc.2004.059006.
Riley AW, Spiel G, Coghill D, Döpfner M, Falissard B, Lorenzo MJ, Preuss U, Ralston SJ, ADORE Study Group: Factors related to health-related quality of life (HRQoL) among children with ADHD in Europe at entry into treatment. Eur Child Adolesc Psychiatry. 2006, 15 (Suppl1): 138-145.
Hakkaart-van Roijen L, Zwirs BWC, Bouwmans C, Tan SS, Schulpen TW, Vlasveld L, Buitelaar JK: Societal cost and quality of life of children suffering from attention deficit hyperactivity disorder (ADHD). Eur Child Adolesc Psychiatry. 2007, 16 (5): 316-326. 10.1007/s00787-007-0603-6.
Bastiaens L: Both atomoxetine and stimulants improve quality of life in an ADHD population treated in a community clinic. Psychiatr Q. 2008, 79 (2): 133-137. 10.1007/s11126-008-9070-6.
Wallander JL, Schmitt M, Koot HM: Quality of life measurement in children and adolescents: issues, instruments, and applications. J Clin Psychol. 2001, 57 (4): 571-585. 10.1002/jclp.1029.
Cheng JY, Chen RY, Ko JS, Ng EM: Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents-meta-analysis and meta-regression analysis. Psychopharmacology (Berl). 2007, 194 (2): 197-209. 10.1007/s00213-007-0840-x.
Jensen PS, Hinshaw SP, Swanson JM, Greenhill LL, Conners CK, Arnold LE, Abikoff HB, Elliott G, Hechtman L, Hoza B, March JS, Newcorn JH, Severe JB, Vitiello B, Wells K, Wigal T: Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): implications and applications for primary care providers. Dev Behav Pediatr. 2001, 22 (1): 60-73.
Kelsey DK, Sumner CR, Casat CD, Coury DL, Quintana H, Saylor KE, Sutton VK, Gonzales J, Malcolm SK, Schuh KJ, Allen AJ: Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics. 2004, 114 (1): e1-8. 10.1542/peds.114.1.e1.
Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee FR, Spencer T, Atomoxetine ADHD Study Group: Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics. 2001, 108 (5): E83-10.1542/peds.108.5.e83.
Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C, Newcorn J, Sallee FR, Sangal RB, Saylor K, West S, Kelsey D, Wernicke J, Trapp NJ, Harder D: Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002, 159 (11): 1896-1901. 10.1176/appi.ajp.159.11.1896.
Spencer T, Heiligenstein JH, Biederman J, Faries DE, Kratochvil CJ, Conners CK, Potter WZ: Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002, 63 (12): 1140-1147.
Buitelaar JK, Danckaerts M, Gillberg C, Zuddas A, Becker K, Bouvard M, Fagan J, Gadoros J, Harpin V, Hazell P, Johnson M, Lerman-Sagie T, Soutullo CA, Wolanczyk T, Zeiner P, Fouche DS, Krikke-Workel J, Zhang S, Michelson D, Atomoxetine International Study Group: A prospective, multicenter, open-label assessment of atomoxetine in non-North American children and adolescents with ADHD. Eur Child Adolesc Psychiatry. 2004, 13 (4): 249-257. 10.1007/s00787-004-0401-3.
Perwien AR, Faries DE, Kratochvil CJ, Sumner CR, Kelsey DK, Allen AJ: Improvement in health-related quality of life in children with ADHD: an analysis of placebo controlled studies of atomoxetine. J Dev Behav Pediatr. 2004, 25 (4): 264-271. 10.1097/00004703-200408000-00006.
Perwien AR, Kratochvil CJ, Faries DE, Vaughan BS, Spencer T, Brown RT: Atomoxetine treatment in children and adolescents with attention-deficit hyperactivity disorder: what are the long-term health-related quality-of-life outcomes?. J Child Adolesc Psychopharmacol. 2006, 16 (6): 713-724. 10.1089/cap.2006.16.713.
Matza LS, Stoeckl MN, Shorr JM, Johnston JA: Impact of atomoxetine on health-related quality of life and functional status in patients with ADHD. Expert Rev Pharmacoeconomics Outcomes Res. 2006, 6 (4): 379-390. 10.1586/14737167.6.4.379.
Brown RT, Perwien A, Faries DE, Kratochvil CJ, Vaughan BS: Atomoxetine in the management of children with ADHD: effects on quality of life and school functioning. Clin Pediatr (Phila). 2006, 45 (9): 819-827. 10.1177/0009922806294219.
Prasad S, Harpin V, Poole L, Zeitlin H, Jamdar S, Puvanendran on behalf of the SUNBEAM Study Group: A multicentre, randomised, open-label study of atomoxetine compared with standard current therapy in UK children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Curr Med Res Opin. 2007, 23 (2): 379-394. 10.1185/030079906X167309.
Wehmeier PM, Dittmann RW, Schacht A, Minarzyk A, Lehmann M, Sevecke K, Lehmkuhl G: Effectiveness of atomoxetine and quality of life in children with attention-deficit/hyperactivity disorder as perceived by patients, parents and physicians in an open-label study. J Child Adolesc Psychopharmacol. 2007, 17: 813-830. 10.1089/cap.2007.0025.
Wehmeier PM, Schacht A, Lehmann M, Dittmann RW, Silva SG, March JS: Emotional well-being in children and adolescents treated with atomoxetine for attention-deficit/hyperactivity disorder: Findings from a patient, parent and physician perspective using items from the pediatric adverse event rating scale (PAERS). Child Adolesc Psychiatry Ment Health. 2008, 2 (1): 11-10.1186/1753-2000-2-11.
Barkley RA, Fischer M, Smallish L, Fletcher K: The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002, 111 (2): 279-289. 10.1037/0021-843X.111.2.279.
Fayyad J, De Graaf R, Kessler RC, Alonso J, Angermeyer M, Demyttenaere K, De Girolamo G, Haro JM, Karam EG, Lara C, Lépine JP, Ormel J, Posada-Villa J, Zaslavsky AM, Jin R: Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007, 190 (5): 402-409. 10.1192/bjp.bp.106.034389.
Jasinski DR, Faries DE, Moore RJ, Schuh LM, Allen AJ: Abuse liability assessment of atomoxetine in a drug-abusing population. Drug and Alcohol Dependence. 2008, 95: 140-146. 10.1016/j.drugalcdep.2008.01.008.
Wolraich ML, Wibbelsman CJ, Brown TE, Evans SW, Gotlieb EM, Knight JR, Ross EC, Schubiner HH, Wender EH, Wilens T: Attention-Deficit/Hyperactivity Disorder among adolescents: a review of the diagnosis, treatment, and clinical implications. Pediatrics. 2005, 115 (6): 1734-1746. 10.1542/peds.2004-1959.
Barkley RA: Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002, 63 (12): 10-15.
Murphy K, Barkley RA: Prevalence of DSM-IV Symptoms of ADHD in adult licensed drivers: Implication for clinical diagnosis. J Atten Disord. 1996, 1 (3): 147-161. 10.1177/108705479600100303.
Kordon A, Kahl KG, Wahl K: A new understanding of attention-deficit disorders--beyond the age-at-onset criterion of DSM-IV. Eur Arch Psyhiatry Clin Neurosci. 2006, 256 (1): i47-54. 10.1007/s00406-006-1007-1.
Riley AW, Robertson J, Forrest CB, Green B, Rebok G, Starfield B: Manual for the Child Health and Illness Profile-Child Edition (CHIP-CETM). 2001, Baltimore, MD: The Johns Hopkins University
Riley AW, Forrest CB, Starfield B, Rebok GW, Robertson JA, Green BF: The Parent Report Form of the CHIP-Child Edition: reliability and validity. Med Care. 2004, 42 (3): 210-220. 10.1097/01.mlr.0000114909.33878.ca.
Riley AW, Chan KS, Prasad S, Poole L: A global measure of child health-related quality of life: reliability and validity of the Child Health and Illness Profile - Child Edition (CHIP-CE) global score. J Med Economics. 2007, 10 (2): 91-106. 10.3111/200710091106.
Svanborg P, Thernlund G, Gustafsson PA, Hägglöf B, Poole L, Kadesjö B: Efficacy and safety of atomoxetine as add-on to psychoeducation in the treatment of attention-deficit/hyperactivity disorder: a randomized, double-blind placebo controlled study in stimulant-naïve Swedish children and adolescents. Eur Child Adolesc Psychiatry. 2009, 18 (4): 240-249. 10.1007/s00787-008-0725-5.
Escobar R, Montoya A, Polavieja P, Cardo E, Artigas J, Hervas A, Fuentes J: Evaluation of patients' and parents' quality of life in a randomized placebo-controlled atomoxetine study in attention- deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009, 19 (3): 253-263. 10.1089/cap.2008.0109.
Dell'Agnello G, Maschiatto D, Bravaccio C, Calamoneri F, Masi G, Curatolo P, Besana D, Mancini F, Rossi A, Poole L, Escobar R, Zuddas A, LYCY Study Group: Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: A placebo-controlled Italian study. Eur Neuropsychopharmacol. 2009, 19 (11): 822-834.
Dickson RA, Jackiewicz G, Khattak S: Change in ADHD symtoms and functional outcomes in Canadian Children during 3 Months of atomoxetine treatment. Poster presented at the 27th Annual Conference of the Canadian Academy of Child and Adolescent Psychiatry (CACAP). 2007, Montreal, Quebec
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Text Revision (DSM-IV-TR). 2000, Washington, DC: American Psychiatric Association, Fourth
DuPaul GJ, Power TJ, Anastopoulos AD, Reid R: ADHD Rating Scale-IV: checklists, norms, and clinical interpretations. 1998, New York: Guilford
Swanson J: School-Based Assessment and Interventions for ADD. 1992, KC Publishing, Irvine, 184.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997, 36 (7): 980-989. 10.1097/00004583-199707000-00021.
Guy W, ed: ECDEU assessment manual for psychopharmacology: publication ADM 76-338. 1996, Washington, DC: US Department of Health, Education and Welfare, 218-222.
Coghill D, Danckaerts M, Sonuga-Barke E, Sergeant J, ADHD European Guidelines Group: Practitioner review: quality of life in child mental health - conceptual challenges and practical choices. J Child Psychol Psychiatry. 2009, 50 (5): 544-561. 10.1111/j.1469-7610.2009.02008.x.
Goodman D, Faraone SV, Adler LA, Dirks B, Hamdani M, Weisler R: Interpreting ADHD Rating Scale Scores: Linking ADHD Rating Scale Scores and CGI Levels in Two Randomized Controlled Trials of Lisdexamfetamine Dimesylate in ADHD. Primary Psychiatry. 2010, 17 (3): 44-52.
Steinhausen HC, Nøvick TS, Baldursson G, Curatolo P, Lorenzo MJ, Rodrigues Pereira R, Ralston SJ, Rothenberger A, ADORE Study Group: Co-existing psychiatric problems in ADHD in the ADORE cohort. Eur Child Adolesc Psychiatry. 2006, 15 (Suppl. 1): 125-129.
Escobar R, Schacht A, Wehmeier PM, Wagner T: Quality of life and ADHD core symptoms: A pooled analysis of 5 non-US atomoxetine clinical trials. J Clin Psychopharmacol. 2010, 30 (2): 145-151. 10.1097/JCP.0b013e3181d21763.
Riley AW, Green BF, Forrest CB, Starfield B, Kang M, Ensminger ME: A taxonomy of adolescent health: development of the adolescent health profile-types. Medical Care. 1998, 36 (8): 1228-1236. 10.1097/00005650-199808000-00010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The research was funded by Eli Lilly and Company. PMW, AS, RE, and NS are full-time employees and stakeholders of Eli Lilly. VH has received research grants and speaker honoraria from Eli Lilly and has served on several advisory boards for Eli Lilly.
Authors' contributions
PMW, AS, and RE developed the meta-analysis on which this manuscript is based. All Authors participated in interpreting the data. PMW and AS drafted the manuscript. RE, NS and VH revised the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wehmeier, P.M., Schacht, A., Escobar, R. et al. Differences between children and adolescents in treatment response to atomoxetine and the correlation between health-related quality of life and Attention Deficit/Hyperactivity Disorder core symptoms: Meta-analysis of five atomoxetine trials. Child Adolesc Psychiatry Ment Health 4, 30 (2010). https://doi.org/10.1186/1753-2000-4-30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1753-2000-4-30