Abstract
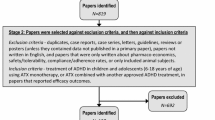
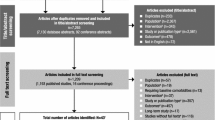
Attention-Deficit/Hyperactivity Disorder (ADHD) impacts a significant number of children and adolescents and often leads to deleterious functional impairment. Psychostimulant medication has historically been the first line of pharmacological intervention, though recent years have seen greater attention paid to non-stimulant alternatives. The objective of the present study was to conduct the most comprehensive meta-analysis to date evaluating the efficacy of atomoxetine in reducing core symptomatology of ADHD according to parent report. Selection criteria were applied, and studies were located by searching electronic databases, review of reference sections, and contact with expert researchers; article searching began on 10/01/2013, and the final search was conducted on 09/01/2014. A total of 42 studies met inclusion criteria—33 with control groups and 9 without—for a total sample of 8398 individuals. For those receiving atomoxetine, the summary pre–post (e.g., standardized mean gain) effect size estimate was 1.37 (95% CI [1.24, 1.51], p < .001); atomoxetine was found to statistically significantly outperform control conditions overall (Z = 4.07, p < .001), though results differed by the type of control group; for instance, when comparing atomoxetine to alternative medications as controls, significant differences were no longer present. The non-stimulant atomoxetine led to significant improvement in core ADHD symptomatology and should be considered as a viable pharmacological treatment option for ADHD.
Similar content being viewed by others
References
References marked with an asterisk (*) indicate studies included in the meta-analysis
Allen AJ, Michelson D (2002) Drug development process for a product with a primary pediatric indication. J Clin Psychiatry 63(12):44–49
*Allen AJ, Kurlan RM, Gilbert DL, Coffey BJ, Linder SL, Lewis DW et al (2005) Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology 65(12):1941–1949. doi:10.1212/01.wnl.0000188869.58300.a7
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Arlington
*Bangs ME, Emslie GJ, Spencer TJ, Ramsey JL, Carlson C, Bartky EJ et al (2007) Efficacy and safety of atomoxetine in adolescents with attention-deficit/hyperactivity disorder and major depression. J Child Adolesc Psychopharmacol 17(4):407–419. doi:10.1089/cap.2007.0066
*Bangs ME, Hazell P, Danckaerts M, Hoare P, Coghill DR, Wehmeier PM et al (2008) Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics 121(2):314–320. doi:10.1542/peds.2006-1880
Becker BJ (1988) Synthesizing standardized mean-change measures. Br J Math Stat Psychol 41:257–278. doi:10.1111/j.2044-8317.1988.tb00901.x
*Block SL, Kelsey D, Coury D, Lewis D, Quintana H, Sutton V et al (2009) Once-daily atomoxetine for treating pediatric attention-deficit/hyperactivity disorder: comparison of morning and evening dosing. Clin Pediatr 48(7):723–733. doi:10.1177/0009922809335321
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to meta-analysis. Wiley, West Sussex
Bradley C (1937) The behavior of children receiving benzedrine. Am J Psychiatry 94:577–585
Brunwasser SM, Gillham JE, Kim ES (2009) A meta-analytic review of the Penn Resiliency Program’s effect on depressive symptoms. J Consult Clin Psychol 77:1042–1054. doi:10.1037/a0017671
*Buitelaar JK, Danckaerts M, Gillberg C, Zuddas A, Becker K, Bouvard M et al (2004) A prospective, multicenter, open-label assessment of atomoxetine in non-North American children and adolescents with ADHD. Eur Child Adolesc Psychiatry 13(4):249–257. doi:10.1007/s00787-004-0401-3
Buitelaar JK, Wilens TE, Zhang S, Ning Y, Feldman PD (2009) Comparison of symptomatic versus functional changes in children and adolescents with ADHD during randomized, double-blind treatment with psychostimulants, atomoxetine, or placebo. J Child Psychol Psychiatry 50(3):335–342. doi:10.1111/j.1469-7610.2008.01960.x
Bymaster FP, Katner JS, Nelson DL, Hemrick-Leucke SK, Threlkeld PG, Heiligenstein JH et al (2002) Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27:699–711. doi:10.1016/S0893-133X(02)00346-9
Cho S, Lee SI, Yoo H, Song DH, Ahn DH, Shin DW et al (2011) A randomized, open-label assessment of response to various doses of atomoxetine in Korean pediatric outpatients with ADHD. Korean Neuropsychiatr Assoc 8:141–148. doi:10.4306/pi.2011.8.2.141
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Erlbaum, Hillsdale
Cooper H, Hedges LV (1994) The handbook of research synthesis. Russel Sage Foundation, New York
*de Jong CGW, Van De Voorde S, Roeyers H, Raymaekers R, Allen AJ, Knijiff S et al (2009) Differential effects of atomoxetine on executive functioning and lexical decision in attention-deficit/hyperactivity disorder and reading disorder. J Child Adolesc Psychopharmacol 19(6):699–707. doi:10.1089/cap.2009.0029
*Dell’Agnello G, Maschietto D, Bravaccio C, Calamoneri F, Masi G, Curatolo P et al (2009) Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a placebo-controlled italian study. Eur Neuropsychopharmacol 19:822–834. doi:10.1016/j.euroneuro.2009.07.008
* Dittman RW, Schact A, Helsberg K, Schneider-Fresenius C, Lehmann M, Lehmkuhl G, Wehmeier PM (2011) Atomoxetine versus placebo in children and adolescents with attentiondeficit/ hyperactivity disorder and comorbid oppositional defiant disorder: A double-blind, randomized, multicenter trial in Germany. J Child Adolesc Psychopharmacol 21:97–110
*Dittmann RW, Cardo E, Nagy P, Anderson CS, Bloomfield R, Caballero B et al (2013) Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperacitivty disorder: a head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs 27:1081–1092. doi:10.1007/s40263-013-0104-8
Dittman RW, Schact A, Helsberg K, Schneider-Fresenius C, Lehmann M, Lehmkuhl G, Wehmeier PM (2011) Atomoxetine versus placebo in children and adolescents with attentiondeficit/ hyperactivity disorder and comorbid oppositional defiant disorder: a double-blind, randomized, multicenter trial in Germany. J Child Adolesc Psychopharmacol 21:97–110
*Gau SSF, Huang Y, Soong W, Chou M, Chou W, Shang C et al (2007) A randomized, double-blind, placebo-controlled clinical trial on once-daily atomoxetine hydrochloride in Taiwanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 17(4):447–459. doi:10.1089/cap.2007.0091
*Geller D, Donnelly C, Lopez F, Rubin R, Newcorn J, Sutton V et al (2007) Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 46:1119–1127. doi:10.1097/chi.0b013e3180ca8385
Hazell PL, Kohn MR, Dickson R, Walton RJ, Granger RE, van Wyk GW (2011) Core ADHD symptom improvement with atomoxetine versus methylphenidate: a direct comparison meta-analysis. J Atten Disord 15(8):674–683. doi:10.1177/1087054710379737
Hedges LV (1982) Estimation of effect size from a series of independent studies. Psychol Bull 92:490–499. doi:10.1037/0033-2909.92.2.490
Hedges LV, Vevea JL (1998) Fixed- and random-effects models in meta-analysis. Psychol Methods 3:486–504. doi:10.1037/1082-989X.3.4.486
Higgins J, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Br Med J 327:557–560. doi:10.1136/bmj.327.7414.557
Hodgson K, Hutchinson AD, Denson L (2014) Nonpharmacological treatments for ADHD: a meta-analytic review. J Atten Disord 18:275–282
Hoekstra PJ (2011) Is there potential for the treatment of children with ADHD beyond psychostimulants? Eur Child Adolesc Psychiatry 20:431–432. doi:10.1007/s00787-011-0212-2
Hofman SG, Sawyer AT, Witt AA, Oh D (2010) The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol 78:169–183. doi:10.1037/a0018555
*Kelsey DK, Sumner CR, Casat CD, Coury DL, Quintana H, Saylor KE et al (2004) Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics 114:e1–e8
*Kemner JE, Starr HL, Ciccone PE, Hooper-Wood CG, Crockett RS (2005) Outcomes of OROS methylphenidate compared with atomoxetine in children with ADHD: a multicenter, randomized prospective study. Adv Ther 22:498–512
Kishimoto T, Nitta M, Borenstein M, Jane JM, Correll CU (2013) Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry 74:957–965. doi:10.4088/JCP.13r08440
*Kratochvil CJ, Bohac D, Harrington M, Baker N, May D, Burke WJ (2001) An open-label trial of tomoxetine in pediatric attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 11(2):167–170
*Kratochvil CJ, Heiligenstein JH, Dittmann R, Spencer TJ, Biederman J, Wernicke J et al (2002) Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 41(7):776–784. doi:10.1097/00004583-200207000-00008
*Kratochvil C, Newcorn J, Arnold E (2005) Atomoxetine alone or combined with fluoxetine for treating ADHD comorbid with depressive or anxiety symptoms. J Am Acad Child Adolesc Psychiatry 44(9):915–924. doi:10.1097/01.chi.0000169012.81536.38
Kratochvil CJ, Wilens TE, Greenhill LL, Gao H, Baker KD, Feldman PD et al (2006) Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 45(8):919–927. doi:10.1097/01.chi.0000222788.34229.68
Kratochvil CJ, Milton DR, Vaughn BS, Greenhill LL (2008) Acute atomoxetine treatment of younger and older children with ADHD: a meta-analysis of tolerability and efficacy. Child Adolesc Psychiatry Ment Health. doi:10.1186/1753-2000-2-25
*Kratochvil CJ, Vaughan BS, Stoner JA, Daughton JM, Lubberstedt BD, Murray DW et al (2011) A double-blind, placebo-controlled study of atomoxetine in young children with ADHD. Pediatrics 127(4):e862–e868. doi:10.1542/peds.2010-0825
Kratz O, Studer P, Baack J, Malcherek S, Erbe K, Moll G, Heinrich H (2012) Differential effects of methylphenidate and atomoxetine on attentional processes in children with ADHD: an event-related potential study using the attention network test. Prog Neuropsychopharmacol Biol Psychiatry 37(1):81–89. doi:10.1016/j.pnpbp.2011.12.008
Lipsey MW, Wilson DB (2000) Practical meta-analysis. Applied social research methods series, vol 49. Sage, Thousand Oaks
*Martenyi F, Zavadenko NN, Jarkova NB, Yarosk AA, Soldatenkova VO, Bardenstein LM, Zykov VP (2010) Atomoxetine in children and adolescents with attention deficit/hyperactivity disorder: a 6-week, randomized, placebo-controlled, double-blind trial in Russia. Eur Child Adolesc Psychiatry 19:57–66. doi:10.1007/s00787-009-0042-7
*Maziade M, Rouleau N, Lee B, Rogers A, Davis L, Dickson R (2009) Atomoxetine and neuropsychological function in children and attention-deficit/hyperactivity disorder: results of a pilot study. J Child Adolesc Psychopharmacol 19(6):709–718. doi:10.1089/cap.2008.0166
*Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee R et al (2001) Atomoxetine in the treatment of children and adolescents with ADHD: a randomized, placebo-controlled, dose-response study. Pediatrics 108(5):83
*Michelson D, Allen AJ, Busner J, Casat C, Dunn D, Kratochvil C et al (2002) Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 159:1896–1901. doi:10.1176/appi.ajp.159.11.1896
*Montoya A, Hervas A, Cardo E, Artigas J, Mardomingo M, Alda J et al (2009) Evaluation of atomoxetine for first-line treatment of newly diagnosed, treatment-naïve children and adolescents with attention deficit/hyperactivity disorder. Curr Med Res Opin 25(11):2745–2754. doi:10.1185/03007990903316152
Moses LE, Mostellar F, Buehler JH (2002) Comparing results of large clinical trials to those of meta-analyses. Stat Med 21:793–800
Newcorn J, Kratochvil C, Allen AJ, Casat C, Ruff D, Moore R, Michelson D (2008) Atomoxetine and osmotically released methylphenidate for the treatment of attention-deficit/hyperactivity disorder: acute comparison and differential response. Am J Psychiatry 165:721–730. doi:10.1176/appi.ajp.2007.05091676
Orwin RG (1983) A fail-safe N for effect size in meta-analysis. J Educ Stat 8:157–159. doi:10.2307/1164923
Perwein AR, Faries DE, Kratochvil CJ, Sumner CR, Kelsey DK, Allen AJ (2004) Improvement in health-related quality of life in children with ADHD: an analysis of placebo controlled studies in atomoxetine. Dev Behav Pediatr 25(4):264–271
Polzer J, Bangs MK, Zhang S, Dellva MA, Tauscher-Wisniewski S, Acharya N et al (2007) Meta-analysis of aggression or hostility events in randomized, controlled clinical trials of atomoxetine for ADHD. J Biol Psychiatry 61(5):713–719. doi:10.1016/j.biopsych.2006.05.044
Popper CW (2000) Pharmacologic alternatives to psychostimulants for the treatment of attention deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am 9:605–646
Posner K, Melvin GA, Murray DW, Gugga SS, Fisher P, Skrobala A et al (2007) Clinical presentation of ADHD in preschool children: preschoolers with ADHD treatment study (PATS). J Child Adolesc Psychopharmacol 17:547–562
*Prasad S, Harpin V, Poole L, Zeitlin H, Jamdar S, Puvanendran K (2007) A multi-centre, randomised, open-label study of atomoxetine compared with standard current therapy in UK children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Curr Med Res Opin 23(2):379–394. doi:10.1185/030079906X167309
Rosenthal R (1979) The “file drawer problem” and tolerance for null results. Psychol Bull 86:638–641. doi:10.1037/0033-2909.86.3.638
Rosenthal R (1984) Meta-analytic procedures for social research. Applied social research methods series, vol 6. Sage, Beverly Hills
Rosenthal R (1991) Meta-analytic procedures for social research. Sage, London
Rosenthal R (1993) Meta-analytic procedures for social research. Sage, Newbury Park
Rosenthal R, DiMatteo MR (2001) Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol 52:59–82. doi:10.1146/annurev.psych.52.1.59
*Sangal RB, Owens J, Allen AJ, Sutton V, Schuh K, Kelsey D (2006) Effects of atomoxetine and methylphenidate on sleep in children with ADHD. SLEEP 29(12):1573–1585
Schwartz S, Correll CU (2014) Efficacy and safety of atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: results from a comprehensive meta-analysis and metaregression. J Am Acad Child Adolesc Psychiatry 53:174–187. doi:10.1016/j.jaac.2013.11.005
*Spencer T, Biederman J, Heiligenstein J, Wilens T, Faries D, Prince J et al (2001) An open-label, dose ranging study of atomoxetine in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 11(3):251–265. doi:10.1089/10445460152595577
*Spencer TJ, Heiligenstein JH, Biederman J, Faries DE, Kratochvil CJ, Conners CK, Potter WZ (2002) Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry 63:1140–1147. doi:10.4088/JCP.v63n1209
Sterling TD (1959) Publication decisions and their possible effects on inferences drawn from tests of significance—or vice versa. J Am Stat Assoc 54:30–34. doi:10.2307/2282137
Stewart RE, Chambless DL (2009) Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol 77:595–606. doi:10.1037/a0016032
*Sumner CR, Gathercole S, Greenbaum M, Rubin R, Williams D, Hollandbeck M, Wietecha L (2009) Atomoxetine for the treatment of attention-deficit/hyperactivity disorder (ADHD) in children with ADHD and dyslexia. Child Adolesc Psychiatry Ment Health. doi:10.1186/1753-2000-3-40
*Svanborg P, Thernlund G, Gustafsson PA, Hagglof B, Poole L, Kadesjo B (2009) Efficacy and safety of atomoxetine as add-on to psychoeducation in the treatment of attention-deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in stimulant naïve Swedish children and adolescents. Eur J Child Adolesc Psychiatry 18:240–249. doi:10.1007/s00787-008-0725-5
*Takahashi M, Takita Y, Yamazaki K, Hayashi T, Ichikawa H, Kambayashi Y et al (2009) A randomized, double-blind, placebo-controlled study of atomoxetine in Japanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19(4):341–350. doi:10.1089/cap.2008.0154
Tanaka Y, Rohde LA, Jin L, Feldman PD, Upadhyaya HP (2013) A meta-analysis of the consistency of atomoxetine treatment effects in pediatric patients with attention-deficit/hyperactivity disorder from 15 clinical trials across four geographic regions. J Child Adolesc Psychopharmacol 23:262–270
*Thurstone C, Riggs PD, Salomonsen-Sautel S, Mikulich-Gilbertson SK (2010) Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance abuse disorder. J Am Acad Child Adolesc Psychiatry 49(6):573–582
Visser SA, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour R et al (2014) Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry 53:34–46
*Wang Y, Zheng Y, Du Y, Song DH, Shin YJ, Cho SC et al (2007) Atomoxetine versus methylphenidate in pediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust N Z J Psychiatry 41:222–230. doi:10.1080/00048670601057767
*Wehmeier PM, Dittmann RW, Schacht A, Minarzyk A, Lehmann M, Sevecke K, Lehmkuhl G (2007) Effectiveness of atomoxetine and quality of life in children with attention-deficit/hyperactivity disorder as perceived by patients, parent, and physicians in an open-label study. J Child Adolesc Psychopharmacol 17(6):813–829. doi:10.1089/cap.2007.0025
Wehmeier PM, Schacht A, Ulberstad F, Lehmann M, Schneider-Fresenius C, Lehmkuhl G, Banaschewski T (2012) Does atomoxetine improve executive function, inhibitory control, and hyperactivity? Results from a placebo-controlled trial using quantitative measurement technology. J Clin Psychopharmacol 32(5):653–660. doi:10.1097/JCP.0b013e318267c304
*Weiss M, Tannock R, Kratochvil C, Dunn D, Velez-Borras J, Thomason C et al (2005) A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry 44(7):647–655. doi:10.1097/01.chi.0000163280.47221.c9
*Wietecha LA, Williams DW, Herbert M, Melmed RD, Greenbaum M, Schuh K (2009) Atomoxetine treatment in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19(6):719–730. doi:10.1089/cap.2008.074
*Wigal SB, McGough JJ, McCracken JT, Biederman J, Spencer TJ, Posner KL et al (2005) A laboratory school comparison of mixed amphetamine salts extended release (adderall XR®) and atomoxetine (strattera®) in school-aged children with attention deficit/hyperactivity disorder. J Atten Disord 9(1):275–289. doi:10.1177/1087054705281121
Wilens TE, Kratochvil C, Newcorn JH, Gao H (2006a) Do children and adolescents with ADHD respond differently to atomoxetine? J Am Acad Child Adolesc Psychiatry 45(2):149–157. doi:10.1097/01.chi.0000190352.90946.0b
Wilens TE, Newcorn JH, Kratochvil CJ, Gao H, Thomason CK, Rogers AK et al (2006b) Long-term atomoxetine treatment in adolescents with attention-deficit/hyperactivity disorder. J Pediatr 149:112–119
Wilens TE, Gault LM, Childress A, Kratochvil CJ, Bensman L, Hall CM et al (2011) Safety and efficacy of ABT-089 in pediatric attention-deficit/hyperactivity disorder: results from two randomized placebo-controlled clinical trials. J Am Acad Child Adolesc Psychiatry 50:73–84
*Yildiz O, Sismanlar SG, Memik NC, Karakaya I, Agaoglu B (2011) Atomoxetine and methylphenidate treatment in children with ADHD: the efficacy, tolerability and effects on executive functions. Child Psychiatry Hum Dev 42(3):257–269. doi:10.1007/s10578-010-0212-3
Acknowledgements
The authors were not supported by any external funding for the completion of this study. There were no third-party sponsors involved at any stage of the project. The authors have confirmed that they have no competing or potential conflict of interest in relation to this work; neither author has any relationship with Eli Lilly and Company, Ltd, the manufacturer of atomoxetine. The authors appreciate the assistance of the many investigators whose correspondence provided needed data clarification which allowed more studies to be represented in this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Neither author has any personal or financial relationships to disclose with other individuals or organizations that could inappropriately influence or bias the present work.
Rights and permissions
About this article
Cite this article
Gayleard, J.L., Mychailyszyn, M.P. Atomoxetine treatment for children and adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD): a comprehensive meta-analysis of outcomes on parent-rated core symptomatology. ADHD Atten Def Hyp Disord 9, 149–160 (2017). https://doi.org/10.1007/s12402-017-0216-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12402-017-0216-y