Abstract
Objectives
To obtain and compare the protein profiles of supernumerary and normal permanent dental pulp tissues.
Materials and methods
Dental pulp tissues were obtained from supernumerary and normal permanent teeth. Proteins were extracted and analyzed by liquid chromatography-tandem mass spectrometry (LC/MS-MS). Protein identification and quantification from MS data was performed with MaxQuant. Statistical analysis was conducted using Metaboanalyst to identify differentially expressed proteins (DEPs) (P-value < 0.05, fold-change > 2). Gene Ontology enrichment analyses were performed with gProfiler.
Results
A total of 3,534 proteins were found in normal dental pulp tissue and 1,093 in supernumerary dental pulp tissue, with 174 DEPs between the two groups. This analysis revealed similar functional characteristics in terms of cellular component organization, cell differentiation, developmental process, and response to stimulus, alongside exclusive functions unique to normal permanent dental pulp tissues such as healing, vascular development and cell death. Upon examination of DEPs, these proteins were associated with the processes of wound healing and apoptosis.
Conclusions
This study provides a comprehensive understanding of the protein profile of dental pulp tissue, including the first such profiling of supernumerary permanent dental pulp. There are functional differences between the proteomic profiles of supernumerary and normal permanent dental pulp tissue, despite certain biological similarities between the two groups. Differences in protein expression were identified, and the identified DEPs were linked to the healing and apoptosis processes.
Clinical relevance
This discovery enhances our knowledge of supernumerary and normal permanent pulp tissue, and serves as a valuable reference for future studies on supernumerary teeth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One potential developmental problem in children is the presence of supernumerary teeth, which are defined as any extra tooth formed in normal dentition [1]. The prevalence of supernumerary teeth in permanent dentition is about 0.1–3.8% [2,3,4,5,6,7]. The most common type is a mesiodens, located at the midline of the anterior maxillary region [2, 4, 5], which accounts for about 47–67% of all supernumerary teeth [8, 9]. Several theories have been proposed to explain the formation of supernumerary teeth, but the main etiology is still unclear [2, 4,5,6,7, 10]. Although supernumerary teeth have been categorized as developmental anomalies and the presence of these teeth was considered a sign of disorder, previous studies have reported using supernumerary teeth as a replacement for normal teeth and as alternative sources for tissue regeneration [7, 11, 12].
Dental pulp is the soft connective tissue located in the center of the tooth, which is surrounded by dental hard tissue [13, 14]. The major components of dental pulp are odontoblasts, fibroblasts, pulp stem cells, nerves, and blood vessels [15]. This pulp tissue is crucial in tooth formation and development, possesses the ability to regenerate, and plays a crucial role in a tooth’s defensive processes [16, 17].
Proteomics is the large-scale study of the interaction, structure, and function of proteins in order to understand the nature of a tissue, organ, organism, or disease [18]. As such, obtaining proteomics data for a particular tissue enhances our comprehension of its function and structure. To date, current literature on dental pulp tissue has focused mainly on the proteomic profile of normal permanent teeth [19,20,21,22,23]. The proteome of supernumerary dental pulp tissue has never been investigated, despite such teeth being far from fully understood. This study is interested in building a solid understanding of the differences between supernumerary dental pulp tissue and normal dental pulp tissue. We specifically aimed to determine the protein profile of supernumerary dental pulp tissue and compare it with that of normal dental pulp tissue.
Methods
Subject selection
The inclusion criteria were permanent teeth obtained from individuals who were not taking any medication and were in good health. The teeth were free of carious lesions and had normal pulpal diagnosis. Additionally, all teeth must be in either stage G or H of tooth development, according to Demirjian’s classification [24]. Stage G is defined as the stage in which the root canal walls are parallel and the apical ends are open, while stage H is defined as the stage in which the roots’ apexes are closed.
There were two groups in this study, with six subjects in each group (n = 6). For the supernumerary tooth group, pulp tissue samples were obtained from supernumerary permanent teeth located in the anterior maxillary region (mesiodens) of children aged less than 10 years. For normal permanent teeth, pulp tissue samples were obtained from third molars of adults aged less than 30 years. Teeth were extracted or surgically removed at the Dental Clinic of Faculty of Dentistry, Mahidol University. Patients and/or their legal guardians provided informed consent before tooth extraction. Exclusion criteria involved teeth with pathologies such as dental caries or pulpitis, patients in an unhealthy condition, and patients with syndromes such as cleft lip, cleft palate, Gardner syndrome, and cleidocranial dysostosis.
Sample preparation
Subsequent to tooth extraction, the specimens were immediately preserved on ice (0 °C), then samples were immediately frozen with liquid nitrogen and stored at -80 °C until further experiment. On the experimental day, cementum and any residual soft tissue, including periodontal ligament, epithelial tissue, and apical papilla if presented, were mechanically removed from the teeth using periodontal curettes. The teeth were then cleansed with normal saline, and part of the enamel, dentin, and cementum were cut vertically with a high-speed bur under water coolant to avoid pulp tissue damage. Subsequently, each tooth was split using a dental elevator to expose the pulp, which was carefully removed using a sterile dental curette. The dental pulp tissue from each tooth was placed in a microcentrifuge tube and stored on ice (0 °C).
Protein extraction and quantification
The protein extraction method was based on previous studies [19, 20]. Briefly, 100 µL of lysis buffer (Sigma-Aldrich, Missouri, United States) and 10 µL of Protease inhibitor cocktail (Roche, Mannheim, Germany) were added to the pulp tissue samples, after which each sample was ground and homogenized using a manual tissue homogenizer, with all handling at 0–4 °C. Next, the samples were vortexed and incubated on ice, followed by centrifugation at 20,000 g for 30 min at 4 °C. Then, the supernatants were collected and protein concentration was determined using a bicinchoninic acid protein assay kit according to the standard microplate procedure protocol [25].
Mass spectrometry
Samples containing 5 µg of protein underwent in-solution digestion. Disulfide bonds were reduced using 5 mM dithiothreitol in 10 mM ammonium bicarbonate (AMBIC), and sulfhydryl groups were alkylated with 15 mM iodoacetamide at room temperature in the dark. Afterwards, sequencing grade porcine trypsin was employed for protein digestion. The resulting tryptic peptides were dried, resuspended in 0.1% formic acid, and subjected to nano-liquid chromatography tandem mass spectrometry (nanoLC-MS/MS). Specifically, the prepared samples were injected into an Ultimate3000 Nano/Capillary LC System (Thermo Scientific, UK) coupled to a ZenoTOF 7600 mass spectrometer (SCIEX, Framingham, MA, USA). Each sample underwent nanoLC/MS-MS analysis in triplicate [26].
Data analysis
The acquired mass spectrometry raw data were analyzed with MaxQuant (version 2.4.2.0) for protein identification and quantification in individual samples. The Andromeda search engine was used to correlate MS/MS spectra to the UniProt Homo sapiens database [27]. MaxQuant’s standard label-free quantitation utilized specific settings, which required missed cleavages to be less than two, a 0.6 Dalton mass tolerance, trypsin digestion, fixed modification involving carbamidomethylation of cysteine, and variable modifications. Protein identification required peptides that have at least seven amino acid lengths and a minimum of one unique peptide. For downstream data analyses, proteins were considered identified if a minimum of two peptides were detected, including at least one unique peptide. Protein false discovery rate was set at 1%, estimated through reversed search sequences. The search allowed a maximum of five modifications per peptide, and referenced a FASTA file comprising proteins from the Homo sapiens proteome obtained from Uniprot. The software automatically incorporated potential contaminants from the contaminants.fasta file provided by MaxQuant into the search space [26]. The raw mass-spectrometric data were deposited in jPOST Repository https://repository.jpostdb.org (accession: PDX050860) [28].
The resulting MaxQuant ProteinGroups.txt file was imported into MetaboAnalyst 5.0 to perform orthogonal partial least squares discriminant analysis (OPLS-DA) and generate heatmaps and volcano plots. Proteins with statistically significant differential expression were identified based on fold-change (> 2.0) and P-value (< 0.05).
Gene Ontology (GO) annotation enrichment analysis including biological process, cellular component, and molecular function terms was performed using gProfiler (https://biit.cs.ut.ee/gprofiler/gost, version e110_eg57_p18_4b54a898).
Result
Each group consisted of six pulp tissue samples from different individuals. In the supernumerary permanent tooth group, teeth were obtained from three girls and three boys between the ages of six and ten years old (mean age: 7.62 years). Two of the teeth in this group were in Demirjian’s stage G of tooth development, while the other four were in stage H of tooth development [24]. In the normal permanent tooth group, teeth were collected from three women and three men between the ages of 20 and 25 years old (mean age: 21.5 years). Out of the six teeth in this group, two were in stage G of tooth development, while four were in stage H, according to Demirjian’s classification [24]. Details on the collected teeth, including age and gender of the donors, are presented in Supplementary Table 1.
After obtaining the data from MaxQuant protein identification and quantitation, keratin proteins were excluded from the analysis to reduce the possibility of contamination from MS analysis [29]. As a results, a total of 3,534 proteins were identified in normal permanent dental pulp tissues (Supplementary Table 2), versus 1,093 identified in supernumerary permanent dental pulp tissues (Supplementary Table 3). Figure 1 illustrates the distribution and clustering of protein abundances across each group, between samples, and within replicates.
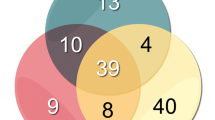
Protein abundance and functional characteristics were evaluated and compared across tissue conditions. Fig. S1 depicts the major biological process and molecular function terms associated with proteins detected in normal permanent dental pulp tissue. A comparison of the functional characteristics of supernumerary and normal permanent dental pulp tissue revealed several similarities and differences in the percentage of proteins found in each group. The two groups shared similar functions in terms of organization and development, differentiation, and response to stimulus. While different functions were primarily associated with apoptosis and cell death, healing, and vasculature development, as shown in Fig. 2.
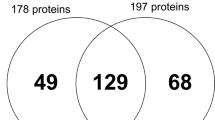
Further analysis of differently expressed proteins (DEPs) found 174 proteins that were differently expressed between supernumerary permanent and normal permanent dental pulp tissue (P-value < 0.05 and fold-change > 2.0), as shown in Supplementary Tables 4 and Fig. 3. For DEPs, enriched biological process terms were mainly related to responses to stress and stimuli, wound healing, and regulation of cell death and apoptosis, while enriched molecular function terms related to the binding of proteins and ions (Fig. 4).
Discussion
Supernumerary tooth is one of the most common developmental anomalies [1]. Presence of a supernumerary tooth may cause displacement or impaction of the adjacent permanent teeth [9]. Several theories have been postulated to explain the development and formation of supernumerary teeth, including their potential function as an alternative replacement of normal permanent teeth [12, 30]; however, the definitive etiology and main function of supernumerary teeth, especially at cellular and molecular levels, remains unclear [2, 4,5,6,7, 10].
Dental pulp tissue has been extensively investigated due to its crucial role in tooth development, maintenance, and function [16, 17]. However, the majority of current research, including proteomic profiling, has focused mainly on the dental pulp of normal permanent teeth [19,20,21,22,23]. Investigation into the structure and function of supernumerary tooth dental pulp is needed, as the proteomic profile cannot be assumed identical to that of a normal permanent tooth. This study is the first to investigate the proteomic profile of supernumerary dental pulp tissue and identify significantly different expression of proteins that may be related to supernumerary teeth.
Mesiodens of the permanent dentition were chosen as the representative of supernumerary permanent teeth in this study because that type is the most common supernumerary tooth and routinely needs to be removed due to complications [1,2,3,4,5,6]. Third molars were chosen as a representative of normal permanent teeth because they are typically maligned or impacted, indicating the need for removal [31]. The dental pulp tissue in this study was obtained from healthy subjects, and all teeth were sound. Each group contains an equal number of subjects from both sexes to minimize the impact of sex differences on the functions and response processes of pulp tissue [2, 32]. Furthermore, as the stage of tooth development has an impact on the biological functions of dental pulp, to minimize these effects, the teeth obtained from each group were in a similar stage of tooth development, with two teeth in each group in stage G and the other four teeth in each group in stage H of tooth development, according to Demirjian’s classification [24].
To reduce epithelial cell contamination, all remaining soft tissue and cementum were mechanically removed with a periodontal curette. Although complete elimination of non-specific protein contamination may not be possible, these contaminants can be reduced. Keratin proteins were excluded from the analysis for the reason that they are commonly regarded as contaminants [29].
OPLS-DA revealed distinct intergroup differences along with intragroup sample homogeneity, reflected in the heatmap of protein expression intensities. The distinct difference revealed by this analysis suggests that specific proteins are expressed differently in supernumerary and normal permanent dental pulp tissues.
Overall, 3,534 proteins were detected in normal permanent dental pulp tissues (Supplementary Table 2), which is within the range of 66 to 4,332 proteins detected in previous studies [19,20,21,22,23], (Supplementary Table 5). The wide variation in total identified proteins in dental pulp tissue may be due to different studies employing different methods of protein extraction, labeling, and separation; mass spectrometry techniques; and criteria for protein identification [19,20,21,22,23]. Despite differences in total detected proteins, GO enrichment analysis based on biological process terms in the present study highlighted similar functions as compared to previous studies of normal permanent dental pulp tissue (Fig. S1) [19,20,21, 23]. For supernumerary permanent teeth, a total of 1,093 proteins were detected (Supplementary Table 3). Interestingly, according to previous reports and this study, there have only been roughly 66 − 4,332 identified proteins in dental pulp tissue thus far (Supplementary Table 5) [19,20,21,22,23]. This is a relatively low number when compared to the number of proteins found in other tissue or in a single cell that have been previously reported [33, 34]. It is still unknown why there are so few proteins found in dental pulp; more research is required to solve this mystery. This could, however, also draw attention to how distinctive and special dental pulp tissue is.
Comparison of the functional characteristics of supernumerary and normal permanent dental pulp tissue revealed that dental pulp tissue from both groups displayed similar functions in terms of cellular component organization, biogenesis, cell differentiation, and developmental processes. These are the major functions of dental pulp that play a crucial role in tooth development, maturation, and the regenerative process [16, 17]. Moreover, similar functions of supernumerary and normal permanent dental pulp tissue were also found in terms of response to stimulus, an important defense mechanism that protects the pulp from any harmful stimulus in order to maintain the vitality of the tooth [13].
While supernumerary and normal permanent dental pulp tissue shared several functional characteristics, differences in protein functions were also observed. Differences were found in the biological processes of wound healing and vasculature development, which clearly are the important mechanisms for tissue regeneration [35]. These functions were only detected in the dental pulp of a normal permanent tooth. Moreover, the function of cell death and regulation of apoptosis process, which normally occur as part of the regenerative process and wound healing of dental pulp [36, 37], were only identified in normal permanent dental pulp tissue.
Furthermore, comparative analysis of DEPs between supernumerary permanent and normal permanent dental pulp tissue identified 174 proteins that were significantly differentially expressed (Supplementary Table 4). GO enrichment analysis of the DEPs revealed enrichment of molecular functions related to protein and ion binding. Differences in functional characteristics between supernumerary and normal permanent dental pulp tissue were also confirmed by DEPs. The biological functions of DEPs were mainly related to responses to stress and stimuli, wound healing, and regulation of cell death and apoptosis. Interestingly, it has been proposed that the development of a supernumerary tooth is significantly influenced by control of the apoptotic process [38, 39].
Possible limitations of this study that may impact its findings primarily concern the small sample size, which is a consequence of the limited number of patients with mesiodens available throughout the study period; this is due to the prevalence of mesiodens in permanent dentition being less than 3.8% [2,3,4,5,6,7]. Additionally, the study encountered challenges related to age differences between the two groups, as mesiodens complications typically require removal in children during the mixed dentition phase, whereas third molar removal is required during late adolescence or adulthood. Consequently, it is impractical to collect both supernumerary permanent and normal permanent dental pulp tissues from donors of the same age, and also from the same donor. Despite the difference in chronological age between the two groups of donors, our study mainly focused on dental age, specifically referring to the stage of tooth formation. Both groups exhibited similar stages of tooth development, categorized as tooth development stages G and H according to Demirjian’s classification [24]. In spite of these limitations, the collected samples are sufficient for statistical analysis and screening purposes in this initial exploration of the characteristics of supernumerary permanent dental pulp tissue. Future studies should validate DEPs to confirm the findings and include a larger sample size for more specific investigations.
In conclusion, this study provides a comprehensive understanding of the proteomic profile of permanent dental pulp tissue, and is furthermore the first to investigate the protein profile of supernumerary permanent dental pulp tissue. Dental pulp tissue of normal permanent and supernumerary permanent teeth displayed similar functional characteristics in terms of cellular component organization, cell differentiation, developmental process, and response to stimulus. While the functional characteristics of healing, vascular development and cell death were only detected in normal permanent dental pulp. Differences in protein expression were identified between supernumerary and normal dental pulp tissue of permanent teeth, these proteins were found to be associated with the processes of wound healing and apoptosis.
Data availability
Data is provided within the manuscript or supplementary information and the raw mass-spectrometric data were deposited in jPOST Repository https://repository.jpostdb.org (accession: PDX050860).
References
Demiriz L, Durmuşlar MC, Mısır AF (2015) Prevalence and characteristics of supernumerary teeth: a survey on 7348 people. J Int Soc Prev Community Dent 5(Suppl 1):S39. https://doi.org/10.4103/2231-0762.156151
Meighani G, Pakdaman A (2010) Diagnosis and management of supernumerary (mesiodens): a review of the literature. J Dent (Tehran) 7(1):41
Lucas Penalva P, Perez-Albacete Martinez C, Ramírez Fernandez M, Mate Sánchez De Val J, Calvo Guirado J (2015) Mesiodens: etiology, diagnosis and treatment: a literature review. BAOJ Dentistry 1(002). https://doi.org/10.24947/baojd/1/1/102
Russell KA, Folwarczna MA (2003) Mesiodens-diagnosis and management of a common supernumerary tooth. J Can Dent Assoc 69(6):362–367
Muhamad A, Moti M, Ornit C, Uri Z (2018) Histological and chemical analyses of mesiodens development and mineralization. Arch Oral Biol 87:191–195. https://doi.org/10.1016/j.archoralbio.2017.12.020
Gallas MM, García A (2000) Retention of permanent incisors by mesiodens: a family affair. Br Dent J 188(2):63–64. https://doi.org/10.1038/sj.bdj.4800390
Lu X, Liu SF, Wang HH, Yu F, Liu JJ, Zhao YM et al (2019) A biological study of supernumerary teeth derived dental pulp stem cells based on RNA-seq analysis. Int Endod J 52(6):819–828. https://doi.org/10.1111/iej.13060
Fernández Montenegro P, Valmaseda Castellón E, Berini Aytés L, Gay Escoda C (2006) Retrospective study of 145 supernumerary teeth. Med Oral Patol Oral Cir Bucal 11(4):E339–E344
Ata-Ali F, Ata-Ali J, Peñarrocha-Oltra D, Peñarrocha-Diago M (2014) Prevalence, etiology, diagnosis, treatment and complications of supernumerary teeth. J Clin Exp Dent 6(4):e414. https://doi.org/10.4317/jced.51499
Mallineni SK, Nuvvula S (2015) Management of supernumerary teeth in children: a narrative overview of published literature. J Cranio Max Dis 4(1):62–68. https://doi.org/10.4103/2278-9588.151907
Demir T, Ates U, Cehreli B, Cehreli ZC (2008) Autotransplantation of a supernumerary incisor as a replacement for fused tooth: 24-month follow-up. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106(4):e1–e6. https://doi.org/10.1016/j.tripleo.2008.06.004
Tirali RE, Sar C, Ates U, Kizilkaya M, Cehreli SB (2013) Autotransplantation of a supernumerary tooth to replace a misaligned incisor with abnormal dimensions and morphology: 2-year follow-up. Case Rep Dent. https://doi.org/10.1155/2013/146343
Yu C, Abbott PV (2007) An overview of the dental pulp: its functions and responses to injury. Aust Dent J 52. https://doi.org/10.1111/j.1834-7819.2007.tb00525.x. S4-S6
Vavpotič M, Turk T, Martinčič DŠ, Balažic J (2009) Characteristics of the number of odontoblasts in human dental pulp post-mortem. Forensic Sci Int 193(1–3):122–126. https://doi.org/10.1016/j.forsciint.2009.09.023
Hosoya A, Nakamura H (2015) Ability of stem and progenitor cells in the dental pulp to form hard tissue. Jpn Dent Sci Rev 51(3):75–83. https://doi.org/10.1016/j.jdsr.2015.03.002
Tatullo M, Marrelli M, Shakesheff KM, White LJ (2015) Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med 9(11):1205–1216. https://doi.org/10.1002/term.1899
Shi X, Mao J, Liu Y (2020) Pulp stem cells derived from human permanent and deciduous teeth: biological characteristics and therapeutic applications. Stem Cells Transl Med 9(4):445–464. https://doi.org/10.1002/sctm.19-0398
Al-Amrani S, Al-Jabri Z, Al-Zaabi A, Alshekaili J, Al-Khabori M (2021) Proteomics: concepts and applications in human medicine. World J Biol Chem 12(5):57–69. https://doi.org/10.4331/wjbc.v12.i5.57
Eckhardt A, Jágr M, Pataridis S, Mikšík I (2014) Proteomic analysis of human tooth pulp: proteomics of human tooth. J Endod 40(12):1961–1966. https://doi.org/10.1016/j.joen.2014.07.001
Silva PAO, Lima SMF, Freire MS, Murad AM, Franco OL, Rezende TMB (2021) Proteomic analysis of human dental pulp in different clinical diagnosis. Clin Oral Investig 25(5):3285–3295. https://doi.org/10.1007/s00784-020-03660-3
Feridouni Khamaneh Y, Kiani P, Miller RD, Schlüter H, Friedrich RE (2021) Complementing the pulp proteome via sampling with a picosecond infrared laser (PIRL). Clin Oral Investig 25:6757–6768. https://doi.org/10.1007/s00784-021-03962-0
Loureiro C, Buzalaf MAR, Pessan JP, Moraes FRN, Pelá VT, Ventura TMO et al (2020) Comparative analysis of the Proteomic Profile of the Dental Pulp in different conditions. A pilot study. Braz Dent J 31(3):319–336. https://doi.org/10.1590/0103-6440202003167
Eckhard U, Marino G, Abbey SR, Tharmarajah G, Matthew I, Overall CM (2015) The human dental pulp proteome and N-terminome: levering the unexplored potential of semitryptic peptides enriched by TAILS to identify missing proteins in the human proteome project in underexplored tissues. J Proteome Res 14(9):3568–3582. https://doi.org/10.1021/acs.jproteome.5b00579
Demirjian A, Goldstein H, Tanner JM (1973) A new system of dental age assessment. Hum Biol. 211–227
Scientific T (2020) Pierce BCA protein assay kit user guide. Illinois, USA
Songjang W, Paiyabhroma N, Jumroon N, Jiraviriyakul A, Nernpermpisooth N, Seenak P et al (2023) Proteomic profiling of early secreted proteins in response to Lipopolysaccharide-Induced Vascular endothelial cell EA.hy926 Injury. Biomedicines 11(11). https://doi.org/10.3390/biomedicines11113065
Tyanova S, Temu T, Cox J (2016) The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc 11(12):2301–2319. https://doi.org/10.1038/nprot.2016.136
Okuda S, Watanabe Y, Moriya Y, Kawano S, Yamamoto T, Matsumoto M et al (2017) jPOSTrepo: an international standard data repository for proteomes. Nucleic Acids Res 45(D1):D1107–D1111. https://doi.org/10.1093/nar/gkw1080
Hodge K, Ten Have S, Hutton L, Lamond AI (2013) Cleaning up the masses: exclusion lists to reduce contamination with HPLC-MS/MS. J Proteom 88:92–103. https://doi.org/10.1016/j.jprot.2013.02.023
Takahashi K, Kiso H, Murashima-Suginami A, Tokita Y, Sugai M, Tabata Y et al (2020) Development of tooth regenerative medicine strategies by controlling the number of teeth using targeted molecular therapy. Inflamm Regen 40:1–9. https://doi.org/10.1186/s41232-020-00130-x
Normando D (2015) Third molars: to extract or not to extract? Dent Press J Orthod 20(4):17–18. https://doi.org/10.1590/2176-9451.20.4.017-018.edt
Rich-Edwards JW, Kaiser UB, Chen GL, Manson JE, Goldstein JM (2018) Sex and Gender Differences Research Design for Basic, Clinical, and Population studies: essentials for investigators. Endocr Rev 39(4):424–439. https://doi.org/10.1210/er.2017-00246
Sinitcyn P, Richards AL, Weatheritt RJ, Brademan DR, Marx H, Shishkova E et al (2023) Global detection of human variants and isoforms by deep proteome sequencing. Nat Biotechnol 41:1776–1786. https://doi.org/10.1038/s41587-023-01714-x
Kawashima Y, Nagai H, Konno R, Ishikawa M, Nakajima D, Sato H et al (2022) Single-Shot 10K Proteome Approach: Over 10,000 Protein Identifications by Data-Independent Acquisition-Based Single-Shot Proteomics with Ion Mobility Spectrometry. J Proteome Res. 3;21(6):1418–1427. https://doi.org/10.1021/acs.jproteome.2c00023
Lo Sicco C, Tasso R, Reverberi D, Cilli M, Pfeffer U, Cancedda R (2015) Identification of a new cell population constitutively circulating in healthy conditions and endowed with a homing ability toward injured sites. Sci Rep 5(1):16574
Okai Y, Harada K, Ohura K, Arita K (2012) Study on apoptosis in human deciduous tooth pulp cells. J Hard Tissue Biol 21(4):413–420
Yano J, Kitamura C, Nishihara T, Tokuda M, Washio A, Chen KK et al (2011) Apoptosis and survivability of human dental pulp cells under exposure to Bis-GMA. J Appl Oral Sci 19:218–222. https://doi.org/10.1590/s1678-77572011000300007
Peterková R, Peterka M, Lesot H (2003) The developing mouse dentition: a new tool for apoptosis study. Ann N Y Acad Sci 1010:453–466. https://doi.org/10.1196/annals.1299.083
Lagronova-Churava S, Spoutil F, Vojtechova S, Lesot H, Peterka M, Klein OD et al (2013) The dynamics of supernumerary tooth development are differentially regulated by sprouty genes. J Exp Zool B Mol Dev Evol 320(5):307–320. https://doi.org/10.1002/jez.b.22502
Funding
Not applicable.
Open access funding provided by Mahidol University
Author information
Authors and Affiliations
Contributions
Conception and study design: S.H.; Methodology: S.H., S.R., R.S.; Investigation: K.L., S.R., Data acquisition & analysis: K.L., S.R.; Visualization: K.L.; Supervision: S.H., S.R., R.S.; Writing- Original draft preparation: S.H., K.L.; Writing- Reviewing & Editing: S.H., S.R., R.S., K.L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethical Institutional Review Board, Faculty of Dentistry and the Faculty of Pharmacy, Mahidol University (COE.No.MU-DT/PY-IRB 2023/005.2001).
Informed consent
Informed consent was obtained from all individual participants and/or their legal guardians.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lertruangpanya, K., Roytrakul, S., Surarit, R. et al. Comparative proteomic analysis of dental pulp from supernumerary and normal permanent teeth. Clin Oral Invest 28, 321 (2024). https://doi.org/10.1007/s00784-024-05698-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05698-z