Abstract
Objective
To determine the effect of clindamycin in the prevention of infection after oral surgery.
Material and Methods
This systematic review and meta-analysis followed the PRISMA statement, the PICO-framework and included only randomized controlled clinical trials. In all studies clindamycin was administered to prevent infections in patients who underwent oral surgery. Two independent researchers conducted the search, data extraction and risk of bias assessment. Included studies were classified by the type of oral surgery. Besides, data of patients, procedures and outcome variables were collected. Risk ratios (RR) and 95% confidence intervals (CI) were calculated by using Mantel–Haenszel model and the number needed to treat (NNT). Finally, any potential sources of heterogeneity were estimated.
Results
Seven trials of 540 articles met the inclusion criteria and were included in the qualitative synthesis. Four articles assessing the effect of oral clindamycin in third molar surgery were quantitatively analyzed. The overall RR was 0.66 (95% CI = 0.38–1.16), being non-statistically significant (p = 0.15). There was no heterogeneity between the studies I2 = 0, p = 0.44. The NNT was 29 (95% CI = 12- -57).
Conclusions
The effectiveness of clindamycin could not be evaluated except in third molar extraction. Oral clindamycin is ineffective in preventing infection in third molar surgery.
Clinical Relevance
There is a lack of high-quality evidence supporting the prescription of clindamycin to prevent infections after oral surgery, despite being frequently prescribed as an alternative for penicillin-allergic patients. Oral clindamycin has not been shown to be effective after third molar extractions.
Similar content being viewed by others
Introduction
Despite the recognized economic and public health implications of the indiscriminate use of antibiotics, professionals prescribe very frequently preventive antibiotics in common oral surgeries such as third molar extractions and oral implant placements in healthy patients [1, 2].
Besides, several surveys conducted in different countries have shown that many professionals continue to prescribe preventive antibiotics after different oral surgeries, in order to prevent infectious complication [3,4,5].
Numerous clinical trials have been carried out to assess the effectiveness of different antibiotic treatments to prevent infection after dental extractions, as we can appreciate in the Cochrane review update of 2021 [6]. Indeed, there is no consensus on the use of antibiotics for preventing surgical infection associated with oral implant placement in healthy patients [7,8,9,10,11].
Penicillin and other antibiotics from its group are the most frequently prescribed in dentistry. Nevertheless, some important questions are brought up relating to patients allergic to them. Clindamycin is widely used in oral surgeres as an alternative preventive treatment in patients allergic to amoxicillin [12,13,14]. In fact, previous studies reported a remarkable effectiveness of clindamycin in reducing the incidence of infectious and inflammatory complications after third molar surgery such as dry socket [15]. However, recent evidence suggests a lack of benefits [14].
Indeed, despite being commonly used as an alternative in penicillin-allergic patients, the effect of clindamycin on oral surgery has not been yet exactly determined in the current literature [16]. For these reasons, it was considered necessary to perform a systematic review and, if it was possible, to conduct a meta-analysis on this topic.
The aim of this study was to assess the effect of clindamycin (with any kind of route of administration, regimen or dosage) to prevent infectious complications in patients who underwent any type of oral surgery.
Material and methods
This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Prior to conduct the review, its methods were established. The study protocol has been registered, and approved in PROSPERO with the registration number CRD4202122624. It can be accessed on the following link.
https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=226241
The null hypothesis (H0) was tested with a significance level of p = 0.05, since the preventive use of clindamycin is not effective in reducing infection in oral surgery.
Eligibility criteria
Only randomized clinical trials (RCT) controlled with placebo or without any treatment were included, regardless of whether they were double-blinded or not. At least patients from one of the groups must have received preventive clindamycin (with any kind of route of administration, regimen or dosage) to prevent infectious complications after any type of oral surgery procedure. The articles were classified according to the type of oral surgery, in which the effectiveness of clindamycin was tested.
All studies that did not meet the inclusion criteria were excluded, particularly noteworthy are those trials in which the control group received an antibiotic treatment.
Information sources
The following electronic databases were used for conducting the search: Pubmed/Medline, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, Embase Ovid and Scopus. Manual search was also carried out. All databases were searched up to January 2021.
Search
The search strategy was based on the PICO-framework. Population (P): Patients were assessed for inclusion in the analysis regardless of their age, gender, previous pathologies or habits, such as smoking. All studies evaluating any type of oral surgical procedure were included. Intervention (I): Antibiotic prophylaxis with clindamycin administered orally, intravenously or topically and prescribed before, during and/or after oral surgery. Comparison (C): Placebo or no treatment gave peri-operatively. Outcome (O): The outcome variables included all signs of postoperative infection (pain, fever, swelling, trismus, and wound or surgical site infection), dry socket, other related complications and adverse events. Two independent researchers performed the study selection until January 2021.
The electronic search in the PubMed/Medline database was carried out by using MeSH thesaurus and search algorithms connected with Boolean operators as key words for titles and abstracts. This is one of the different search strategies used: ("clindamycin"[MeSH Terms] OR "clindamycin"[All Fields] OR "clindamycine"[All Fields]) AND ("surgery, oral"[MeSH Terms] OR ("surgery"[All Fields] AND "oral"[All Fields]) OR "oral surgery"[All Fields] OR ("oral"[All Fields] AND "surgery"[All Fields]) OR "oral surgery"[All Fields] OR "oral surgical procedures"[MeSH Terms] OR ("oral"[All Fields] AND "surgical"[All Fields] AND "procedures"[All Fields]) OR "oral surgical procedures"[All Fields] OR ("oral"[All Fields] AND "surgery"[All Fields]).
No restrictions were used on the language or date of publication. The filters activated were: humans and clinical trials.
Study selection
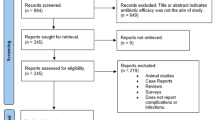
The search strategy produced the results shown in Fig. 1. The databases not listed in this figure did not yield any relevant publications. Two independent researchers performed the selection of studies (IA and AF), a third researcher was requested in case of conflict (FR). The included and excluded articles with the reasons for exclusion were recorded in Table 1.
Data collection process
A data collection protocol was designed, in which each selected study was independently reviewed by two investigators (IA and NAL), and differences were resolved by consulting a third analyst (FR). When there was no explicit data in the main text, calculations were performed using the results in tables or figures, when it was possible. In case of lack or doubt about data of interest in the article, the authors were contacted.
Data items
Table 2 included all data recorded in each study. Studies were classified according to the type of oral surgery performed. Apart from that, when more than one antibiotic was tested in the same study, only the information regarding the patients who were treated with clindamycin and those who belonged to the control groups was collected.
Risk of bias in individual studies
The Cochrane Collaboration's tool was used to assess the individual risk of bias of each RCT included in quantitative analysis (Fig. 2). The bias in each study was analyzed using the recommended approach for assessing risk of bias in studies included in Cochrane reviews.
Summary measures
The effectiveness of the treatment was assessed considering the relative risk (RR). The differences in incidences between the treatment and control groups or attributable risk were utilized to assess the clinical significance of the treatment with clindamycin. Furthermore, the number needed to treat (NNT) was calculated.
Synthesis of results
The analysis was carried out using StataIC 13 (Stata-Corp LP, College Station, College Station, TX) and Review Manager (RevMan) 5.2 version (Copenhagen: The Cochrane Collaboration, 2012) software. We assessed the heterogeneity of the different studies using the I2 statistic. The overall relative risk, resulting from combining outcomes from the different studies, was calculated with inverse variance-weighted Mantel–Haenszel (MH) model. Empirical correction was used for the studies with zero effect sizes in one of their arms, and any studies with a zero effect size in both arms were excluded from the analysis.
Results
Study selection
We identified 540 records in both the databases and manual search (Fig. 1). After removing duplicates, 38 articles were selected for the full-text assessment. After full-text assessment, 7 were included in qualitative synthesis. First, all articles that did not analyze the infection clinically were excluded. Nine articles [38,39,40,41,42,43,44,45,46] studied bacteremia, three articles [47,48,49] studied the influence of clindamycin on the oral microbiome. Bulut et al. (2001) [50] studied the levels of the acute phase of proteins. One article [51] could not be found and it was excluded. Afterwards, the articles were classified according to the type of oral surgery in which the effectiveness of clindamycin was tested. Table 1 shows the studies that were included and those that were excluded with their reasons.
Study characteristics
Table 2 shows the studied variables of the included studies: one study was performed on endodontic surgery and, six studies on third molar surgery. Hamiti-Krasniqi et al. (2014) [35], tested topical clindamycin in the prevention of dry socket, while Halpern and Dodson (2007) [12] used intravenous clindamycin (600 mg IV 1 h before surgery). Both studies showed lower infection rates in patients treated with clindamycin than in the placebo group. In the rest of the clinical trials, the treatment was with oral clindamycin, varying in their regimens and dosages. The follow-up period throughout the studies ranged from 1 to 4 weeks.
Only four trials allowed us to pool information on the effect of oral clindamycin in third molar extractions. For this reason, we decided to continue with a quantitative analysis testing the null hypothesis (H0), with a significance level of p = 0.05, that the preventive use of oral clindamycin is not effective in reducing infection in third molar surgery.
Risk of bias within studies
Risk of bias of each study is presented in Fig. 2. Despite the fact that some studies were not of high quality and that they dealt with different doses, the quantitative analysis was perform including the four articles [31, 33, 34, 37] in which the efficacy of oral clindamycin in third molar surgery was studied.
Summary measures
The four studies in which oral clindamycin was prescribed to prevent infectious complications after third molar extraction were the only included ones. The quantitative analysis involved 486 extractions, 245 of them treated with clindamycin and 241 from the control group (treated with placebo or with no treatment). There were 19 and 27 reported infection, dry socket or other events in respective group.
The Forest Plot (Fig. 3) shows the graphic representation of the RR and 95% CI estimates performed with the samples of the 4 included studies. The overall RR extracted from all the studies indicated that there was no statistical benefit, and oral clindamycin may not be effective in the prevention of infectious complications after third molar extractions.
Synthesis of results
The heterogeneity measured from the I2 test was 0 (p = 0.44), the null hypothesis of absence of heterogeneity between the results of the studies included in this meta-analysis could not be rejected. The Q statistic also supports the assumption of homogeneity between studies.
The overall RR, by using the Mantel–Haenszel (MH) method was found to be 0.66, with a 95% CI of 0.38 to 1.16, being non-statistically significant (p = 0,15). This range also included the value 1, indicating that clindamycin treatment may not prevent the development of infectious complications (dry socket, infection, or both conditions at the same time) following third molar extractions.
Analysis of clinical significance
The NNT was 29 and it ranged between 12 and -57. This means that between 12 and infinity patients would need to be treated with oral clindamycin to prevent a single case of infection after third molars extraction. These results indicated that oral clindamycin may be ineffective in preventing infections following third molar extraction.
Discussion
The principal findings of this systematic review and meta-analysis were the small number of studies available, focusing on the effect of prophylactic clindamycin in oral surgery procedures, despite being the antibiotic of choice in patients with hypersensitivity reactions to penicillins [12,13,14, 16, 33].
The quantitative analysis carried out on four studies that evaluated the effect of oral clindamycin in third molar extractions showed the ineffectiveness of clindamycin preventing infection complications.
Furthermore, the main weaknesses of this study lie in the small number of publications that could be included. Only seven clinical trials [12, 28, 31, 33,34,35, 37] met the inclusion criteria: six on third molar extractions, one in endodontic surgery [28] and no one on oral implant surgery. In the rest of oral surgical interventions [17,18,19,20,21,22,23,24,25,26,27, 29], the authors did not use a control group with placebo or without any treatment. This may be due to ethical reasons. Nevertheless, absence of a control group impedes the effectiveness assessment of the tested treatments.
Some RCT [13, 32] analyzed the preventive effect of amoxicillin, replacing it for clindamycin when the patient was allergic to penicillin. However, most studies did not specify the sample size of each antibiotic or the number of infected patients according to the antibiotic that was finally used.
Another aspect to take into account is the sample size of each study. In the quantitative analysis, the total number of extractions was 486: 245 treated with oral clindamycin and 241 belonging to the control group. In addition, we must not forget that each of the trials studied a different antibiotic prescription pattern.
Besides, the risk of bias of each of the studies must also cautiously considered. In fact, there were no signs indicating publication bias in the present review, yet there may be a possibility that small-sized and negative studies might not have been published.
Nevertheless, there may be important implications for clinicians emerging from the present study. Nowadays, there is no consensus on the need to prescribe preventive antibiotics in oral surgery such as third molar extractions or oral implant placements in healthy patients. Reviews and meta-analysis have been conducted by using mainly beta-lactam antibiotics for prophylaxis. In 2021 a Cochrane review [6] concluded that there was evidence that prophylactic antibiotics reduce the risk of infection, dry socket and pain, following third molar extractions and resulted in an increase in mild and transient adverse effects. However, due to the increasing prevalence of bacteria which are resistant to treatment by currently available antibiotics, clinicians should consider carefully whether treating 12 healthy patients with antibiotics to prevent one infection (NNT) is likely to do more harm than benefit [6].
Healthy patients allergic to amoxicillin are frequently treated preventively with clindamycin in oral surgery. In the present meta-analysis with oral clindamycin the NNT was 29 (ranging from 12 to -57). These results indicate that oral clindamycin may not only be ineffective in preventing infections after third molar extraction, but it may even have a negative effect. With the limitations of the study, published in 2021 [52] authors state that clindamycin has been associated with a significantly elevated risk of failure of dental implant, and an up to six times increased risk of infection after surgical implant placement. Immediate implants also had a 5.7 to 10 times higher risk of failure.
The NNT is only a part of the information required to make decisions. Therefore, when the clinicians prescribe antibiotics before and/or after oral surgery to prevent infectious complications, other factors such as costs, adverse effects, patient characteristics, and social priorities must also be considered. Recent evidence also implicates clindamycin with a higher adverse-effect profile than amoxicillin, and pseudomembranous colitis is a key adverse outcome of clindamycin with an incidence of 2 to 10% [16].
Educational programs, clinical guidelines, professionals and educators should promote the improvement of the use of prophylactic antibiotics in oral surgery. They should also attempt to reduce the possible gap between the antibiotic prophylaxis usage supported by scientific evidence and the real antibiotic prescriptions performed by professionals.
This review highlights the need for further research focusing on clindamycin, with different dosages and adverse drug reactions, particularly in those surgical procedures where it is frequently prescribed as a prophylactic treatment.
It would also be interesting to review the efficacy of other antibiotics such as clarithromycin, azithromycin and metronidazole that are also used as preventive treatment in oral surgery procedures in patients allergic to amoxicillin. Clarithromycin is another acceptable penicillin substitute. This drug has a more limited spectrum of activity than clindamycin but has some advantages over erythromycin. Clarithromycin is effective against facultative anaerobes and some of the obligate anaerobic bacteria. Metronidazole is a synthetic antibiotic that is highly effective against obligate anaerobes but is not effective against facultative anaerobic bacteria.
In conclusion, there was not enough evidence to evaluate the effectiveness of preventive clindamycin in oral surgical interventions other than third molar extraction. The null hypothesis that oral clindamycin is not effective in preventing infection in third molar surgery regardless of the dosage used may be accepted.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Reference
Chen C, Gilpin N, Walsh L (2020) Discrepancy in Therapeutic and Prophylactic Antibiotic Prescribing in General Dentists and Maxillofacial Specialists in Australia. Antibiot (Basel, Switzerland) 9(8):492. https://doi.org/10.3390/antibiotics9080492
Williams R (2020) Antibiotic prophylaxis during dental implant placement in the UK. Br Dent J 229(12):787–792. https://doi.org/10.1038/s41415-020-2352-6
Yalcin-Ülker GM, Cakir M, Meral DG (2020) Antibiotic prescribing habits of the clinicians dealing with dental implant surgery in Turkey: a questionnaire study. Int J Implant Dent 6(1):66. https://doi.org/10.1186/s40729-020-00252-4
Rodríguez Sánchez F, Arteagoitia I, Teughels W, Rodríguez Andrés C, Quirynen M (2020) Antibiotic dosage prescribed in oral implant surgery: A meta-analysis of cross-sectional surveys. PLoS ONE 15(8):e0236981. https://doi.org/10.1371/journal.pone.0236981
Tomczyk S, Whitten T, Holzbauer SM, Lynfield R (2018) Combating antibiotic resistance: a survey on the antibiotic-prescribing habits of dentists. Gen Dent 66(5):61–68
Lodi G, Azzi L, Varoni EM, Pentenero M, Del Fabbro M, Carrassi A, Sardella A, Manfredi M (2021) Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst Rev 2(2):CD003811
Esposito M, Grusovin MG, Worthington HV (2013) Interventions for replacing missing teeth: antibiotics at dental implant placement to prevent complications. Cochrane Database Syst Rev 2013(7):CD004152. https://doi.org/10.1002/14651858.CD004152.pub4
Braun RS, Chambrone L, Khouly I (1939) (2019) Prophylactic antibiotic regimens in dental implant failure: A systematic review and meta-analysis. J Am Dent Associ 150(6):e61–e91. https://doi.org/10.1016/j.adaj.2018.10.015
Park J, Tennant M, Walsh LJ, Kruger E (2018) Is there a consensus on antibiotic usage for dental implant placement in healthy patients? Aust Dent J 63(1):25–33. https://doi.org/10.1111/adj.12535
Kim AS, Abdelhay N, Levin L, Walters JD, Gibson MP (2020) Antibiotic prophylaxis for implant placement: a systematic review of effects on reduction of implant failure. Br Dent J 228(12):943–951. https://doi.org/10.1038/s41415-020-1649-9
Rodríguez Sánchez F, Rodríguez Andrés C, Arteagoitia I (2018) Which antibiotic regimen prevents implant failure or infection after dental implant surgery? A systematic review and meta-analysis. J Craniomaxillofac Surg : Off Publ Eur Assoc Craniomaxillofac Surg 46(4):722–736. https://doi.org/10.1016/j.jcms.2018.02.004
Halpern LR, Dodson TB (2007) Does prophylactic administration of systemic antibiotics prevent postoperative inflammatory complications after third molar surgery? J Oral Maxillofacial Surg : Off J Am Assoc Oral Maxillofacial Surg 65(2):177–185. https://doi.org/10.1016/j.joms.2006.10.016
Xue P, Wang J, Wu B, Ma Y, Wu F, Hou R (2015) Efficacy of antibiotic prophylaxis on postoperative inflammatory complications in Chinese patients having impacted mandibular third molars removed: a split-mouth, double-blind, self-controlled, clinical trial. Br J Oral Maxillofac Surg 53(5):416–420. https://doi.org/10.1016/j.bjoms.2015.02.001
Basma HS, Misch CM (2021) Extraction Socket Grafting and Ridge Augmentation Failures Associated with Clindamycin Antibiotic Therapy: A Retrospective Study. Int J Oral and Maxillofacial Implants 36(1):122–125. https://doi.org/10.11607/jomi.8461
Kupfer SR (1995) Prevention of dry socket with clindamycin. A retrospective study. N Y State Dent J 61(6):30–33
Azher S, Patel A (2021) Antibiotics in Dentoalveolar Surgery, a Closer Look at Infection, Alveolar Osteitis and Adverse Drug Reaction. J Oral Maxillofacial Surg : Off J Am Assoc Oral Maxillofacial Surg S0278–2391(21):00405–00415. https://doi.org/10.1016/j.joms.2021.04.019 (Advance online publication)
Miles BA, Potter JK, Ellis E 3rd (2006) The efficacy of postoperative antibiotic regimens in the open treatment of mandibular fractures: a prospective randomized trial. J Oral Maxillofacial Surg : Off J Am Assoc Oral Maxillofacial Surg 64(4):576–582. https://doi.org/10.1016/j.joms.2006.01.003
Lindeboom JA, Tuk JG, Kroon FH, van den Akker HP (2005) A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone grafting procedures: single-dose clindamycin versus 24-hour clindamycin prophylaxis. Mund-, Kiefer- und Gesichtschirurgie : MKG 9(6):384–388. https://doi.org/10.1007/s10006-005-0650-4
Lindeboom JA, Frenken JW, Tuk JG, Kroon FH (2006) A randomized prospective controlled trial of antibiotic prophylaxis in intraoral bone-grafting procedures: preoperative single-dose penicillin versus preoperative single-dose clindamycin. Int J Oral Maxillofac Surg 35(5):433–436. https://doi.org/10.1016/j.ijom.2006.01.003
Klinge A, Khalil D, Klinge B, Lund B, Naimi-Akbar A, Tranaeus S, Hultin M (2020) Prophylactic antibiotics for staged bone augmentation in implant dentistry. Acta Odontol Scand 78(1):64–73. https://doi.org/10.1080/00016357.2019.1656819
Lindeboom JA, Baas EM, Kroon FH (2003) Prophylactic single-dose administration of 600 mg clindamycin versus 4-time administration of 600 mg clindamycin in orthognathic surgery: A prospective randomized study in bilateral mandibular sagittal ramus osteotomies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 95(2):145–149. https://doi.org/10.1067/moe.2003.54
Baqain ZH, Hyde N, Patrikidou A, Harris M (2004) Antibiotic prophylaxis for orthognathic surgery: a prospective, randomised clinical trial. Br J Oral Maxillofac Surg 42(6):506–510. https://doi.org/10.1016/j.bjoms.2004.06.010
Davis CM, Gregoire CE, Davis I, Steeves TW (2017) Prevalence of Surgical Site Infections Following Orthognathic Surgery: A Double-Blind, Randomized Controlled Trial on a 3-Day Versus 1-Day Postoperative Antibiotic Regimen. J Oral Maxillofacial Surg : Off J Am Assoc Oral Maxillofacial Surg 75(4):796–804. https://doi.org/10.1016/j.joms.2016.09.038
Righi M, Manfredi R, Farneti G, Pasquini E, Romei Bugliari D, Cenacchi V (1995) Clindamycin/cefonicid in head and neck oncologic surgery: one-day prophylaxis is as effective as a three-day schedule. J Chemother (Florence, Italy) 7(3):216–220. https://doi.org/10.1179/joc.1995.7.3.216
Mann W, Maurer J, Wolfensberger M, Riechelmann H, Daschner F (1990) Perioperative Antibiotikaprophylaxe in der Kopf-Hals-Chirurgie [Perioperative preventive use of antibiotics in head and neck surgery]. HNO 38(6):197–201
Clayman GL, Raad II, Hankins PD, Weber RS (1993) Bacteriologic profile of surgical infection after antibiotic prophylaxis. Head Neck 15(6):526–531. https://doi.org/10.1002/hed.2880150609
Raslan N, Mansour O, Assfoura L (2017) Evaluation of antibiotic mix in Non-instrumentation Endodontic Treatment of necrotic primary molars. Eur J Paediatr Dent 18(4):285–290. https://doi.org/10.23804/ejpd.2017.18.04.04
Lindeboom JA, Frenken JW, Valkenburg P, van den Akker HP (2005) The role of preoperative prophylactic antibiotic administration in periapical endodontic surgery: a randomized, prospective double-blind placebo-controlled study. Int Endod J 38(12):877–881. https://doi.org/10.1111/j.1365-2591.2005.01030.x
Laird WR, Stenhouse D, Macfarlane TW (1972) Control of post-operative infection A comparative evaluation of clindamycin and phenoxymethylpenicillin. Bri Dent J 133(3):106–109. https://doi.org/10.1038/sj.bdj.4802883
Bystedt H, Nord CE (1980) Effect of antibiotic treatment on post-operative infections after surgical removal of mandibular third molars. Swed Dent J 4(1–2):27–38
Poeschl PW, Eckel D, Poeschl E (2004) Postoperative prophylactic antibiotic treatment in third molar surgery–a necessity? J Oral Maxillofacial Surg : Off J Am Assoc Oral Maxillofacial Surg 62(1):3–9. https://doi.org/10.1016/j.joms.2003.05.004
Foy SP, Shugars DA, Phillips C, Marciani RD, Conrad SM, White RP Jr (2004) The impact of intravenous antibiotics on health-related quality of life outcomes and clinical recovery after third molar surgery. J Oral Maxillofacial Surg : Off J Am Assoc of Oral Maxillofacial Surg 62(1):15–21. https://doi.org/10.1016/j.joms.2003.04.003
Kaczmarzyk T, Wichlinski J, Stypulkowska J, Zaleska M, Panas M, Woron J (2007) Single-dose and multi-dose clindamycin therapy fails to demonstrate efficacy in preventing infectious and inflammatory complications in third molar surgery. Int J Oral Maxillofac Surg 36(5):417–422. https://doi.org/10.1016/j.ijom.2006.12.003
Adde CA, Soares MS, Romano MM, Carnaval TG, Sampaio RM, Aldarvis FP, Federico LR (2012) Clinical and surgical evaluation of the indication of postoperative antibiotic prescription in third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol 114(5 Suppl):S26–S31. https://doi.org/10.1016/j.tripleo.2011.08.018
Hamiti-Krasniqi V, Agani Z, Shtino G, Loxha M, Ahmedi J, Rexhepi A (2014) Impact of Local Application of Clindamycin in Preventing Dry Socket after Third Mandibular Molar Extraction. Open J Stomatol 4:463–469. https://doi.org/10.4236/ojst.2014.49062
Xue P, Hou R, Shang L, Ma Y, Wu F, Zhang S (2014) Zhonghua kou qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi =. Chinese J Stomatol 49(10):603–606
Kaposvári I, Körmöczi K, László ZB, Oberna F, Horváth F, Joób-Fancsaly Á (2017) A preoperatív antibiotikus és antiszeptikus kezelés hatása a műtéti úton eltávolított alsó bölcsességfogak sebgyógyulására – prospektív randomizált vizsgálat [Prospective randomized study regarding the effect of the preoperative antibiotic and chlorhexidine rinse on wound healing after mandibular third molar surgery]. Orv Hetil 158(1):13–19. https://doi.org/10.1556/650.2017.30645
Wiesenbaugh JM Jr (1971) Comparison of oral penicillin G and clindamycin as prophylactic antibiotics in oral surgery. Oral Surg, Oral Med, Oral Pathol 31(3):302–311. https://doi.org/10.1016/0030-4220(71)90151-4
Katoh H (1992) Incidence of transient bacteremia following dental surgery–prophylactic use of cefuroxime, ceftriaxone or clindamycin. Tokai J Exp Clin Med 17(3–4):109–113
Göker K, Güvener O (1992) Antibacterial effects of ofloxacin, clindamycin and sultamicillin on surgical removal of impacted third molars. J Marmara Univ Dent Fac 1(3):237–249
Aitken C, Cannell H, Sefton AM, Kerawala C, Seymour A, Murphy M, Whiley RA, Williams JD (1995) Comparative efficacy of oral doses of clindamycin and erythromycin in the prevention of bacteraemia. Br Dent J 178(11):418–422. https://doi.org/10.1038/sj.bdj.4808789
Hall G, Nord CE, Heimdahl A (1996) Elimination of bacteraemia after dental extraction: comparison of erythromycin and clindamycin for prophylaxis of infective endocarditis. J Antimicrob Chemother 37(4):783–795. https://doi.org/10.1093/jac/37.4.783
Roberts GJ, Watts R, Longhurst P, Gardner P (1998) Bacteremia of dental origin and antimicrobial sensitivity following oral surgical procedures in children. Pediatr Dent 20(1):28–36
Diz Dios P, Tomás Carmona I, Limeres Posse J, Medina Henríquez J, Fernández Feijoo J, Alvarez Fernández M (2006) Comparative efficacies of amoxicillin, clindamycin, and moxifloxacin in prevention of bacteremia following dental extractions. Antimicrob Agents Chemother 50(9):2996–3002. https://doi.org/10.1128/AAC.01550-05
Maharaj B, Coovadia Y, Vayej AC (2012) A comparative study of amoxicillin, clindamycin and chlorhexidine in the prevention of post-extraction bacteraemia. Cardiovasc J Afr 23(9):491–494. https://doi.org/10.5830/CVJA-2012-049
Limeres Posse J, Álvarez Fernández M, Fernández Feijoo J, Medina Henríquez J, Lockhart PB, Chu VH, Diz Dios P (2016) Intravenous amoxicillin/clavulanate for the prevention of bacteraemia following dental procedures: a randomized clinical trial. J Antimicrob Chemother 71(7):2022–2030. https://doi.org/10.1093/jac/dkw081
Kirchner JC, Edberg SC, Sasaki CT (1988) The use of topical oral antibiotics in head and neck prophylaxis: is it justified? Laryngoscope 98(1):26–29. https://doi.org/10.1288/00005537-198801000-00007
Grandis JR, Vickers RM, Rihs JD, Yu VL, Wagner RL, Kachman KK, Johnson JT (1994) The efficacy of topical antibiotic prophylaxis for contaminated head and neck surgery. Laryngoscope 104(6 Pt 1):719–724. https://doi.org/10.1288/00005537-199406000-00011
Grandis JR, Vickers RM, Rihs JD, Yu VL, Johnson JT (1994) Efficacy of topical amoxicillin plus clavulanate/ticarcillin plus clavulanate and clindamycin in contaminated head and neck surgery: effect of antibiotic spectra and duration of therapy. J Infect Dis 170(3):729–732. https://doi.org/10.1093/infdis/170.3.729
Bulut E, Bulut S, Etikan I, Koseoglu O (2001) The value of routine antibiotic prophylaxis in mandibular third molar surgery: acute-phase protein levels as indicators of infection. J Oral Sci 43(2):117–122. https://doi.org/10.2334/josnusd.43.117
Chapnick P, Diamond LH (1992) A review of dry socket: a double-blind study on the effectiveness of clindamycin in reducing the incidence of dry socket. J (Can Dent Assoc) 58(1):43–52
Salgado-Peralvo A-O, Peña-Cardelles J-F, Kewalramani N, Ortiz-García I, Jiménez-Guerra Á, Uribarri A, Velasco-Ortega E, Moreno-Muñoz J, Núñez-Márquez E, Monsalve-Guil L (2021) Is Penicillin Allergy a Risk Factor for Early Dental Implant Failure? Syst Rev Antibiot 10:1227. https://doi.org/10.3390/antibiotics10101227
Acknowledgements
Open Access funding provided by University of the Basque Country
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Open Access funding provided by University of the Basque Country.
Author information
Authors and Affiliations
Contributions
All authors conducted the study read the articles, interpreted and analyzed the data. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval does not apply to systematic reviews.
Informed consent
Informed consent does not apply to systematic reviews.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arteagoitia, I., Sánchez, F.R., Figueras, A. et al. Is clindamycin effective in preventing infectious complications after oral surgery? Systematic review and meta-analysis of randomized controlled trials. Clin Oral Invest 26, 4467–4478 (2022). https://doi.org/10.1007/s00784-022-04411-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-022-04411-2