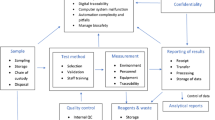
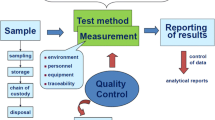
Abstract
Many higher education institutions in the world have testing laboratories linked, or not, to their teaching and/or research programs. However, only a small proportion of these laboratories have accreditation in accordance with the ISO/IEC 17025 standard. The ISO/IEC 17025 establishes the management and technical requirements necessary for the implementation and maintenance of a quality management system (QMS) in laboratories that perform testing, calibration and/or sampling activities, being used by them to demonstrate their competence in carrying out their activities. One of the requirements of the current version of the standard is the need to carry out risk management (RM), introduced with the demand for risk-based thinking. The objective of this research was to propose a system for the implementation of RM in laboratories, through mapping, identification, classification, critical analysis, and treatment of risks. The research considered the history of actions taken, the risks verified by the laboratory team, the evaluation of their impacts and the probabilities of their occurrence, their classification and the identification of actions necessary to accept, mitigate or eliminate these risks. The system proposed was applied in a testing laboratory at a university in southern Brazil, enabling the maintenance of its accreditation.
Similar content being viewed by others
References
Grochau IH, Ferreira CA, Ferreira ZJ, ten Caten CS (2010) Implementation of a quality management system in university test laboratories: a brief review and new proposals. Accredit Qual Assur 15(12):681–689
International Organization for Standardization (ISO) (2017) ISO/IEC 17025 - General requirements for the competence of testing and calibration laboratories. Geneva
Grochau IH, ten Caten CST, Forte MMC (2018) Motivations, benefits and challenges on ISO/IEC 17025 accreditation of Higher Education Institution laboratories. Accredit Qual Assur 23(3):183–188. https://doi.org/10.1007/s00769-018-1317-9
Grochau IH, ten Caten CST, Forte MMC (2017) Current American landscape in laboratory accreditation according to ISO/IEC 17025. Accredit Qual Assur 22(2):57–62. https://doi.org/10.1007/s00769-017-1248-x
Grochau IH, Leal DKB, ten Caten CST (2020) European current landscape in laboratory accreditation. Accredit Qual Assur 25:303–310. https://doi.org/10.1007/s00769-020-01440-w
Ribeiro LMS, Beijo LA, Salgado EG, Nogueira DA (2019) Modelling of ISO 9001 certifications for the American countries: a Bayesian approach. Total Qual Manag Bus Excell. https://doi.org/10.1080/14783363.2019.1696672
International Organization for Standardization (ISO) (2015) ISO 9001 - Quality management systems - Requirements, Geneva
Cagnin F, Oliveira MC, Miguel PAC (2019) Assessment of ISO 9001: 2015 implementation: focus on risk management approach requirements compliance in an automotive company. Total Qual Manag Bus Excell. https://doi.org/10.1080/14783363.2019.1677151
Wong SK (2017) Risk-based thinking for chemical testing. Accredit Qual Assur 22:103–108. https://doi.org/10.1007/s00769-017-1256-x
Samani MA, Ismail N, Leman Z, Zulkifli N (2019) Development of a conceptual model for risk-based quality management system. Total Qual Manag Bus Excell 30(5–6):483–498. https://doi.org/10.1080/14783363.2017.1310617
Zou X, Isa CR, Rahman M (2019) Valuation of enterprise risk management in the manufacturing industry. Total Qual Manag Bus Excell 30(11–12):1389–1410. https://doi.org/10.1080/14783363.2017.1369877
Fonseca LM, Domingues JP, Baylina-Machado P, Harder D (2019) ISO 9001:2015 adoption: a multicountry empirical research. J Ind Eng Manag 12(1):27–50. https://doi.org/10.3926/jiem.2745
Grochau IH, ten Caten CST (2012) A process approach to ISO/IEC 17025 in the implementation of a quality management system in testing laboratories. Accredit QualAssur 17:519–527. https://doi.org/10.1007/s00769-012-0905-3
Sedrez CS, Fernandes FC (2011) Gestão de Riscos nas Universidades e Centros Universitários do Estado de Santa Catarina. Revista Gestão Universitária na América Latina, Florianópolis, Edição especial 2011, p. 70–93. Available at: https://periodicos.ufsc.br/index.php/gual/article/view/24829. Access at: 10 Jul 2019
Wen D, Sun X, Yan D (2020) The quality movement: where are we going? Past, present and future. Total Qual Manag Bus Excell. https://doi.org/10.1080/14783363.2020.1801342
Casteleiro C, Mendes SL (2020) Exploring the influence of quality management systems in work engagement and psychological empowerment in private institutions of social solidarity. Total Qual Manag Bus Excell. https://doi.org/10.1080/14783363.2020.1832460
Ikram M, Zhang Q, Sroufe R (2020) Future of quality management system (ISO 9001) certification: novel grey forecasting approach. Total Qual Manag Bus Excell. https://doi.org/10.1080/14783363.2020.1768062
International Organization for Standardization (ISO) (2013) ISO/TR 31004 - Risk management ― Guide to the implementation of ISO 31000. Geneva
International Organization for Standardization (ISO) (2018) ISO 31000 - Risk management ― Guidelines. Geneva
International Organization for Standardization (ISO) (2009) ISO/IEC 31010 - Risk management — Risk assessment techniques. Geneva
Pacana A (2018) Supervision of measuring equipment based on risk management and ISO 9001. Prod Eng Arch 21:8–11
Acknowledgements
The authors gratefully acknowledge the financial support from the Luiz Englert Foundation and the Brazilian government agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, and Financiadora de Estudos e Projetos – FINEP (SIBRATEC project).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, F.R., Grochau, I.H. & Veit, H.M. System proposal for implementation of risk management in the context of ISO/IEC 17025. Accred Qual Assur 26, 271–278 (2021). https://doi.org/10.1007/s00769-021-01484-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-021-01484-6