Abstract
The relative stabilities of phases within the two systems monazite-(Ce) – fluorapatite – allanite-(Ce) and xenotime-(Y) – (Y,HREE)-rich fluorapatite – (Y,HREE)-rich epidote have been tested experimentally as a function of pressure and temperature in systems roughly replicating granitic to pelitic composition with high and moderate bulk CaO/Na2O ratios over a wide range of P-T conditions from 200 to 1000 MPa and 450 to 750 °C via four sets of experiments. These included (1) monazite-(Ce), labradorite, sanidine, biotite, muscovite, SiO2, CaF2, and 2 M Ca(OH)2; (2) monazite-(Ce), albite, sanidine, biotite, muscovite, SiO2, CaF2, Na2Si2O5, and H2O; (3) xenotime-(Y), labradorite, sanidine, biotite, muscovite, garnet, SiO2, CaF2, and 2 M Ca(OH)2; and (4) xenotime-(Y), albite, sanidine, biotite, muscovite, garnet, SiO2, CaF2, Na2Si2O5, and H2O. Monazite-(Ce) breakdown was documented in experimental sets (1) and (2). In experimental set (1), the Ca high activity (estimated bulk CaO/Na2O ratio of 13.3) promoted the formation of REE-rich epidote, allanite-(Ce), REE-rich fluorapatite, and fluorcalciobritholite at the expense of monazite-(Ce). In contrast, a bulk CaO/Na2O ratio of ~1.0 in runs in set (2) prevented the formation of REE-rich epidote and allanite-(Ce). The reacted monazite-(Ce) was partially replaced by REE-rich fluorapatite-fluorcalciobritholite in all runs, REE-rich steacyite in experiments at 450 °C, 200–1000 MPa, and 550 °C, 200–600 MPa, and minor cheralite in runs at 650–750 °C, 200–1000 MPa. The experimental results support previous natural observations and thermodynamic modeling of phase equilibria, which demonstrate that an increased CaO bulk content expands the stability field of allanite-(Ce) relative to monazite-(Ce) at higher temperatures indicating that the relative stabilities of monazite-(Ce) and allanite-(Ce) depend on the bulk CaO/Na2O ratio. The experiments also provide new insights into the re-equilibration of monazite-(Ce) via fluid-aided coupled dissolution-reprecipitation, which affects the Th-U-Pb system in runs at 450 °C, 200–1000 MPa, and 550 °C, 200–600 MPa. A lack of compositional alteration in the Th, U, and Pb in monazite-(Ce) at 550 °C, 800–1000 MPa, and in experiments at 650–750 °C, 200–1000 MPa indicates the limited influence of fluid-mediated alteration on volume diffusion under high P-T conditions. Experimental sets (3) and (4) resulted in xenotime-(Y) breakdown and partial replacement by (Y,REE)-rich fluorapatite to Y-rich fluorcalciobritholite. Additionally, (Y,HREE)-rich epidote formed at the expense of xenotime-(Y) in three runs with 2 M Ca(OH)2 fluid, at 550 °C, 800 MPa; 650 °C, 800 MPa; and 650 °C, 1000 MPa similar to the experiments involving monazite-(Ce). These results confirm that replacement of xenotime-(Y) by (Y,HREE)-rich epidote is induced by a high Ca bulk content with a high CaO/Na2O ratio. These experiments demonstrate also that the relative stabilities of xenotime-(Y) and (Y,HREE)-rich epidote are strongly controlled by pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monazite [(LREE,Th,U)PO4], xenotime-(Y) [(Y,HREE)PO4], fluorapatite [(Ca,LREE,Si,Na)5(PO4)3F], and allanite [(Ca,REE)(Al2,Fe2+)(Si2O7)(SiO4)O(OH)] are common accessory minerals in various types of igneous, metamorphic, and sedimentary rocks and represent the principal hosts of rare earth elements (REEs) in the Earth’s crust. They are widely used as geochronometers, including U-Pb and Th-U-Pb dating of monazite (Williams et al. 2007) and xenotime-(Y) (Hetherington et al. 2008), Lu-Hf, U-Pb, fission track and (U-Th)/He dating of apatite (Chew and Spikings 2015), and U-Pb dating of allanite (Gregory et al. 2007; Darling et al. 2012). Distribution of Y between co-existing monazite and xenotime-(Y) (Gratz and Heinrich 1997; Heinrich et al. 1997; Andrehs and Heinrich 1998) or monazite and garnet (Pyle et al. 2001) can be utilized as geothermometers. In addition to these important applications, monazite, xenotime-(Y), apatite, and allanite can also be used for studying metasomatic processes; the nature of the fluids responsible for these processes (Putnis 2009; Budzyń et al. 2011; Harlov et al. 2011; Harlov 2015); and to constrain timing of mineral-fluid interactions (Williams et al. 2011; Villa and Williams 2013).
The relative stabilities of monazite, allanite, and fluorapatite are of particular interest in studies of igneous and metamorphic processes. For example, in metamorphosed rocks of granitic or pelitic composition, monazite-(Ce) alteration and breakdown involve formation of complex textures involving fluorapatite, allanite-(Ce), epidote [Ca2(Al2,Fe3+)(Si2O7)(SiO4)O(OH)], and ThSiO4 or ThO2, mediated by a fluid with high Ca and F activities (Broska and Siman 1998; Finger et al. 1998; Broska et al. 2005; Petrík et al. 2006; Janots et al. 2008; Ondrejka et al. 2012). More rarely, monazite-(Ce) can be replaced by secondary fluorapatite and monazite, but not allanite-(Ce) (Rasmussen and Muhling 2007; Budzyń et al. 2010). The diversity of products is strictly related to the P-T conditions, bulk rock composition, and composition of the fluid. Several experimental studies have determined the stabilities of monazite-(Ce), allanite-(Ce), and fluorapatite relative to each other. Experiments at 450–500 °C and 450–610 MPa utilizing silicate mineral assemblages and various fluids, including H2O, 2 M NaOH, 2 M KOH, 1 M/2 M Ca(OH)2, 1 M HCl, NaCl + H2O, KCl + H2O, CaCl2 + H2O, CaCO3 + H2O, and Na2Si2O5 + H2O, tested the stability of monazite-(Ce) relative to allanite-(Ce) and fluorapatite as a function of P-T-X conditions (Budzyń et al. 2011). Monazite-(Ce) partially broke down to fluorapatite and/or britholite in most of the experiments. In the presence of Ca-bearing fluids, allanite-(Ce) and REE-rich epidote also formed. In the experiment at 450 °C and 450 MPa with Na2Si2O5 + H2O, alteration of monazite-(Ce) via fluid-mediated coupled dissolution-reprecipitation resulted in the resetting of monazite Th-U-Pb clock far below the diffusional closure temperature (Williams et al. 2011). Recently, the stability of monazite-(Ce) was tested in the presence of silicate mineral assemblages under conditions of 250–350 °C and 200–400 MPa (Budzyń et al. 2015). Monazite-(Ce) was stable in the presence of 2 M Ca(OH)2, where some fluorapatite to fluorcalciobritholite [(Ca,REE)5(SiO4,PO4)3F] formed, but no allanite. The presence of Na2Si2O5 + H2O promoted the strong alteration of the monazite-(Ce) and the formation of steacyite [(K,□)(Na,Ca)2(Th,U)Si8O20] enriched in REE, and fluorcalciobritholite. Furthermore, fluid induced monazite-(Ce) alteration promoted mass transfer with partial removal of Th, U, and Pb via fluid-mediated coupled dissolution-reprecipitation resulting in significant disturbance of the original ages without resetting in contrast to 450 °C and 450 MPa experiment in Williams et al. (2011).
These experiments were based on available data from monazite-allanite relations in metamorphosed granites and pelites (Broska and Siman 1998; Finger et al. 1998; Janots et al. 2006), and the thermodynamic modeling of the relative stabilities of monazite-(La) and dissakisite-(La), Mg-equivalent of allanite (Janots et al. 2007). Later thermodynamic modeling by Spear (2010), which utilized the average whole rock metapelite data of Shaw (1956), showed that increasing the CaO content from 2.17 to 4.34 wt.% shifts the transition of allanite-(Ce) to monazite-(Ce) from ~400–550 °C, depending on a pressure, to ~480 °C at 200 MPa and ~750 °C at 1000 MPa, thereby expanding the allanite-(Ce) stability field. Fluorapatite was stable over the entire calculated P-T range (100–1100 MPa and 350–800 °C) considered. In the experiments of Budzyń et al. (2011), those with a significantly increased bulk CaO content (~11.3–17.7 wt.%) resulted in allanite-(Ce) and fluorapatite-britholite formation at 450–500 °C and 400–610 MPa in agreement with Spear’s (2010) thermodynamic modeling.
Xenotime-(Y) is recognized as a robust geochronometer, which is resistant to alteration (Rasmussen 2005). In addition, volume diffusion of Pb has been experimentally demonstrated to be slower than in zircon or monazite (Cherniak 2006). Similar to monazite-(Ce), fluid-mediated alteration has also led to the replacement of xenotime-(Y) by corona-like textures including (Y,HREE)-rich fluorapatite and (Y,HREE)-rich epidote in granitic rocks (Broska et al. 2005; Broska and Petrík 2015) or fluorapatite and hingganite-(Y) in pegmatite (Majka et al. 2011). The P-T conditions for such alterations are not well constrained. During progressive metamorphism of pelites, xenotime-(Y) breaks down before the growth of garnet. This is then followed by the breakdown of HREE-rich clinozoisite to xenotime-(Y) at 560–610 °C, depending on bulk composition of rock, particularly the bulk CaO/Na2O ratio (Janots et al. 2008). Because xenotime-(Y) is less abundant than monazite in nature, our knowledge about its stability during metamorphism, particularly in relation to (Y,HREE)-rich epidote and (Y,HREE)-rich fluorapatite, is still limited.
In this work, the relative stabilities of monazite-(Ce), allanite-(Ce), and fluorapatite are explored in a series of experiments which expand on the previous experiments of Budzyń et al. (2011) to a broader range of P-T conditions at 200 to 1000 MPa and 450 to 750 °C. The study aims to determine (i) if allanite-(Ce) and/or fluorapatite will form at the expense of monazite-(Ce) in experiments utilizing monazite-(Ce) plus a silicate mineral assemblage and a 2 M Ca(OH)2 fluid or a Na2Si2O5 + H2O fluid over the above P-T range; and (ii) the impact of fluid-mediated alteration on the remobilization of Th, U, and Pb in the monazite-(Ce) structure in the presence of a Na2Si2O5 + H2O, with regards to its impact on geochronology. Starting mixes with both fluids had an elevated bulk Ca content that should promote fluorapatite and allanite-(Ce) formation. A simultaneous set of parallel experiments for xenotime-(Y) were aimed at determining the P-T stability relations between xenotime-(Y), (Y,HREE)-rich epidote, and (Y,HREE)-rich fluorapatite. This study also aims to estimate to what degree Y + REE incorporation into epidote-group minerals is a function of fluid composition or P-T conditions or both in a REE-dominated system.
Experimental and analytical methods
Experiments
Experiments were performed utilizing cold-seal autoclaves on a hydrothermal line and the piston-cylinder apparatus. Pressures and temperatures ranged from 200 to 1000 MPa and from 450 to 750 °C. Each run involved four sets of experiments, two for monazite-(Ce) and two for xenotime-(Y), each one corresponding to two different fluids, 2 M Ca(OH)2 or Na2Si2O5 + H2O (Table 1). In each set of experiments, coarse grains (50–250 μm) of monazite-(Ce) and xenotime-(Y) were used to explore reactions, primarily focused on the partial replacement of monazite-(Ce) and xenotime-(Y) by (Y,REE)-enriched epidote and fluorapatite. In order to effect these reactions in an approximation of a metapelitic, geochemical environment, quartz, albite, biotite, garnet, labradorite, muscovite, K-feldspar, and CaF2 from diverse sources were added to the system. These provided the materials necessary for the reactions: monazite-(Ce) + annite + quartz + Ca + F (in fluid-I) = fluorapatite + allanite-(Ce) + fluid-II (Broska and Siman 1998), and xenotime-(Y) + annite + anorthite + fluid = (Y,HREE)-rich apatite + (Y,HREE)-rich epidote + muscovite (Broska et al. 2005). These experiments were not aimed at achieving thermobarometric equilibria between a group of silicate minerals from a diverse set of sources. Rather, these coarse grains of monazite-(Ce) and xenotime-(Y) were used to induce (Y,REE)-bearing epidote and apatite reaction textures to form, which is the chief focus of this study.
The Burnet monazite-(Ce) used in experiments originates from a pegmatite from Burnet County, Texas, USA. This monazite-(Ce) (10.18–12.54 wt.% ThO2; 0.28–0.40 wt.% UO2; 0.51–0.65 wt.% PbO; see supplementary Table S1 for average composition) was selected to test the relative mobility of Y, REE, Th, U, and Pb. A fragment of the Burnet monazite-(Ce) was crushed and sieved to a 50–250 μm fraction. Optically clear to slightly foggy, reddish-brown grains were hand picked out under a binocular microscope. The separated grains were washed in ethanol in an ultrasonic bath. Under high-contrast back-scattered electrons (BSE) imaging, the monazite-(Ce) grains show faint zonation and patchiness in cross section, which is related to slight variations in the ThO2 content.
The xenotime-(Y) used for experiments is a part of a gem quality, euhedral crystal from a pegmatite in the North-West Frontier Province (NWFP), Pakistan. A fragment of the xenotime-(Y) crystal was crushed and sieved to obtain a 50–250 μm grain size fraction. Optically clear grains with no inclusions of foreign mineral phases were hand picked out using a binocular microscope. The xenotime-(Y) separate was then washed in ethanol in an ultrasonic bath.
Four mineral starting mixes were prepared, two for monazite-(Ce) and two for xenotime-(Y) in the presence of silicate minerals. The silicate starting mixes roughly replicate the composition of granitic to pelitic rocks in which the altered monazite-(Ce) and xenotime-(Y) are most commonly found. Each set of experiments have a high bulk CaO content with respect to natural granites and pelites, i.e. estimated 10.08 wt.% in set (1), 5.91 wt.% in set (2), 9.21 wt.% in set (3) and 5.56 wt.% in set (4) (Table 2). The two silicate starting mixes were prepared for the monazite-(Ce) and xenotime-(Y) experiments (Table 1). These included (1) monazite-(Ce), labradorite (Ab37An60Kfs3; Chihuahua, Mexico), sanidine (Eifel region, Germany), biotite (migmatitic gneiss, Sikkim Himalaya, India), muscovite (pegmatite, Siedlimowice, Sudety Mts., Poland), SiO2, CaF2, and 2 M Ca(OH)2; (2) monazite-(Ce), hydrothermal albite (Ab100; Rožňava, Slovak Republic), sanidine, biotite, muscovite, SiO2, CaF2, Na2Si2O5, and doubly distilled H2O; (3) xenotime-(Y), labradorite, sanidine, biotite, muscovite, garnet (Gore Mt., USA), SiO2, CaF2, and 2 M Ca(OH)2 fluid; and (4) xenotime-(Y), albite, sanidine, biotite, muscovite, garnet, SiO2, CaF2, Na2Si2O5, and doubly distilled H2O (Table 1). The 2 M Ca(OH)2 solution was prepared using a Ca(OH)2 chemical reagent (Merck) and doubly distilled H2O. Synthetic amorphous SiO2 was used instead of quartz to increase the reaction rates. CaF2 (suprapur; Merck 2842) was used as a source of Ca and F to form fluorapatite. Labradorite was used in experiments with 2 M Ca(OH)2 as an additional source of Ca, whereas albite was used in experiments with Na2Si2O5 + H2O to increase the Na bulk content. Experiments with xenotime-(Y) included garnet to test Y partitioning between xenotime-(Y) and garnet. The estimated average bulk composition of the silicate mixes and all other components used in each capsule charge, calculated based on the average composition of each starting phase, are presented in Table 2. High amounts of monazite-(Ce) and xenotime-(Y) in the starting mixes were used in order that these phases might be easily found in the grain mounts. This, however, significantly increased the bulk REE and P contents in the experiments compared to their actual relative abundances in natural rocks.
The minerals in the silicate mixes were crushed and sieved to obtain a 50 to 250 μm fraction. Foreign and cloudy mineral grains were hand picked out under the binocular microscope. The Au capsules, 15 mm long and 3 mm wide (outer diameter 3.0 mm, inner diameter 2.6 mm), were loaded with ~5 mg of doubly distilled H2O, 20–36 mg of mixed solids (Table 1), and arc-welded shut using a Lampert PUK-04 precision welding device. The Au capsules were checked for leaks by weighing, heating in a 105 °C oven overnight, and then weighed again.
Experiments at 200 to 400 MPa and 450 to 750 °C (Table 1) were performed in standard cold-seal, 6 mm bore, René metal autoclaves with H2O as the pressure medium. Four gently flattened Au capsules, two with monazite-(Ce) [experimental sets (1) and (2)] and two with xenotime-(Y) [experimental sets (3) and (4)], were placed in each autoclave. During the run, the experiments were buffered at approximately the Ni-NiO oxygen buffer due to the presence of Ni metal filler rods, which occupied the bore of the autoclave not occupied by the Au capsules. Temperatures were measured externally by a thermocouple tip inserted into the end of autoclave near the Au capsules. Thermocouples are accurate to within ±3 °C. No variation in temperature was observed during each run. The maximum temperature gradient along the length of a capsule was approximately 5 °C at 750 °C. The temperature gradient was measured in a sealed autoclave on a hydrothermal line at 400 MPa by placing two thermocouples in contact with both ends of a 1 cm long Au capsule and placing a third thermocouple in contact with the center of the capsule. For the case of 750 °C, as measured by the central thermocouple, the thermal gradient was then determined by the difference between this temperature and the two temperatures measured at either end of the capsule. Pressure on the hydrothermal line was calibrated against a pressure transducer calibrated against a Heise gauge manometer for which the quoted pressure is accurate to ±5 MPa. The autoclaves were quenched after the run using compressed air, reaching temperatures of ~100 °C within 1 min.
Experiments at 600 to 1000 MPa and 450 to 750 °C (Table 1) were performed using the piston-cylinder apparatus (Johannes et al. 1971; Johannes 1973). An NaCl assembly with a graphite oven was used in the 450, 500, and 650 °C runs, and a CaF2 assembly with a graphite oven was used in the 750 °C runs. In the case of the CaF2 assembly, the actual pressure during the experimental run was corrected for friction (cf. Harlov and Milke 2002). Four gently flattened Au capsules [two for the monazite-(Ce) and two for the xenotime-(Y) experiments] were positioned vertically with the Ni-Cr thermocouple tip placed approximately halfway up alongside of one of the capsules (Fig. 1). Biotite sheets were used to separate the capsules. Estimated maximum thermal gradients along the length of the capsule are ±20 °C and were estimated in the same manner as described above for the hydrothermal autoclave experiments. Estimated uncertainty in pressure is ±50 MPa (cf. Harlov and Milke 2002). At the start of a run, the pressure was taken up to approximately 10–15 % below run conditions, and then the temperature was brought up to the desired value. Thermal expansion caused the pressure to increase to the approximate target value. The pressure was then adjusted to the desired value, and automatically maintained within a preset range during the course of the experiment. During the run, the presence of the graphite oven buffered the experiment to the C-CO-CO2 oxygen buffer. Quench was achieved by turning off the current, such that H2O-cooling jacket cooled down the NaCl or CaF2 assembly to below 50 °C within about 15 s.
After each run, the capsules were carefully examined, cleaned, weighed, and opened. The pH of the fluid within the capsule was measured using litmus paper. The capsules were then dried at 105 °C overnight. A portion of the extracted experiment was mounted in epoxy and polished for back scattered electron (BSE) imaging and electron probe micro analysis. A second part of the extracted experimental products was sprinkled on an adhesive carbon mount and carbon coated for BSE imaging. Product phase dimensions were measured in cross section in polished grain mounts. Because the exposures strongly depend dimensionally on the cross cut of the minerals during grinding down and polishing, particularly in the replacement textures, image analysis was not applied in order to avoid misleading volumetric data.
Analytical methods
The BSE imaging, and preliminary chemical analyses of the starting minerals and the experimental products were performed using a Hitachi S-4700 field emission scanning electron microscope equipped with energy dispersive spectrometer (EDS). Chemical analyses of the mixes and experimental products were performed using a JEOL JXA-8530F HyperProbe Field Emission Electron Probe Microanalyzer (EPMA) equipped with four wavelength spectrometers. Monazite-(Ce) and xenotime-(Y) analyses were collected using a 20 kV accelerating voltage, and a 40 nA beam current with a 3–4 μm beam size for monazite-(Ce) and 1 μm beam size for xenotime-(Y). Measured concentrations of REE, U, and Pb were corrected (online and offline) for various interferences following the combined approach of Åmli and Griffin (1975) and Rhede (personal comm. to JM; see also Förster et al. 2012). Fluorapatite, fluorcalciobritholite, REE-rich steacyite, REE-rich epidote, and allanite-(Ce) were analyzed using a 20 kV, 20 nA and a 1–3 μm beam size. Feldspars and micas from runs with monazite-(Ce), and amphibole from runs with monazite-(Ce) and xenotime-(Y), were analyzed using a 15 kV, 10 nA, and 2 μm beam size for the feldspars, a 4 μm beam size for the micas, and a focused beam for the amphibole. Further details on measurement conditions can be found in supplementary Table S2.
Chemical analyses of feldspars, micas, garnets, and titanite from experiments with xenotime-(Y) [sets (3) and (4)], analyses of melt, and compositional X-ray maps of monazite-(Ce) and xenotime-(Y), were performed using a JEOL JXA-8230 SuperProbe Electron Probe Microanalyzer. Measurement conditions can be found in supplementary Table S2. Compositional WDS X-ray maps of altered monazite-(Ce) and xenotime-(Y) were collected using a 15 kV accelerating voltage, 100 nA beam current, 100 ms dwell time, 0.33 μm step size, and a focused beam.
Abbreviations
The abbreviations of mineral names are used according to those proposed by Whitney and Evans (2010). Ab – albite, Aln – allanite-(Ce), Amph – amphibole, Brt – fluorcalciobritholite, Bt – biotite, Bt2 – secondary low-Ti biotite, Btw – bytownite, Cher – cheralite, Chl – chlorite, Crn – corundum, Grt – garnet, Kfs – K-feldspar, Lbr – labradorite, Mnz – monazite-(Ce), rq-Mnz – re-equilibrated monazite-(Ce) with patchy zoning, Ms – muscovite, Ms2 – secondary muscovite, Or – orthoclase, Px – pyroxene, Qz – quartz, REE-Ap – REE-rich fluorapatite, REE-Ep – REE-rich epidote, Stc – REE-rich steacyite, Ttn – titanite, Wo – wollastonite, Xtm – xenotime-(Y), (Y,REE)-Ap – (Y,REE)-rich fluorapatite, YBrt – Y-rich fluorcalciobritholite, (Y,HREE)-Ep – (Y,HREE)-rich epidote.
Experimental results
Experiments with monazite-(Ce) and 2 M Ca(OH)2
Monazite-(Ce) breakdown, including various degrees of dissolution on the surface and formation of new phases, were documented in all runs (Fig. 2a, Table 3). The remaining fluid had a neutral pH in most products, except in runs at 450 °C, 800–1000 MPa; and 550 °C, 1000 MPa, which tended to have a moderately high pH (Table 1). Monazite-(Ce) grains from runs at 450–550 °C and 200–400 MPa are altered mostly along the rims, showing partial dissolution and overgrowth by other mineral phases (Fig. 3b, d and e). In the higher P-T experiments, most of the monazite-(Ce) grains were partially dissolved in the same way, with occasional almost complete replacement by REE-rich epidote-allanite-(Ce) (Fig. 3l). The composition of the monazite-(Ce) in the experimental products is the same as the original Burnet monazite-(Ce), indicating that the remaining monazite-(Ce) was not affected by compositional alteration (Fig. 4a and b; supplementary Table S1). High Th content, in both the altered and original monazite-(Ce), is related to the huttonitic and cheralitic substitutions (Fig. 5a and b).
BSE images showing the results from experiments with monazite-(Ce) and 2 M Ca(OH)2; a small crystals of REE-rich epidote formed in the run at 450 °C, 200 MPa, and 16 days; b altered monazite-(Ce) overgrown by secondary fluorcalciobritholite and allanite-(Ce) at 450 °C, 400 MPa, and 18 days; c allanite-(Ce) overgrowing altered monazite-(Ce) from the run at 450 °C, 1000 MPa and 16 days; d–e REE-rich fluorapatite-fluorcalciobritholite and allanite-(Ce) overgrowing altered monazite-(Ce) in experiments at 550 °C, 200 MPa, and 12 days (d) and 550 °C, 400 MPa, and 6 days (e); f altered monazite-(Ce) overgrown by numerous tabular crystals of allanite-(Ce) at 550 °C, 800 MPa, and 8 days; g hexagonal prisms of fluorcalciobritholite formed on the altered monazite-(Ce) surface (650 °C, 200 MPa, 6 days); h tabular crystals of allanite-(Ce) and minute hexagonal prisms of fluorcalciobritholite on the altered surface of the monazite-(Ce) (650 °C, 200 MPa, 6 days); i labradorite with developed K-feldspar rim (650 °C, 200 MPa, 6 days); j monazite-(Ce) partially replaced by an aggregate of REE-rich fluorapatite and fluorcalciobritholite (650 °C, 400 MPa, 6 days); k altered monazite-(Ce) grains overgrown by large crystals of allanite-(Ce) (650 °C, 600 MPa, 6 days); l remnants of altered monazite-(Ce) replaced by allanite-(Ce) (650 °C, 800 MPa, 6 days); m overgrowths of fluorcalciobritholite, allanite-(Ce), and REE-rich epidote grains on altered monazite-(Ce), showing a decreasing distribution of REE in the products as a function of the distance from the monazite-(Ce) going outwards (650 °C, 1000 MPa, 6 days); n bytownite formed at the expense of labradorite, showing a melt-filled porosity (750 °C, 400 MPa, 4 days); o monazite-(Ce) grains overgrown by fluorcalciobritholite and allanite-(Ce) (750 °C, 600 MPa, 4 days); p large crystals of allanite-(Ce) formed at the expense of monazite-(Ce) (750 °C, 1000 MPa, 4 days)
Plots of Pb versus Th + U contents in atoms per formula unit (apfu) showing similar patterns in the Burnet monazite-(Ce) (a), and monazite-(Ce) in products from all runs with 2 M Ca(OH)2 (b); c, d the monazite-(Ce) from runs with Na2Si2O5 + H2O at 450 °C and 550 °C shows significant depletion in Pb, as well as in Th and U, related to compositional alterations in the monazite-(Ce) via fluid-mediated coupled dissolution-reprecipitation; e, f the monazite-(Ce) from runs with Na2Si2O5 + H2O under temperature conditions of 650 °C and 750 °C presents similar patterns as the original Burnet monazite-(Ce)
Elongated, hexagonal prisms of REE-rich fluorapatite-fluorcalciobritholite (several μm to ~20 μm long) formed in most of the experiments (Fig. 3b, d, e, g, h, m and o). REE-rich fluorapatite-fluorcalciobritholite formed either on the monazite-(Ce) grain surface or as aggregates of crystals texturally unrelated to monazite-(Ce). Occasionally, REE-rich fluorapatite-fluorcalciobritholite partially replaces significant parts of the monazite-(Ce) grains forming pseudomorphs (Fig. 3j). The fluorcalciobritholite is characterized by high Y + REE and Th concentrations of 24.7–45.3 wt.% (Y + REE)2O3 and 3.1–10.3 wt.% ThO2 (supplementary Table S3). The U content is relatively low and ranges between 0.1 and 0.3 wt.% UO2. The fluorcalciobritholite phase represents a solid solution between fluorapatite – calciobritholite – fluorbritholite, due to coupled heterovalent substitutions on the M and T sites (cf. Pasero et al. 2010).
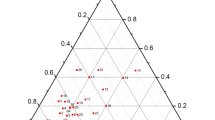
REE-rich epidote-allanite-(Ce) formed in all the runs. The grain size ranges from several to ~50 μm in low P-T runs, to large ~130 μm grains formed at the highest P-T conditions. From the BSE images, it is evident that the degree of monazite-(Ce) replacement by allanite-(Ce) increases with increasing P-T conditions. It should be noted that occasionally fluorcalciobritholite is present in the near vicinity of the monazite-(Ce) grain surface, while the allanite-(Ce) crystals form outer rims around the monazite-(Ce) grains (Fig. 3m). Allanite-(Ce) is characterized by an REE content reaching 19.7 wt.% REE2O3, and a Th concentration of 1.2 wt.% ThO2 on average (supplementary Table S4). The Al vs. Y + REE diagram (after Petrík et al. 1995) shows that the epidote-group minerals are compositionally solid solutions of epidote, clinozoisite, and allanite (Fig. 6). The REE-rich epidote-allanite-(Ce) from these experiments commonly demonstrate REE enrichment in the cores as relatively small areas that, when analyzed, may be contaminated with analyses from neighboring, lower REE content domains.
Al versus Y + REE diagrams (after Petrík et al. 1995), which present compositional variations in the epidote-group minerals formed due to monazite-(Ce) breakdown in the experimental products from runs under temperature conditions of 450 °C (a), 550 °C (b), 650 °C (c), and 750 °C (d)
The starting labradorite grains preserved in all runs occasionally show compositional alterations (Table 3; supplementary Table S5). These include albite rims formed at 450 °C, 800 MPa, and K-feldspar rims formed at 550 °C, 200 MPa; and 650 °C, 200, 400, 1000 MPa. In runs at 750 °C and 400, 800, 1000 MPa, the altered labradorite grain rims achieved a composition of An79-94Ab6-17Kfs1–9 with the porosity filled by melt (Fig. 3n; supplementary Table S5). The starting K-feldspar is present in the products, except runs at 750 °C, 200, 400, and 1000 MPa. The K-feldspar grains are partially to completely compositionally altered in most runs, with the altered domains depleted in Na and Ba relative to the starting Eifel sanidine (supplementary Table S5).
Muscovite was found in run products from the 450 °C, 400–1000 MPa, and the 550 °C, 400, 800, and 1000 MPa experiments (supplementary Table S6). Muscovite was not observed in the products of the remaining experiments. The biotite was observed to be unaltered in all the runs (supplementary Table S6).
Melt formed in experimental runs at 650 °C, 600–1000 MPa; and 750 °C, 200–1000 MPa (Figs. 1a and 3l–p; Table 3). The melt contains 63.56–69.84 wt.% SiO2, and is enriched in 0.81–1.28 wt.% F (supplementary Table S7). Concentrations of 12.91–14.99 wt.% Al2O3, 2.21–4.69 wt.% CaO, 1.33–1.80 wt.% K2O, and 0.14–0.70 wt.% Na2O define a peraluminous composition with an alumina saturation index (ASI) of 2.4–3.5. The CIPW Qz-Ab-Or diagram (supplementary Fig. S1) shows that the melt is dominated by quartz, which places the normative composition of the melt outside that of a granite.
Experiments with monazite-(Ce) and Na2Si2O5 + H2O
All experiments with Na2Si2O5 + H2O resulted in monazite-(Ce) breakdown (Figs. 7, 8 and 9; Table 3). At the end of each experiment, the remaining fluid in the capsule had a high pH (Table 1). Monazite-(Ce) in the 450 °C, 200–1000 MPa; and 550 °C, 200–600 MPa experiments developed dissolution pits on the surface and porosity within altered areas of the grains (Figs. 7a and 8). Some monazite-(Ce) grains are partially replaced by REE-rich steacyite (Figs. 7c and 9). In cross section, the monazite-(Ce) shows patchy zoning with irregular boundaries between bright and dark patches under high contrast BSE imaging. The bright domains have a composition similar to the starting Burnet monazite-(Ce), while the dark domains are depleted in Th, U, and Y (Fig. 8b–d), with almost complete removal of Pb (Fig. 4c and d), and subsequent enrichment in REE, particularly LREE (supplementary Table S1).
BSE images presenting products from experiments with monazite-(Ce) and Na2Si2O5 + H2O; a altered monazite-(Ce) with developed porosity, accompanied by secondary REE-rich steacyite and fluorcalciobritholite (450 °C, 200 MPa, 16 days); b altered K-feldspar with developed albite rim (450 °C, 200 MPa, 16 days); c altered monazite-(Ce) partially replaced by REE-rich steacyite (450 °C, 400 MPa, 18 days); d monazite-(Ce) partially replaced by REE-steacyite, which formed also individual crystals (450 °C, 600 MPa, 16 days); e large crystal of REE-rich steacyite with fluorcalciobritholite inclusions, formed at the expense of monazite-(Ce) (550 °C, 600 MPa, 8 days); f altered surface of monazite-(Ce) overgrown by fluorcalciobritholite and amphibole; the porosity shows a rough parallel orientation (550 °C, 800 MPa, 8 days); g, h altered monazite-(Ce) with a developed porosity oriented parallel to monazite-(Ce) cleavage planes along the rims with overgrowths of cheralite and fluorcalciobritholite (650 °C, 200 MPa, 6 days); i altered monazite-(Ce) overgrown by fluorcalciobritholite prisms and partially mantled by melt (650 °C, 200 MPa, 6 days); j albite partially replaced by K-feldspar with fluorcalciobritholite inclusions (650 °C, 400 MPa, 6 days); k, l strongly altered monazite-(Ce) with oriented porosity, presumably along a preferred crystallographic direction or plane; the porosity is filled with secondary cheralite and fluorcalciobritholite (650 °C, 1000 MPa, 6 days); m sector zoning of REE-rich fluorapatite-fluorcalciobritholite prismatic crystals formed in melt at the expense of monazite-(Ce) (650 °C, 1000 MPa, 6 days); n altered monazite-(Ce) surface overgrown by REE-rich fluorapatite-fluorcalciobritholite (750 °C, 200 MPa, 4 days); o, p altered monazite-(Ce) overgrown by fluorcalciobritholite mantled by melt (750 °C, 1000 MPa, 4 days)
BSE image and compositional WDS X-ray maps of altered monazite-(Ce) from the M12N-23 run at 450 °C and 600 MPa, with Na2Si2O5 + H2O; the monazite-(Ce) shows a porosity related to partial dissolution and patchy zoning formed due to fluid-mediated coupled dissolution-reprecipitation alteration; A altered, U unaltered
Monazite-(Ce), from the runs at P-T conditions of 550 °C, 800 and 1000 MPa, and 650–750 °C, 200–1000 MPa, was strongly reacted, showing large dissolution pits on the surface (Fig. 7f and h), and an oriented porosity across the grains, filled with other minerals. The monazite-(Ce) is overgrown by REE-rich fluorapatite-fluorcalciobritholite (Fig. 7g, h, n and o; supplementary Table S3). Occasionally, most of the monazite-(Ce) is replaced by REE-rich fluorapatite-fluorcalciobritholite formed in pores oriented parallel to each other along grain rims (Fig. 7g) or across whole grains (Fig. 7k and l). Minute, euhedral to elongated, anhedral grains of cheralite are also present as inclusions at the boundary between the REE-rich fluorapatite-fluorcalciobritholite and host monazite-(Ce) (Fig. 7l).
REE-rich fluorapatite-fluorcalciobritholite formed in all the experiments. Beside partially replacing and/or overgrowing monazite-(Ce), the REE-rich fluorapatite-fluorcalciobritholite also formed individual grains or grain aggregates. These individual grains are elongated hexagonal crystals, which range in size from several microns (lowest P-T runs), up to 100 μm long and ~8 μm across (in the moderate to highest P-T runs). Some grains are sector zoned with a fluorcalciobritholite composition in the center, and a REE-rich fluorapatite in triangular sectors expanding from the center of the grain towards the rim (Fig. 7m). The sector zoning is related to trace element incorporation as controlled by the crystal surface structure during growth of the apatite crystals, and subsequent enrichment of selected sectors in REE (Rakovan and Reeder 1996; Rakovan 2002). The crystals in cross section commonly have cores with a REE-rich fluorapatite-fluorcalciobritholite composition, and a fluorcalciobritholite rim (Fig. 7n).
REE-rich steacyite is present only in products from runs at 450 °C, 200–1000 MPa; and 550 °C, 200–600 MPa. It forms subhedral to euhedral tetragonal grains varying in size from 10 μm in the lower P-T runs up to ~100 μm in the 550 °C, 600 MPa run (Fig. 7a, d and e). REE-rich steacyite also partially replaces altered monazite-(Ce), irregularly filling the inner regions or, occasionally, filling roughly oriented, parallel channels (Figs. 7c and d and 9). The composition of REE-rich steacyite is variable, ranging from 10.24 to 27.06 wt.% ThO2, 0.72–2.14 wt.% UO2, and 1.93–9.64 wt.% (Y + REE)2O3 (supplementary Table S8).
Starting albite and K-feldspar are present in products of all runs at 450–650 °C and 200–1000 MPa, whereas none was preserved in the 750 °C experiments (Table 3). Secondary albite rims formed on K-feldspar grains in runs at 450 °C, 200–1000 MPa; 550 °C, 200–600 MPa; and 650 °C, 200 MPa (Fig. 7b; supplementary Table S5). Albite developed on K-feldspar rims in runs at 450–550 °C, 200–1000 MPa; and 650 °C, 200 and 600 MPa (Fig. 7j; supplementary Table S5).
Starting biotite and muscovite are found only in products from runs at 450 °C, 400, 600, and 1000 MPa; and 550 °C, 1000 MPa. The composition of the micas remained unaltered with respect to the starting composition (supplementary Table S6).
Amphibole formed in all runs with Na2Si2O5 + H2O. In experiments at 450, 550, and 650 °C, needle-like crystals of amphibole were too small for EPMA measurements (Fig. 7a, b, d–g, j and k). SEM-EDS analyses indicate that these are Na-(Fe-Mg) amphiboles, suggesting that Na2Si2O5 + H2O and the micas were the main sources of elements for the amphibole. The highest P-T runs, at 750 °C, 600–1000 MPa, promoted the formation of amphibole crystals up to 20 μm long and several microns across with a composition similar to ferritaramite (Leake et al. 1997; Fig. 7o; supplementary Table S9).
Melt formed in runs at 650 °C, 200 and 600–1000 MPa (Fig. 7g–i, k and m), and 750 °C, 200–1000 MPa (Fig. 7o and p). It dominates the experimental products from runs at 750 °C, in which the feldspars have totally disappeared. The melt contains 66.17–70.56 wt.% SiO2 and is characterized by a peraluminous composition (ASI = 3.1–5.9) defined by 13.40–15.65 wt.% Al2O3, 0.18–0.59 wt.% CaO, 0.88–2.18 wt.% Na2O, and 1.16–2.16 wt.% K2O (supplementary Table S7). The F content is 0.71–1.61 wt.%. The Qz-Ab-Or diagram (supplementary Fig. S1) demonstrates that the non-granitic melt is dominated by normative quartz with small amounts of normative albite and orthoclase.
Titanite also formed in most runs with melt (Table 3). Their small size did not allow for accurate EPMA measurements.
Experiments with xenotime-(Y) and 2 M Ca(OH)2
Xenotime-(Y) reacted in all runs with 2 M Ca(OH)2 (Table 4; Figs. 2c and 10). The remaining fluid in the capsule had a pH of 7 except in runs at 450 °C, 800–1000 MPa; and 650 °C, 1000 MPa, where the pH was high (Table 1). Xenotime-(Y) grains from the 450 °C, 200 MPa run show dissolution pits. A few, delicate, micron-sized crystals of Y-rich fluorcalciobritholite formed on the surface (Fig. 10a and b). Unreacted xenotime-(Y) surfaces are relatively common. In all other runs, the xenotime-(Y) is moderately to strongly reacted, with etching on the surface and overgrowth by numerous crystals of Y-rich fluorcalciobritholite (Fig. 10f and g). Strong dissolution occasionally resulted in the replacement of most of the xenotime-(Y) grain along grain fractures, by aggregates of secondary, Y-rich fluorcalciobritholite with only a few remnants of xenotime-(Y) remaining (Fig. 10d). Although experiments at 450 °C and 200 MPa resulted in the lowest degree of alteration, a few xenotime-(Y) grains occasionally remained unreacted even at 750 °C (Fig. 10p). Remnant xenotime-(Y) after the experiment showed no compositional alterations and preserved the composition of the original NWFP xenotime-(Y) (supplementary Table S10).
BSE images of run products from experiments with xenotime-(Y) and 2 M Ca(OH)2; a, b slightly altered xenotime-(Y) overgrown by small crystals of Y-rich fluorcalciobritholite (450 °C, 200 MPa, 16 days); c fractured xenotime-(Y) with Y-rich fluorcalciobritholite filling the fractures (450 °C, 400 MPa, 18 days); d Y-rich fluorcalciobritholite almost completely replacing altered xenotime-(Y) (450 °C, 800 MPa, 16 days); e labradorite partially replaced by K-feldspar (550 °C, 200 MPa, 12 days); f numerous hexagonal prisms of Y-rich fluorcalciobritholite formed on the surface of the xenotime-(Y) (550 °C, 400 MPa, 6 days); g hexagonal, sector-zoned, prismatic crystals of (Y,REE)-rich fluorapatite and Y-rich fluorcalciobritholite, which have formed on the xenotime-(Y) surface (550 °C, 800 MPa, 8 days); h (Y,HREE)-rich epidote formed at the expense of altered xenotime-(Y) (hexagonal prisms of Y-rich fluorcalciobritholite formed on the xenotime-(Y) surface (550 °C, 800 MPa, 8 days); i, j Y-rich fluorcalciobritholite overgrowths on altered xenotime-(Y) (650 °C, 200 MPa, 6 days); k (Y,HREE)-rich epidote formed at the expense of altered xenotime-(Y) in experiments at 650 °C, 800 MPa, 6 days (k) and 650 °C, 1000 MPa, 6 days (l); m altered xenotime-(Y) overgrown by Y-rich fluorcalciobritholite (650 °C, 1000 MPa, 6 days); n recrystallized muscovite in run at 650 °C, 1000 MPa, 6 days; o small crystals of pyroxene formed in melt (750 °C, 1000 MPa, 4 days); p slightly altered xenotime-(Y) with overgrowths of minute Y-rich fluorcalciobritholite crystals (750 °C, 200 MPa, 4 days); altered garnet is partially replaced by an aggregate of secondary low-Ti biotite and iron oxides
Yttrium-rich fluorcalciobritholite formed on the xenotime-(Y) grain surface in all the runs and commonly grew along fractures in the xenotime-(Y) grains. The elongated crystals of Y-rich fluorcalciobritholite, up to 20 μm long and a few microns thick, show zoning with (Y,REE)-rich fluorapatite cores and Y-rich fluorcalciobritholite rims (Fig. 10g). Due to the small size of the Y-rich fluorcalciobritholite crystals, EPMA measurements provide a mixed composition consisting of the cores and the rims (supplementary Table S11).
(Y,HREE)-rich epidote formed in three runs with 2 M Ca(OH)2, i.e. at 550 °C, 800 MPa; 650 °C, 800 MPa; and 650 °C, 1000 MPa. The (Y,HREE)-rich epidote forms elongated crystals, up to ~120 μm long and 20 μm thick, and is present as individual grains or grain aggregates not associated with xenotime-(Y) (Fig. 10h, k and l). The average Y and total REE contents in the (Y,HREE)-rich epidote are 4.06 wt.% Y2O3 and 1.71 wt.% REE2O3 in the 650 °C, 1000 MPa run; and 1.77 wt.% Y2O3 and 0.54 wt.% REE2O3 in the 650 °C, 800 MPa run (supplementary Table S12). According to the Al versus Y + REE plot in Fig. 11, the (Y,HREE)-rich epidote represents a solid solution between allanite, epidote, and clinozoisite.
Al versus Y + REE diagram (after Petrík et al. 1995), which shows compositional variations in the epidote-group minerals formed due to the alteration of xenotime-(Y) in experiments at 650 °C, 800 MPa, and 650 °C, 1000 MPa
Labradorite is present in the products of all runs at 450, 550, 650 °C; and in the products of the 750 °C, 200, 600 MPa runs. Labradorite was rimmed by albite in two runs at 550 °C, 800 and 1000 MPa (supplementary Table S13). Secondary K-feldspar rims formed at 550 °C, 200 and 400 MPa; and 550 °C, 200 and 1000 MPa. The starting labradorite was not found in the experimental products at 750 °C, 400, 800, and 1000 MPa, where the feldspar has a bytownite composition and melt-filled porosity. Remnants of the starting K-feldspar were preserved in all the runs at 450, 550, and 650 °C. K-feldspar was not found in the experimental products from the 750 °C runs.
Biotite is present in products from all runs at 450, 550, and 650 °C. In runs at 750 °C, secondary biotite formed (Fig. 10p), which is characterized by low Ti concentrations (0.09–0.45 wt.% TiO2) compared to the 4.09 wt.% TiO2 content in the starting biotite (supplementary Table S14). Muscovite is preserved in all runs at 450 °C, three runs at 550 °C, 600–1000 MPa, and two runs at 650 °C, 600 and 800 MPa. Small, 10–50 μm flakes of secondary muscovite formed at 650 °C, 1000 MPa. In the remaining experimental products, i.e. 550 °C, 200 and 400 MPa; 650 °C, 200 and 400 MPa; and 750 °C, 200–1000 MPa, muscovite disappeared.
Garnet was preserved unreacted in all runs at 450, 550, and 650 °C (supplementary Table S15). All experiments at 750 °C, 200–1000 MPa resulted in the breakdown of the garnet rims with partial replacement by low-Ti biotite (Fig. 10p). The remaining garnet cores preserved the original composition. Although the xenotime-(Y) experiments included garnet to test the partitioning of Y between xenotime-(Y) and garnet in terms of geothermometric applications, no change in Y or HREE enrichment in garnet, compared to the original garnet, was found. This indicates that garnet was not a stable phase in the 750 °C experiments since it did not recrystallize and incorporate Y, HREE but rather broke down to biotite. In contrast, it was non-reactive in the lower temperature experiments either because it was a stable phase or more likely due to lower reaction rates such that the garnet was metastable.
Clinopyroxene formed in runs at 750 °C, 200–1000 MPa, where it occurs as euhedral crystals up to 4 × 20 μm in size (Fig. 10o). Titanite is found only in the high temperature runs with 2 M Ca(OH)2. It incorporates up to 3.91 wt.% Y2O3 and 2.53 wt.% REE2O3 (based on EDS analyses), presumably from the xenotime-(Y).
Melt formed in runs at 650 °C, 200, 800, and 1000 MPa (Fig. 10i and m); and in runs at 750 °C, 200–1000 MPa (Fig. 10o and p). The melt composition consisted of 64.26–71.45 wt.% SiO2, 10.58–14.79 wt.% Al2O3, 0.20–4.66 wt.% CaO, 0.08–1.32 wt.% Na2O, and 0.77–1.94 wt.% K2O, which defines a peraluminous character for the melt with an ASI = 2.6–4.0 (supplementary Table S16). The F content is 0.60–1.31 wt.%. Similar to experiments with monazite-(Ce), the non-granitic melt is dominated by normative quartz (supplementary Fig. S1).
Experiments with xenotime-(Y) and Na2Si2O5 + H2O
All experiments with Na2Si2O5 + H2O resulted in xenotime-(Y) breakdown and the formation of new phases (Table 3; Figs. 2, 12 and 13). The remaining fluid had a high pH, except two runs in which the fluid had a neutral pH (450 °C, 200 MPa and 550 °C, 400 MPa; Table 1). Xenotime-(Y) shows dissolution pits and some etching on the surface. In all runs numerous crystals of Y-rich fluorcalciobritholite formed on the xenotime-(Y) surface as masses of individual grains, which overgrew and partially replaced the xenotime-(Y) both on the surface and along internal fractures. In most of the 450–550 °C experiments, the xenotime-(Y) is occasionally preserved completely unreacted. In all runs, the remnant xenotime-(Y) reflects the composition of the starting NWFP xenotime-(Y), showing no compositional alteration (supplementary Table S10).
BSE images of run products from experiments with xenotime-(Y) and Na2Si2O5 + H2O; a aggregate of Y-rich fluorcalciobritholite prisms from the experiment at 450 °C, 400 MPa, and 18 days; b xenotime-(Y) crystal showing delicate dissolution and formation of some Y-rich fluorcalciobritholite crystals (450 °C, 600 MPa, 16 days); c altered garnet partially replaced by secondary mica (550 °C, 200 MPa, 12 days); d hexagonal prismatic crystals of Y-rich fluorcalciobritholite formed on the altered xenotime-(Y) surface (550 °C, 400 MPa, 6 days); e altered albite almost completely replaced by K-feldspar with inclusions of Y-rich fluorcalciobritholite with REE-rich fluorapatite cores (550 °C, 600 MPa, 8 days); f aggregate of hexagonal prismatic, crystals of Y-rich fluorcalciobritholite (650 °C, 200 MPa, 6 days); g zoned crystals of REE-rich fluorapatite and Y-rich fluorcalciobritholite in melt (650 °C, 200 MPa, 6 days); h sector-zoned crystals of REE-rich fluorapatite and Y-rich fluorcalciobritholite, partially overgrowing altered xenotime-(Y) (650 °C, 200 MPa, 6 days); i altered xenotime-(Y) partially replaced by REE-rich fluorapatite and Y-rich fluorcalciobritholite (650 °C, 600 MPa, 6 days); j Y-rich fluorcalciobritholite almost completely replacing altered xenotime-(Y) (650 °C, 1000 MPa, 6 days); k secondary biotite formed at the expense of garnet breakdown (650 °C, 1000 MPa, 6 days); l large crystals of REE-rich titanite (650 °C, 1000 MPa, 6 days); m altered xenotime-(Y) overgrown by Y-rich fluorcalciobritholite (750 °C, 400 MPa, 4 days); n secondary, low-Ti biotite formed at the expense of garnet breakdown (750 °C, 600 MPa, 4 days); o altered xenotime-(Y) overgrown by Y-rich fluorcalciobritholite (750 °C, 800 MPa, 4 days); p secondary, low-Ti biotite formed at the expense of garnet breakdown (750 °C, 1000 MPa, 4 days)
In all the runs, Y-rich fluorcalciobritholite formed euhedral to subhedral, hexagonal crystals whose size varied from a few microns in length to several microns thick and up to 100 μm long. The size of crystals in the reaction rims was independent with respect to temperature conditions, whereas the amount of Y-rich fluorcalciobritholite formed increased with increasing temperature. Beside overgrowth and partial replacement of xenotime-(Y), Y-rich fluorcalciobritholite formed aggregates of individual grains commonly dispersed in the melt in the 650–750 °C runs (Fig. 12o). The Y-rich fluorcalciobritholite commonly shows sector zoning, related to crystal surface structure control of trace element incorporation (cf. Rakovan and Reeder 1996; Rakovan 2002), with (Y,REE)-rich fluorapatite cores and Y-rich fluorcalciobritholite rims (Fig. 12g and h; supplementary Table S11).
Albite was preserved in all the runs at 450, 550, and 650 °C, but not in the 750 °C experiments. K-feldspar rims formed on albite at 450 °C, 200–800 MPa; and at 550 °C, 200, 600, and 800 MPa (supplementary Table S13). K-feldspar was found in the products from all 450, 550, and 650 °C runs, and in one run at 750 °C, 600 MPa. Albite rims developed on K-feldspar in almost all the runs, i.e. 450 °C, 200–800 MPa; 550 °C, 200–800 MPa; 650 °C, 400, 800, and 1000 MPa; and 750 °C, 600 MPa (supplementary Table S13).
Biotite is present unaltered in products from most runs. Only some starting biotite was preserved in the products from the 750 °C temperature experiments (Table 3). Secondary low-Ti biotite formed aggregates of small flakes in runs at 550 °C, 200 MPa; 650 °C, 200–1000 MPa; and 750 °C, 200–1000 MPa (Fig. 12k and p; supplementary Table S14). Muscovite was present only in the products of four runs at 450 °C, 200, 600, 800, and 1000 MPa, whereas it was not found in the products from the remaining experiments.
Garnet is preserved unaltered only in runs at 450 °C, 200–1000 MPa. In runs at 550 °C, the garnet grains reacted along the rims and fractures, with partial replacement by secondary low-Ti biotite, chlorite, and/or Na-K feldspar (Fig. 12c). The remaining garnet preserved the original composition (supplementary Table S15) indicating no recrystallization had occurred during partial alteration to biotite (supplementary Fig. S2). Garnet was not found in the products from runs at 650 and 750 °C. Experiments under these temperature conditions resulted in complete breakdown of garnet judging from the presence of aggregates of secondary, low-Ti biotite and/or secondary albite occasionally forming post-garnet pseudomorphs.
Amphibole formed in all the runs. In runs at 450, 550, and 650 °C, needle-like crystals of Na-(Fe-Mg) amphiboles were identified using EDS analysis (Fig. 12a, b, g, i and l). Experiments at 750 °C promoted the formation of amphibole grains up to 40 μm across, with a composition similar to ferritaramite (Leake et al. 1997; Fig. 12o and p; supplementary Table S17).
Titanite was found in the products from experiments at 650 °C, 200, 600, 800, and 1000 MPa; and 750 °C, 200 and 1000 MPa. Commonly it forms small grains, although occasional grains, up to 150 μm long and 40 μm across, are present (Fig. 12l). The titanite contains 5.07–6.30 wt.% (Y + REE)2O3 (supplementary Table S18).
Melt formed in runs at 650 °C, 200, 600, 800, and 1000 MPa (Fig. 12g, h, k and l), and 750 °C, 200–1000 MPa (Fig. 12m–p). The non-granitic, peraluminous melt (ASI = 1.9–4.7) contains 62.07–70.71 wt.% SiO2, 8.85–16.98 wt.% Al2O3, 0.15–1.96 wt.% CaO, 1.34–3.36 wt.% Na2O, and 1.46–2.07 K2O (supplementary Table S16). The F content is 0.86–2.06 wt.%. The non-granitic melt is dominated by normative quartz with minor normative albite and orthoclase according to the Qz-Ab-Or diagram (supplementary Fig. S1).
Discussion
Interpretation of the experimental results: runs with monazite-(Ce)
Similar to what was found in Budzyń et al. (2011), the results from these experiments demonstrate that the two fluids used were highly reactive with respect to both monazite-(Ce) and xenotime-(Y) over the broad P-T range considered. The neutral pH of the fluid in the 2 M Ca(OH)2 runs suggests that all of the Ca(OH)2 was used up as the main source of Ca for the apatites and REE-rich epidote-allanite-(Ce). This was the case except in the high pressure experiments at 450–550 °C, where the remaining fluid still maintained a high pH suggesting slower reaction rates. In contrast, the high pH character of the remaining fluid from runs with Na2Si2O5 + H2O indicates that not all the Na supplied by the Na2Si2O5 was used up.
Conversion of monazite-(Ce) to REE-rich epidote and allanite-(Ce), over the entire P-T range considered for the 2 M Ca(OH)2 experiments, indicates that the significantly increased Ca bulk content (10.08 wt.% CaO; Table 2), compared to natural rocks of metapelitic to granitic composition [e.g. 2.17 wt.% CaO for pelites; Shaw (1956)], was high enough to expand the stability field of allanite-(Ce) to at least 750 °C and 1000 MPa. At the same time, the relative size and abundance of the allanite-(Ce) grains increased with increasing temperature, though this was less dependent on pressure. Here, monazite-(Ce) served as the source of REE for the REE-epidote and allanite-(Ce). The chemical variability in either product phase is related to the coupled substitutions REE3+ + Fe2+ = Ca2+ + Fe3+ and REE3+ + Fe2+ = Ca2+ + Al3+ (Petrík et al. 1995). The data indicate high variations in the allanite-(Ce) REE content (Fig. 6), with significant enrichment in the 750 °C, 200 MPa run.
REE-rich fluorapatite-fluorcalciobritholite also formed in most of the experiments due to the presence of monazite-(Ce) and CaF2. Differences in the apatite mineral chemistry and abundances in the experimental products are mainly related to the degree of monazite-(Ce) breakdown and the growth ratio of these phases, which are themselves related to increasing reaction kinetics with increasing temperature and pressure. Monazite-(Ce) breakdown to REE-rich fluorapatite-fluorcalciobritholite, and REE-epidote-allanite-(Ce), most likely occurred via the reaction monazite-(Ce) + annite + quartz + Ca + F (in fluid-I) = fluorapatite + allanite-(Ce) + fluid-II (Broska and Siman 1998). Although in most of the runs biotite appears unaltered, some biotite, apart from the muscovite and feldspars, must have reacted as the primary source of Fe for the REE-epidote and allanite-(Ce).
The unaltered monazite-(Ce) cores demonstrate that the interior of the grains was shielded from fluid-aided alteration, except for partial dissolution near the surface, which occasionally progressed towards the cores of some of the monazite-(Ce) grains from the 650 and 750 °C runs (Fig. 3l). These alteration textures are the result of coupled dissolution-reprecipitation, which is induced by a fluid front infiltrating the parent phase, and leaving behind a chemically altered phase (Putnis 2002, 2009; Harlov et al. 2011; Putnis and Austrheim 2012). In nature, coupled dissolution-reprecipitation is widely recognized as being responsible for mineral replacement reactions and pseudomorphism (Putnis 2002, 2009). The pseudomorphic replacement of the monazite-(Ce) by REE-rich fluorapatite-fluorcalciobritholite required a supply of external components (Si, Ca, F) from the fluid into the altered areas of the monazite-(Ce). The textural setting of the reaction phases replacing monazite-(Ce) (Fig. 3e, g, h and m) indicates that local REE, Th, and U, released from the altered monazite-(Ce), were incorporated into the newly formed apatites.
Partial replacement of monazite-(Ce) grains in these experiments, occasionally takes the form of a succession of REE-bearing minerals in the form of a corona (Fig. 3m). In this succession, REE-rich fluorapatite-fluorcalciobritholite forms in close contact to the monazite-(Ce), followed by allanite-(Ce). The outer part of corona-like texture consists of REE-rich epidote overgrowing the allanite-(Ce). The corona demonstrates the limited distance over which REE mobilization occurs as reflected by the decreasing REE content in the sequence of secondary phases surrounding the monazite-(Ce). Similar conclusions have been made for natural examples of the partial- to complete replacement of monazite by a sequence of apatite, allanite, and epidote zones in metamorphosed granitic rocks and pelites (e.g. Broska and Siman 1998; Finger et al. 1998; Broska et al. 2005; Majka and Budzyń 2006; Petrík et al. 2006; Budzyń et al. 2010; Ondrejka et al. 2012; Budzyń and Jastrzębski 2016; Finger et al. 2016; Lo Pò et al. 2016). The occurrence of ThSiO4 or ThO2 only in the inner zone mantling the monazite, as inclusions in apatite (Finger et al. 1998; Ondrejka et al. 2012; Budzyń et al. 2010), indicates that Th transport occurs over a much more limited distance from the monazite compared to the REEs.
The similar form and size of REE-rich fluorapatite-fluorcalciobritholite crystals in all the experiments indicate that their growth is relatively independent of the P-T conditions, although higher amounts of these crystals formed in high temperature runs with Na2Si2O5 + H2O, where REE-rich fluorapatite-fluorcalciobritholite were the primary phases replacing monazite-(Ce) (cf. Figs. 7a and e vs. 7f–p). REE enrichment in the product apatites is the result of two coupled substitution reactions REE3+ + Si4+ = Ca2+ + P5+ and REE3+ + Na+ = 2 Ca2+ (Pan and Fleet 2002). REE substitution into apatites from the 2 M Ca(OH)2 experiments is dominated by Si (12.23–15.59 wt.% SiO2, <0.01 wt.% Na2O), whereas both REE substitutions occur in apatites from the runs with Na2Si2O5 + H2O (0.82–15.62 wt.% SiO2, 0.58–4.52 wt.% Na2O; supplementary Table S3). An increase in the REE concentration in apatite from the 2 M Ca(OH)2 experiments from 27.66 to 44.09 wt.% (Y + REE)2O3 in runs from 450 to 750 °C (supplementary Table S3) indicates a direct correlation with increasing temperature conditions. The apatites from the experiments with Na2Si2O5 + H2O show similar increase from 14.25 to 39.47 wt.% (Y + REE)2O3 in the 450–550 °C runs to 36.94–42.60 wt.% (Y + REE)2O3 in the 650–750 °C runs (supplementary Table S3). These results also support those of Krenn et al. (2012) in a series of monazite-allanite-fluorapatite experiments. Krenn et al. (2012) documented an increase in the Na2O-SiO2-(Y + REE)2O3 content in fluorapatite [0.17–4.86 wt.% SiO2, 0.47–3.38 wt.% Na2O, 2.53–29.19 wt.% (Y + REE)2O3] with an increase in the pressure and temperature from 0.5 to 4 GPa and 650 to 900 °C. These experiments, along with the results from this study, support previous work suggesting that high activities of Si and Na in the fluid/melt promote the incorporation of REEs into apatite (Pan and Fleet 2002).
In the Na2Si2O5 + H2O experiments, the altered domains in the monazite-(Ce) are characterized by a fluid-induced, pervasive, interconnected nano- and micro-porosity (Figs. 7a and c and 8), which is characteristic of a coupled dissolution-reprecipitation process (Putnis 2002, 2009; Harlov et al. 2007, 2011; Putnis and Austrheim 2012). These domains are also characterized by depletion in Th and U, and the almost complete removal of Pb (supplementary Table S1) in runs at 450 °C, 200–1000 MPa; and 550 °C, 200–600 MPa. In runs at higher P-T conditions, a fluid-induced porosity occurs, along with numerous tiny inclusions of cheralite, apparently oriented parallel to a specific crystallographic plane in the monazite-(Ce) (Fig. 7g, k and l). The Burnet monazite-(Ce) (11.43 wt.% ThO2, 0.34 wt.% UO2, 0.59 wt.% PbO), used in these experiments, is relatively rich in Th, which would imply a greater degree of metamictization, and therefore a greater susceptibility to fluid-aided alteration via coupled dissolution-reprecipitation.
In the experiments utilizing Na2Si2O5 + H2O, altered domains in the monazite-(Ce) from the 450–550 °C experiments supplied Th and REE to the REE-rich steacyite, REE-rich fluorapatite, and Y-rich fluorcalciobritholite. The textural setting indicates that the Th was either transported by the fluid to form individual crystals of REE-rich steacyite, or that transport of Th was limited and REE-rich steacyite partially replaced monazite-(Ce). In the experimental replacement of monazite-(Ce), mobility of Th has also been documented by the formation of REE-rich steacyite at 250–350 °C and 200–400 MPa by Budzyń et al. (2015) or turkestanite {(K,□)(Ca,Na)2(Th,U)[Si8O20] nH2O} at 450–500 °C, 450–610 MPa (Budzyń et al. 2011). Natural occurrences of steacyite and turkestanite are known from several alkaline complexes (Pautov et al. 1997, 2004; Kabalov et al. 1998; Petersen et al. 1999; Vilalva and Vlach 2010).
In Na2Si2O5 + H2O experiments at 650–750 °C, reacted areas in the monazite-(Ce) are characterized by a REE-rich fluorapatite-, Y-rich fluorcalciobritholite- and cheralite-filled lenticular porosity (Fig. 7f, g, k and l). The lenticular shape of the pores (Fig. 7l) suggests preferred dissolution along a certain crystallographic direction (or crystallographic plane) in the monazite-(Ce). Cheralite formation, along inner pore surfaces in the reacted monazite-(Ce), indicates that Th transport occurred over only short distances, whereas REE were mobilized across the pore volumes to form REE-rich fluorapatite and Y-rich fluorcalciobritholite.
The experiments also document the albitization of K-feldspar grain rims (Figs. 3i and 7b and j) via coupled dissolution-reprecipitation (e.g. Putnis 2002, 2009; Norberg et al. 2011, 2013). The re-equilibrated albitic domains in the K-feldspar are characterized by both a micro-porosity and the presence of REE-rich fluorapatite or fluorcalciobritholite inclusions. These suggest fluid aided mass transfer of P, Ca, and REE from the outside the feldspar structure into the albitized regions, which would have then allowed for these inclusions to form.
Previous experimental data have shown that monazite solubility increases in felsic melts under water-saturated conditions (Montel 1993), whereas it is low in peraluminous melts (Montel 1986; Wolf and London 1995). In a series of monazite alteration experiments, Harlov et al. (2011) showed that an increase in the Al content in the fluid/melt limits the reactivity of monazite. In the experiments from this study, feldspars and micas partially to completely broke down to provide the components for a peraluminous melt in the high temperature experiments (supplementary Tables S7, S16). However no decrease in the reactivity of monazite-(Ce) was seen despite the melt composition. This would imply that the dissolution and reactivity of monazite is mostly related to the actual fluid/melt chemistry with no direct dependence on the Al content.
The results from this study have implications with regard to monazite Th-U-Pb geochronology. Previous experiments have shown that fluid-aided coupled dissolution-reprecipitation alteration can disturb the Th-U-Pb system in monazite (Teufel and Heinrich 1997; Seydoux-Guillaume et al. 2002; Harlov et al. 2007, 2011; Harlov and Hetherington 2010; Hetherington et al. 2010; Grand’Homme et al. 2016). In experiments utilizing Na2Si2O5 + H2O, partial loss of Pb in the altered areas of the monazite-(Ce) was seen at 250–350 °C, 200–400 MPa (Budzyń et al. 2015). Here, for the same mineral assemblage and fluid (Na2Si2O5 + H2O), Pb in altered areas of the monazite-(Ce) is almost completely removed for experiments at 450 °C, 200–1000 MPa and 550 °C, 200–600 MPa (Fig. 4c and d; supplementary Table S1), which is in good agreement with similar results reported by Williams et al. (2011). These temperatures are far below the diffusional closure temperature for Pb (i.e. above 800–900 °C; Cherniak et al. 2004; Gardes et al. 2006). However in both the 2 M Ca(OH)2 and Na2Si2O5 + H2O experiments, the unreacted monazite-(Ce) cores show no evidence of having experienced solid-state diffusion over the entire range of temperatures and pressures considered in these experiments. This indicates that significant dissolution, alteration, and partial replacement of the monazite-(Ce) grain by altered monazite-(Ce) or new phases does not affect the composition of the core over the duration of the experiment, even under conditions of 650–750 °C and subsequent partial melting of the initial mineral mix. Instead, the monazite-(Ce) core (or unaltered domains in patchy zoned grains) retains its original composition, within EPMA detection limits, in an alkali-rich fluid environment in a P-T window from 450 to at least 750 °C, and 200 to 1000 MPa.
Interpretation of the experimental results: runs with xenotime-(Y)
Similar to the experiments involving monazite-(Ce), the experiments involving xenotime-(Y) indicate that it is highly reactive with both 2 M Ca(OH)2 and Na2Si2O5 + H2O and that the reaction products are relatively similar to those found for experiments involving monazite-(Ce) over the same P-T range (Tables 1 and 3). Yttrium-rich fluorcalciobritholite always formed either as individual crystals or, more commonly, formed overgrowths on xenotime-(Y) grains indicating relatively high mobilization of Y + REE, more specifically Y + HREE. However, Y + REE abundances in Y-rich fluorcalciobritholite show no obvious pattern relative to the P-T conditions (supplementary Table S11) unlike the apatites from the experiments involving monazite-(Ce). The higher abundance of unaltered xenotime-(Y) grains in the low temperature run products, compared to the paucity of unreacted grains in high P-T runs, suggests that xenotime-(Y) reactivity is relatively temperature dependent. Some experiments also document partial replacement of the xenotime-(Y) by Y-rich fluorcalciobritholite, which could be interpreted as having been driven, at least partially, by coupled dissolution-reprecipitation (Figs. 10c and i and 12i and m).
Previous experiments involving the metasomatic alteration of xenotime-(Y) in the presence of alkali-bearing fluids (Na2Si2O5 + H2O, NaF + H2O), along with the addition of SiO2, Al2O3, ThO2, and ThSiO4, has documented enrichment in ThSiO4 in altered areas of the xenotime-(Y) via coupled dissolution-reprecipitation (Harlov and Wirth 2012). More recent experiments at 250–350 °C and 200–400 MPa have also resulted in the strong alteration of xenotime-(Y) in the presence of Na2Si2O5 + H2O (Budzyń and Kozub-Budzyń 2015). In the experiments from this study, altered areas within the xenotime-(Y), enriched or depleted in REE, were not observed. This indicates that the components released from the altered xenotime-(Y) were incorporated primarily into the Y-rich fluorcalciobritholite.
Although the 2 M Ca(OH)2 fluid was highly aggressive in all runs, only three runs (550 °C, 800 MPa; 650 °C, 800 and 1000 MPa) resulted in the formation of (Y,HREE)-rich epidote, which suggests certain differences in the stability relations between xenotime-(Y) – (Y,HREE)-rich epidote compared to monazite – allanite. The experimental data first indicate that the P-T conditions are a crucial factor in controlling the relative stabilities between xenotime-(Y) and (Y,HREE)-rich epidote. Furthermore, the (Y + REE)2O3 content in (Y,HREE)-rich epidote increases from 2.31 wt.% at 800 MPa to 5.76 wt.% at 1000 MPa (supplementary Table S12). This suggests that the substitution of these elements is to some degree a function of pressure. However, because of the small crystal size, accurate analyses of (Y,HREE)-rich epidote could only be obtained in two runs. Hence these experimental results should be used only as a guide when applied to natural interpretations.
Monazite – allanite – fluorapatite and xenotime-(Y) – (Y,HREE)-rich epidote – (Y,HREE)-rich fluorapatite assemblages in nature
The influence of the Ca activity on the stability relations between monazite, allanite, and fluorapatite has been previously described in various rocks of granitic composition (Lee and Dodge 1964; Lee and Bastron 1967; Bea 1996; Bingen et al. 1996; Broska and Siman 1998; Finger et al. 1998; Broska et al. 2000, 2005; Claeson 2002) and metapelites (Janots et al. 2008, 2009). Temperature conditions controlling monazite–allanite phase relations have been constrained to ~400–450 °C in metapelites, where progressive metamorphic reactions involve the breakdown of monazite to form allanite (Smith and Barreiro 1990; Wing et al. 2003; Janots et al. 2006, 2008). In some cases these breakdown reactions were later followed by a reversed reaction resulting in the formation of monazite at the andalusite or kyanite isograd (Wing et al. 2003) or under temperature conditions of ~550–650 °C (Janots et al. 2008; Finger et al. 2016). Thermodynamic modeling by Spear (2010), utilizing Shaw’s (1956) average pelite bulk composition, predicted that increasing CaO from 2.17 to 4.34 wt.% would shift the allanite-(Ce) to monazite-(Ce) transition from 400 °C at 200 MPa and 550 °C at 1000 MPa, to 480 °C at 200 MPa and 750 °C at 1000 MPa (Fig. 6 in Spear 2010).
The experiments with 2 M Ca(OH)2 confirm that a high CaO bulk content (10.08 wt.%, Table 2) shifts the stability of allanite-(Ce) towards higher temperatures and pressures up to the limits set by these experiments, i.e. 750 °C and 1000 MPa. In contrast, the experiments with Na2Si2O5 + H2O, also characterized by a high 5.91 wt.% CaO bulk content, produced no REE-rich epidote or allanite-(Ce) indicating that, depending on the bulk composition, a high Ca content is not necessarily one of the main factors controlling the monazite-(Ce)-to-allanite-(Ce) transition. Spear’s (2010) thermodynamic modeling also demonstrated the significant impact of Al, showing that the temperature of the allanite-(Ce)-to-monazite-(Ce) transition increases with a decrease in the Al2O3 bulk content. Hence, the increased stability of allanite-(Ce) in the experiments to high temperatures might also have been promoted by the low Al2O3 bulk content [10.04 and 7.74 wt.% in experimental sets (1) and (2), respectively, Table 2] compared to 16.57 wt.% Al2O3 in Shaw’s average pelite.
However, in general, the whole rock activity of Na, relative to that of Ca, appears to be one of the major factors in controlling the relative stabilities of monazite vs. allanite and xenotime-(Y) vs. (Y,HREE)-rich epidote. For example, in amphibolite-facies Alpine metapelites, both temperature and the bulk CaO/Na2O ratio control the relative stabilities of allanite, monazite, and xenotime-(Y) (Janots et al. 2008). An increase in temperature to above 586 °C, during progressive metamorphism, resulted in the total breakdown of allanite and the formation of monazite and xenotime-(Y) for CaO/Na2O < 0.54, while allanite remained stable up to 610 °C for CaO/Na2O > 0.93 (Janots et al. 2008). In this study, the CaO/Na2O ratios in the experimental runs with monazite-(Ce) and xenotime-(Y) and 2 M Ca(OH)2 were 13.3 and 13.7, respectively (Table 2), which is much higher than that found to the Alpine metapelites (0.09–3.32 CaO/Na2O; Janots et al. 2008). Consequently, the allanite-(Ce) stability field was shifted to higher temperatures up to and probably higher than 750 °C. In contrast, neither epidote nor allanite-(Ce) formed in the monazite-(Ce) and xenotime-(Y) Na2Si2O5 + H2O experiments with significantly lower CaO/Na2O ratios of 1.0, though still high concentrations of 5.91 and 5.56 wt.% CaO, and low concentrations of 7.74 and 9.25 wt.% Al2O3 (Table 2), respectively, compared to Spear’s (2010) model. The results from these experiments demonstrate that while the CaO/Na2O ratio is important, the total whole rock composition is also important. This implies that the monazite-(Ce)-allanite-(Ce)-fluorapatite thermodynamic model of Spear (2010) should be only applied to those rocks whose whole rock composition approximates Shaw’s (1956) average pelite bulk composition.
Compared to numerous studies on monazite, little has been described regarding the alteration of xenotime-(Y) in nature. The fluid-mediated breakdown and replacement of xenotime-(Y) by (Y,HREE)-rich fluorapatite and (Y,HREE)-rich epidote have been documented in metamorphosed granitic rocks (Broska et al. 2005). However, the P-T conditions of such alterations remain a matter of some speculation. Fluid-aided, compositional alteration of xenotime-(Y) at 450 °C has been described in a quartz-muscovite-chlorite schist, which included metamorphic overgrowths on detrital xenotime-(Y) (Rasmussen et al. 2011). In Alpine metapelites, xenotime-(Y) has been described as being replaced by HREE-rich epidote and fluorapatite at 450–528 °C, followed by a reverse reaction at 560–610 °C, depending on the CaO bulk content and Ca/Na ratios (Janots et al. 2008). These observations are confirmed by the breakdown of xenotime-(Y) to (Y,HREE)-rich epidote in the same temperature range in the 2 M Ca(OH)2 experiments (Fig. 2c; Table 3). In general, the results from the xenotime-(Y) experiments in this study could potentially provide important data for petrological constraints regarding xenotime-(Y) breakdown to (Y,HREE)-rich apatite and (Y,HREE)-rich epidote during the formation of the Veporic nappe system in the Tatric unit, Tribeč Mountains (Broska et al. 2014).
The partitioning of REE and actinides between monazite-(Ce), fluorapatite, and fluorcalciobritholite played a significant role in the experiments. In nature, fluorapatite is an important carrier of REE and actinides with increasing metamorphic grade (Bingen et al. 1996; Spear and Pyle 2002; Finger and Krenn 2007; Hansen and Harlov 2007; Krenn et al. 2009, 2012). The results from this study show that REE can substitute into fluorapatite in extensive amounts under conditions replicating medium- to high-grade metamorphism. However, only minor amounts of REE-rich fluorapatite formed in the experiments, whereas fluorcalciobritholite or Y-rich fluorcalciobritholite were the main products due to high Y + REE bulk content. In nature, these phases are relatively uncommon, and have been reported in post-magmatic assemblages including britholite-(Y), fluorbritholite-(Y), fluorcalciobritholite, secondary monazite, and REE carbonate minerals formed during low-temperature alteration of primary magmatic fluorapatite and allanite-(Ce) in A-type granites from the Western Carpathians in Slovakia (Uher et al. 2015). Britholites also occur in various alkaline rocks such as alkali granites (Lyalina et al. 2014), pegmatites associated with peralkaline granites (Pekov et al. 2011), nepheline syenites (Liferovich and Mitchell 2006; Dumańska-Słowik et al. 2012), and carbonatites (Ahijado et al. 2005; Doroshkevich et al. 2009). The predominance of fluorcalciobritholite or Y-rich fluorcalciobritholite in the products from these experiments appears to have been related to the high percentage of monazite-(Ce) and xenotime-(Y) in the starting mineral mix, as well as the high alkalinity of the starting bulk composition, which includes the fluids used, i.e. 2 M Ca(OH)2 and Na2Si2O5 + H2O. High compositional variations in the REE-rich fluorapatite and fluorcalciobritholite prevent us from making speculations regarding P-T control of REE substitution in the fluorapatite and britholite phases.
The experimental results from this study should be treated with caution when applied to interpretations of natural processes. This is mainly due to the high Y + REE bulk content [8.2–9.4 wt.% (Y + REE)2O3; Table 2] due to the predominance of monazite-(Ce) or xenotime-(Y) in the system, the specific whole rock bulk composition of the starting mineral mix + fluids, and the fact that fluids are in excess compared to natural rock systems. Future experiments should more closely replicate the bulk rock composition used by Spear (2010), i.e. 2.17 to 4.34 wt.% CaO and 1.73 wt.% Na2O. This will require significantly lowering the bulk rock CaO and Na2O and raising the bulk rock Al2O3 over the P-T range considered by Spear (2010). Further lowering of the bulk CaO/Na2O ratio to less than 0.5, which corresponds to Alpine metapelites of Janots et al. (2008), should also help to increase the stability field of monazite relative to that of allanite and hopefully go some way in helping to experimentally map out the allanite-monazite transition as a function of P-T-X.
References
Ahijado A, Casillas R, Nagy G, Fernández C (2005) Sr-rich minerals in a carbonatite skarn, Fuerteventura, Canary Islands (Spain). Mineral Petrol 84:107–127
Åmli R, Griffin W (1975) Microprobe analysis of REE minerals using empirical correction factors. Am Mineral 60:599–606
Anderson JL, Bender EE (1989) Nature and origin of Proterozoic A-type granitic magmatism in the southwestern United States of America. Lithos 23:19–52
Andrehs G, Heinrich W (1998) Experimental determination of REE distributions between monazite and xenotime: potential for temperature-calibrated geochronology. Chem Geol 149:83–96
Aranovich LY, Newton RC, Manning CE (2013) Brine-assisted anatexis: experimental melting in the system haplogranite–H2O–NaCl–KCl at deep-crustal conditions. Earth Planet Sci Lett 374:111–120
Bea F (1996) Residence of REE, Y, Th and U in granites and crustal protoliths; implications for the chemistry of crustal melts. J Petrol 37:521–552
Bingen B, Demaiffe D, Hertogen J (1996) Redistribution of rare earth elements, thorium, and uranium over accessory minerals in the course of amphibolite to granulite facies metamorphism: the role of apatite and monazite in orthogneisses from southwestern Norway. Geochim Cosmochim Acta 60(8):1341–1354
Broska I, Petrík I (2015) Variscan thrusting in I- and S-type granitic rocks of the Tribeč Mountains, Western Carpathians (Slovakia): evidence from mineral compositions and monazite dating. Geol Carpath 66(6):455–471
Broska I, Siman P (1998) The breakdown of monazite in the West-Carpathian Veporic orthogneisses and Tatric granites. Geol Carpath 49:161–167
Broska I, Petrík I, Williams T (2000) Coexisting monazite and allanite in peraluminous granitoids of the Tribeč Mountains, Western Carpathians. Am Mineral 85:22–32
Broska I, Williams CT, Janák M, Nagy G (2005) Alteration and breakdown of xenotime-(Y) and monazite-(Ce) in granitic rocks of the Western Carpathians, Slovakia. Lithos 82:71–83
Broska I, Uher P, Krogh Ravna E, Janák M, Kullerud K, Luptáková J, Bačík P, Vojtko P, Madarás J (2014) Apatite stability in examples from low- to high-metamorphic conditions. In: Bagiński B, Grafka O, Matyszczak W, Macdonald R (eds) Workshop on accessory minerals. University of Warsaw, September, 2014, pp 13–15
Budzyń B, Jastrzębski M (2016) Monazite stability and the maintenance of Th-U-total Pb ages during post-magmatic processes in granitoids and host metasedimentary rocks: a case study from the Sudetes (SW Poland). Geol Quat 60(1):106–123
Budzyń B, Kozub-Budzyń GA (2015) The stability of xenotime in high Ca and Ca-Na systems, under experimental conditions of 250–350 °C and 200–400 MPa: the implications for fluid-mediated low-temperature processes in granitic rocks. Geol Quat 59(2):316–324
Budzyń B, Hetherington CJ, Williams ML, Jercinovic MJ, Michalik M (2010) Fluid-mineral interactions and constraints on monazite alterations during metamorphism. Mineral Mag 74(4):633–655
Budzyń B, Harlov DE, Williams ML, Jercinovic MJ (2011) Experimental determination of stability relations between monazite, fluorapatite, allanite, and REE-epidote as a function of pressure, temperature, and fluid composition. Am Mineral 96:1547–1567
Budzyń B, Konečný P, Kozub-Budzyń GA (2015) Stability of monazite and disturbance of the Th–U–Pb system under experimental conditions of 250–350 °C and 200–400 MPa. Ann Soc Geol Pol 85:405–424
Cherniak DJ (2006) Pb and rare earth elements diffusion in xenotime. Lithos 88:1–14
Cherniak DJ, Watson EB, Grove M, Harrison TM (2004) Pb diffusion in monazite: a combined RBS/SIMS study. Geochim Cosmochim Acta 68:829–840
Chew DM, Spikings RA (2015) Geochronology and thermochronology using apatite: time and temperature, lower crust to surface. Elements 11:189–194
Claeson DT (2002) Stability of REE-bearing minerals in a metaluminous leucotonalite from the Eriksberg gabbro, Transscandinavian Igneous Belt, Sweden. Neues Jb Miner Abh 177:277–291
Darling JR, Storey CD, Engi M (2012) Allanite U-Th-Pb geochronology by laser ablation ICPMS. Chem Geol 292–293:103–115
Doroshkevich AG, Viladkar AG, Ripp GS, Burtseva MV (2009) Hydrothermal REE mineralization in the Amba Dongar carbonatite complex, Gujarat, India. Can Mineral 47:1105–1116
Dumańska-Słowik M, Budzyń B, Heflik W, Sikorska M (2012) Stability relationships of REE-bearing phosphates in an alkali-rich system (nepheline syenite from the Mariupol Massif, SE Ukraine). Acta Geol Pol 62:247–265
Finger F, Krenn E (2007) Three metamorphic monazite generations in a high-pressure rock from the Bohemian Massif and the potentially important role of apatite in stimulating polyphase monazite growth along a PT loop. Lithos 95:103–115
Finger F, Broska I, Roberts MP, Schermaier A (1998) Replacement of primary monazite by apatite-allanite-epidote coronas in an amphibolite facies granite gneiss from the eastern Alps. Am Mineral 83:248–258
Finger F, Krenn E, Schulz B, Harlov D, Schiller D (2016) “Satellite monazites” in polymetamorphic basement rocks of the Alps: their origin and petrological significance. Am Mineral 101:1094–1103
Förster HJ (1998) The chemical composition of REE-Y-Th-U-rich accessory minerals in peraluminous granites of the Erzgebirge-Fichtelgebirge region, Germany, Part I: the monazite-(Ce)-brabantite solid solution series. Am Mineral 83:259–272
Förster HJ, Rhede D, Stein HJ, Romer RL, Tischendorf G (2012) Paired uraninite and molybdenite dating of the Königshain granite: implications for the onset of late-Variscan magmatism in the Lausitz Block. Int J Earth Sci (Geol Rundsch) 101:57–67
Gardes E, Jaoul O, Montel JM, Seydoux-Guillaume AM, Wirth R (2006) Pb diffusion in monazite: an experimental study of Pb2+ + Th4+ ↔ 2Nd3+ interdiffusion. Geochim Cosmochim Acta 70:2325–2336
Grand’Homme A, Janots E, Seydoux-Guillaume AM, Guillaume D, Bosse V, Magnin V (2016) Partial resetting of the U-Th-Pb systems in experimentally altered monazite: nanoscale evidence of incomplete replacement. Geology 44(6):431–434
Gratz R, Heinrich W (1997) Monazite-xenotime thermobarometry: experimental calibration of the miscibility gap in the binary system CePO4–YPO4. Am Mineral 82:772–780
Gregory CJ, Rubatto D, Allen CM, Williams IS, Hermann J, Ireland T (2007) Allanite micro-geochronology: A LA-ICP-MS and SHRIMP U-Th-Pb study. Chem Geol 245:162–182
Hansen EC, Harlov DE (2007) Whole-rock, phosphate, and silicate compositional trends across an amphibolite- to granulite-facies transition, Tamil Nadu, India. J Petrol 48:1641–1680
Harlov DE (2015) Apatite: a fingerprint for metasomatic processses. Elements 11:171–176
Harlov DE, Hetherington CJ (2010) Partial high-grade alteration of monazite using alkali-bearing fluids: experiment and nature. Am Mineral 95:1105–1108
Harlov DE, Milke R (2002) Stability of corundum + quartz relative to kyanite and sillimanite at high temperature and pressure. Am Mineral 87:424–432
Harlov DE, Wirth R (2012) Experimental incorporation of Th into xenotime at middle to lower crustal P-T utilizing alkali-bearing fluids. Am Mineral 97:641–652
Harlov DE, Wirth R, Hetherington CJ (2007) The relative stability of monazite and huttonite at 300–900 °C and 200–1000 MPa: metasomatism and the propagation of metastable mineral phases. Am Mineral 92:1652–1664
Harlov DE, Wirth R, Hetherington CJ (2011) Fluid-mediated partial alteration of monazite: the role of fluids during apparent solid state element mass transfer. Contrib Mineral Petrol 162:329–348
Heinrich W, Andrehs G, Franz G (1997) Monazite-xenotime miscibility gap thermometry. I. an empirical calibration. J Metamorph Geol 15:3–16
Hetherington CJ, Jercinovic MJ, Williams ML, Mahan K (2008) Understanding geologic processes with xenotime: composition, chronology, and a protocol for electron microprobe microanalysis. Chem Geol 254:133–147
Hetherington CJ, Harlov DE, Budzyń B (2010) Experimental initiation of dissolution-reprecipitation reactions in monazite and xenotime: the role of fluid composition. Mineral Petrol 99:165–184
Janots E, Negro F, Brunet F, Goffé B, Engi M, Bouybaouène ML (2006) Evolution of the REE mineralogy in HP–LT metapelites of the Sebtide complex, Rif, Morocco: monazite stability and geochronology. Lithos 87:214–234
Janots E, Brunet F, Goffé B, Poinssot C, Burchard M, Cemic L (2007) Thermochemistry of monazite-(La) and dissakisite-(La): implications for monazite and allanite stability in metapelites. Contrib Mineral Petrol 154:1–14
Janots E, Engi M, Berger A, Allaz J, Schwarz JO, Spandler C (2008) Prograde metamorphic sequence of REE minerals in pelitic rocks of the Central Alps: implications for allanite–monazite–xenotime phase relations from 250 to 610 °C. J Metamorph Geol 26:509–526
Janots E, Engi M, Rubatto D, Berger A, Gregory C, Rahn M (2009) Metamorphic rates in collisional orogeny from in situ allanite and monazite dating. Geology 37:11–14
Janoušek V, Farrow CM, Erban V (2006) Interpretation of whole-rock geochemical data in igneous geochemistry: introducing Geochemical Data Toolkit (GCDkit). J Petrol 47:1255–1259
Johannes W (1973) Eine vereinfachte Piston-Zylinder-Apparatur hoher Genauigkeit. Neues Jb Miner Monat 7(8):337–351
Johannes W, Bell PM, Mao HK, Boettcher AL, Chipman EW, Hays JF, Newton RC, Siefert F (1971) An interlaboratory comparison of piston cylinder pressure calibration using the albite-breakdown reaction. Contrib Mineral Petrol 32:24–38
Kabalov YK, Sokolova EV, Pautov LA, Schneider J (1998) Crystal structure of a new mineral turkestanite: a calcium analogue of steacyite. Crystallog Rep 43:584–588 [Translated from Russian in: Kristallografiya+ 43(4):632–636]
Krenn E, Janák M, Finger F, Broska I, Konečný P (2009) Two types of metamorphic monazite with contrasting La/Nd, Th, and Y signatures in an ultrahigh-pressure metapelite from the Pohorje Mountains, Slovenia: indications for pressure-dependent REE exchange between apatite and monazite? Am Mineral 94:801–815
Krenn E, Harlov DE, Finger F, Wunder B (2012) LREE-redistribution among fluorapatite, monazite, and allanite at high pressures and temperatures. Am Mineral 97:1881–1890
Leake BE, Woolley AR, Arps CES, Birch WD, Gilbert MC, Grice JD, Hawthorne FC, Kato A, Kisch HJ, Krivovichev VG, Linthout K, Laird J, Mandarino JA, Maresch WV, Nickel EH, Rock NMS, Schumacher JC, Smith DC, Stephenson NCN, Ungaretti L, Whittaker EJW, Youzhi G (1997) Nomenclature of amphiboles: report of the subcommittee on amphiboles of the international mineralogical association, commission on new minerals and mineral names. Can Mineral 35:219–246
Lee DE, Bastron H (1967) Fractionation of rare-earth elements in allanite and monazite as related to geology of the Mt. Wheeler mine area, Nevada. Geochim Cosmochim Acta 31:339–356
Lee DE, Dodge FCW (1964) Accessory minerals in some granitic rocks in California and Nevada as a function of calcium content. Am Mineral 49:1660–1669
Liferovich RP, Mitchell RH (2006) Apatite-group minerals from nepheline syenite, Pilansberg alkaline complex, South Africa. Mineral Mag 70:463–484
Linthout K (2007) Tripartite division of the system 2REEPO4 – CaTh(PO4)2 – 2ThSiO4, discreditation of brabantite, and recognition of cheralite as the name for members dominated by CaTh(PO4)2. Can Mineral 45:503–508
Lo Pò D, Braga R, Massonne HJ, Molli G, Montanini A, Theye T (2016) Fluid-induced breakdown of monazite in medium-grade metasedimentary rocks of the Pontremoli basement (Northern Apennines, Italy). J Metamorph Geol 34:63–84
Lyalina LM, Zozulya DR, Savchenko YE, Tarasov MP, Selivanova EA, Tarasova E (2014) Fluorbritholite-(Y) and Yttrialite-(Y) from Silexites of the Keivy Alkali Granites, Kola Peninsula. Geol Ore Deposits 56:589–602
Majka J, Budzyń B (2006) Monazite breakdown in metapelites from Wedel Jarlsberg Land, Svalbard – preliminary results. Mineral Pol 37(1):61–69
Majka J, Pršek J, Budzyń B, Bačík P, Barker A, Łodziński M (2011) Fluorapatite-hingganite-(Y) coronas as products of fluid induced xenotime-(Y) breakdown in the Skoddefjellet pegmatite (Svalbard). Mineral Mag 75:159–167
Montel JM (1986) Experimental determination of the solubility of Ce-monazite in SiO2-Al2O3-K2O-Na2O melts at 800 °C, 2 kbar, under H2O-saturated conditions. Geology 14:659–662
Montel JM (1993) A model for monazite/melt equilibrium and application to the generation of granitic magmas. Chem Geol 110:127–146
Nekvasil H (1988) Calculated effect of anorthite component on the crystallization paths of H2O-undersaturated haplogranitic melts. Am Mineral 73:966–981
Norberg N, Neusser G, Wirth R, Harlov D (2011) Microstructural evolution during experimental albitization of K-rich alkali feldspar. Contrib Mineral Petrol 162:531–546
Norberg N, Harlov D, Neusser G, Wirth R, Rhede D, Morales L (2013) Experimental development of patch perthite from synthetic cryptoperthite: microstructural evolution and chemical re-equilibration. Am Mineral 98:1429–1441
Ondrejka M, Uher P, Putiš M, Broska I, Bačík P, Konečný P, Schmiedt I (2012) Two-stage breakdown of monazite by post-magmatic and metamorphic fluids: an example from the Veporic orthogneiss, Western Carpathians, Slovakia. Lithos 142–143:245–255
Pan Y, Fleet ME (2002) Composition of the apatite-group minerals: substitution mechanisms and controlling factors. In: Kohn MJ, Rakovan J, Hughes JM (Eds) Phosphates: geochemical, geobiological, and materials importance. Rev Mineral Geochem 48:13–49
Pasero M, Kampf A, Ferraris C, Pekov IV, Rakovan J, White TJ (2010) Nomenclature of the apatite supergroup minerals. Eur J Mineral 22:163–179
Pautov LA, Agakhanov AA, Sokolova EV, Kabalov YK (1997) Turkestanite –Th(Ca, Na)2(K1–x,□x)(Si8O20)⋅nH2O: A new mineral with Si-O rings. Zap Vses Mineral O-va 126:45–55
Pautov LA, Agakhanov AA, Sokolova E, Hawthorne FC (2004) Maleevite, BaB2Si2O8, and pekovite, SrB2Si2O8, new mineral species from the Dara-i-Pioz alkaline massif, Northern Tajikistan: description and crystal structure. Can Mineral 42:107–119
Pekov IV, Zubkova NV, Chukanov NV, Husdal TA, Zadov E, Pushcharovsky DY (2011) Fluorbritholite-(Y), (Y, Ca, Ln)5[(Si, P)O4]3F, a new mineral of the britholite group. Neues Jb Miner Abh 188(2):191–197
Petersen OV, Johnsen O, Micheelsen HI (1999) Turkestanite from the Ilímaussaq alkaline complex, South Greenland. Neues Jb Miner Monat 9:424–432
Petrík I, Broska I, Lipka J, Siman P (1995) Granitoid Allanite-(Ce) substitution relations, redox conditions and REE distributions (on an Example of I-Type Granitoids, Western Carpathians, Slovakia). Geol Carpath 46:79–94
Petrík I, Konečný P, Kováčik M, Holický I (2006) Electron microprobe dating of monazite from the Nizke Tatry Mountains orthogneisses (Western Carpathians, Slovakia). Geol Carpath 57:227–242
Putnis A (2002) Mineral replacement reactions: from macroscopic observations to microscopic mechanisms. Mineral Mag 66:689–708
Putnis A (2009) Mineral replacement reactions. In: Putirka KD, Tepley III FJ (Eds) Minerals, inclusions and volcanic processes. Rev Mineral Geochem 70:87–124
Putnis A, Austrheim H (2012) Mechanisms of metasomatism and metamorphism on the local mineral scale: the role of dissolution-reprecipitation during mineral re-equilibration. In: Harlov DE, Austrheim H (eds) Metasomatism and the chemical transformation of rock, lecture notes in earth system sciences. Springer, Berlin, pp 141–170
Pyle JM, Spear FS, Rudnick RL, McDonough WF (2001) Monazite–xenotime–garnet equilibrium in metapelites and a new monazite–garnet thermometer. J Petrol 42:2083–2107
Rakovan J (2002) Growth and surface properties of apatite. In: Kohn MJ, Rakovan J, Hughes JM (Eds) Phosphates: geochemical, geobiological, and materials importance. Rev Mineral Geochem 48:51–86
Rakovan J, Reeder RJ (1996) Intracrystalline rare earth element distributions in apatite: surface structural influences on incorporation during growth. Geochim Cosmochim Acta 60:4435–4445
Rasmussen B (2005) Radiometric dating of sedimentary rocks: the application of diagenetic xenotime geochronology. Earth-Sci Rev 68:197–243
Rasmussen B, Muhling JR (2007) Monazite begets monazite: evidence for dissolution of detrital monazite and reprecipitation of syntectonic monazite during low-grade regional metamorphism. Contrib Mineral Petrol 154:675–689
Rasmussen B, Fletcher IR, Muhling JR (2011) Response of xenotime to prograde metamorphism. Contrib Mineral Petrol 162:1259–1277
Seydoux-Guillaume AM, Paquette JL, Wiedenbeck M, Montel JM, Heinrich W (2002) Experimental resetting of the U-Th-Pb systems in monazite. Chem Geol 191:165–181
Shaw DM (1956) Geochemistry of pelitic rocks. Part III: major elements and general geochemistry. Geol Soc Am Bull 67:919–934
Smith HA, Barreiro B (1990) Monazite U-Pb dating of staurolite grade metamorphism in pelitic schists. Contrib Mineral Petrol 105:602–615
Spear FS (2010) Monazite–allanite phase relations in metapelites. Chem Geol 279:55–62
Spear FS, Pyle JM (2002) Apatite, Monazite, and Xenotime in Metamorphic Rocks. In: Kohn MJ, Rakovan J, Hughes JM (Eds) Phosphates: geochemical, geobiological, and materials importance. Rev Mineral Geochem 48:293–335
Teufel S, Heinrich W (1997) Partial resetting of the U-Pb isotope system in monazite through hydrothermal experiments: an SEM and U-Pb isotope study. Chem Geol 137:273–281
Uher P, Ondrejka M, Bačík P, Broska I, Konečný P (2015) Britholite, monazite, REE carbonates, and calcite: Products of hydrothermal alteration of allanite and apatite in A-type granite from Stupné, Western Carpathians, Slovakia. Lithos 236–237:212–225
Vilalva FCJ, Vlach SRF (2010) Major- and trace-element composition of REE-rich turkestanite from peralkaline granites of the Morro Redondo Complex, Graciosa Province, south Brasil. Mineral Mag 74:645–658
Villa IM, Williams ML (2013) Geochronology of Metasomatic Events. In: Harlov DE, Austrheim H (eds) Metasomatism and the chemical transformation of rock, lecture notes in earth system sciences. Springer, Berlin, pp 171–202
Washington HS (1917) Chemical analyses of igneous rocks. United States Geological Survey Professional Paper 99, 1201 p.
Whitney DL, Evans BW (2010) Abbreviations for names of rock-forming minerals. Am Mineral 95:185–187
Williams ML, Jercinovic MJ, Hetherington CJ (2007) Microprobe monazite geochronology: understanding geologic processes by integrating composition and chronology. Annu Rev Earth Planet Sci 35:137–175
Williams ML, Jercinovic MJ, Harlov DE, Budzyń B, Hetherington CJ (2011) Resetting monazite ages during fluid-related alteration. Chem Geol 283:218–225
Wing B, Ferry JM, Harrison TM (2003) Prograde destruction and formation of monazite and allanite during contact and regional metamorphism of pelites: petrology and geochronology. Contrib Mineral Petrol 145:228–250
Wolf MB, London D (1995) Incongruent dissolution of REE- and Sr-rich apatite in peraluminous granitic liquids: Differential apatite, monazite, and xenotime solubilities during anatexis. Am Mineral 80:765–775
Acknowledgments
The project was funded by the National Science Center of Poland, grant no. 2011/01/D/ST10/04588. The authors are grateful to I. Petrík and two anonymous reviewers for their valuable and constructive comments, and L. Nasdala for editorial handling the manuscript. B. Budzyń acknowledges W. Heinrich for the access to the experimental laboratories of the Deutsche GeoForschungsZentrum, Potsdam. Scanning electron microscope BSE imaging and analyses were performed in the Institute of Geological Sciences, Jagiellonian University, Kraków. A. Zagórska is appreciated for assistance in sample preparation. The Burnet monazite-(Ce) was provided by M.J. Jercinovic and M.L. Williams.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: L. Nasdala
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
The CIPW Qz-Ab-Or diagrams presenting normalized composition of melts formed in experiments with monazite-(Ce) and xenotime-(Y). Diagrams were plotted using GCDkit 4.0 software (Janoušek et al. 2006). Melt normative compositions are compared to: N88 – compositional frequency of granites with normative Ab + Or + Qz ≥80 % (Washington 1917; from Nekvasil 1988); and A13 – compositions of southwestern USA A-type granites (Anderson and Bender 1989; from Aranovich et al. 2013) (DOCX 145 kb)
Supplementary Fig. S2
BSE image and compositional X-ray maps of the altered garnet in experiment with a Na2Si2O5 + H2O fluid; the X12N-06 run at 550 °C, 200 MPa over 12 days; maps were collected using a 15 kV accelerating voltage, 40 nA beam current, 100 ms dwell time, 0.3 μm step size, and a focused beam; slight shift between images is related to mapping in two sessions: (1) Fe, Mg, Ca, Si, K, (2) Mn, Al, Ti, Na (DOCX 8002 kb)
Supplementary Table S1
Average composition of monazite-(Ce) calculated from EPMA analysis results (DOCX 75 kb)
Supplementary Table S2
Conditions of EPMA analyses (DOCX 16 kb)
Supplementary Table S3
Average composition of REE-rich fluorapatite-fluorcalciobritholite from monazite-(Ce) experiments calculated from EPMA analysis results (DOCX 62 kb)
Supplementary Table S4
Average composition of REE-rich epidote-allanite-(Ce) from the products of monazite-(Ce) experiments calculated from EPMA analysis results (DOCX 31 kb)
Supplementary Table S5
Average composition of feldspars in monazite-(Ce) experiments calculated from EPMA analysis results (DOCX 93 kb)
Supplementary Table S6
Average composition of micas in monazite-(Ce) experiments calculated from EPMA analysis results (DOCX 41 kb)
Supplementary Table S7
Average composition of melt from monazite-(Ce) experiments calculated from EPMA analysis results (DOCX 29 kb)
Supplementary Table S8
Average composition of REE-rich steacyite calculated from EPMA analysis results (DOCX 27 kb)
Supplementary Table S9
Average composition of amphibole from monazite-(Ce) experiments calculated from EPMA analysis results (DOCX 14 kb)
Supplementary Table S10
Average composition of xenotime-(Y) calculated from EPMA analysis results (DOCX 61 kb)
Supplementary Table S11
Average composition of Y-rich fluorcalciobritholite from xenotime-(Y) experiments calculated from EPMA analysis results (DOCX 66 kb)
Supplementary Table S12
Average composition of (Y,HREE)-rich epidote from the products of xenotime-(Y) experiments calculated from EPMA analysis results (DOCX 14 kb)
Supplementary Table S13
Average composition of feldspars from xenotime-(Y) experiments calculated from EPMA analysis results (DOCX 91 kb)
Supplementary Table S14
Average composition of micas from xenotime-(Y) experiments calculated from EPMA analysis results (DOCX 84 kb)
Supplementary Table S15
Average composition of garnet from xenotime-(Y) experiments calculated from EPMA analysis results (DOCX 36 kb)
Supplementary Table S16
Average composition of melt from xenotime-(Y) experiments calculated from EPMA analysis results (DOCX 33 kb)
Supplementary Table S17
Average composition of amphibole formed in xenotime-(Y) experiments calculated from EPMA analysis results (DOCX 14 kb)
Supplementary Table S18
Results of the EPMA analyses of titanite from run X12N-08 at 650 °C, 1000 MPa, 6 days (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Budzyń, B., Harlov, D.E., Kozub-Budzyń, G.A. et al. Experimental constraints on the relative stabilities of the two systems monazite-(Ce) – allanite-(Ce) – fluorapatite and xenotime-(Y) – (Y,HREE)-rich epidote – (Y,HREE)-rich fluorapatite, in high Ca and Na-Ca environments under P-T conditions of 200–1000 MPa and 450–750 °C. Miner Petrol 111, 183–217 (2017). https://doi.org/10.1007/s00710-016-0464-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00710-016-0464-0