Abstract
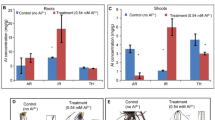
Aluminum stress deteriorates lentil production under acidic soils. Enhanced insight into Al tolerance traits is needed to improve its productivity. Therefore, Al-resistant (L-4602, PAL-8) and Al-sensitive (BM-4, EC-223229) cultivars along with a resistant wild (ILWL-15) were characterized for morpho-physiological traits viz. seedling root architecture (SRA), Al accumulation, and localization via fluorescent and non-fluorescent staining under control and Al-treated conditions. Also, antioxidant activities and organic acid secretion were quantified, and expressions of 10 associated genes were analyzed. Roots of Al-resistant cultivars and wild genotype showed higher root growth, antioxidant enzyme activities, and organic acid secretion than Al-sensitive ones. Among these traits, higher organic acid secretion was influenced by enhanced expression of genes, especially—aluminum sensitive-3 (ALS 3), aluminum-activated malate transporter (ALMT), multidrug and toxic compound extrusion (MATE), citrate synthase (CS), and phospho enol pyruvate carboxylase (PEPC)—which helped in reducing Al and callose accumulation. These genes were located on lentil chromosomes via sequence alignment with lentil draft genome. A strong link between morpho-physiological variation and organic acid secretion was noted which reinforced the prominence of exclusion mechanism. It was complemented by enhanced antioxidant activities at seedling stage which mitigated Al stress effects on SRA. Wild outperformed over cultivars indicating its impregnable evolution which can be exploited to better understand tolerance mechanisms. Al-resistant cultivars had significantly higher seed yield than Al-sensitive and national checks on Al-toxic fields, confirming—tolerance is sustained till reproductive stage in lentil. This study elucidated role of gene families in eliminating Al toxicity that will assist breeders to formulate strategies for developing Al-resistant cultivars.









Similar content being viewed by others
Abbreviations
- Al:
-
Aluminum
- PEPC:
-
Phosphoenolpyruvate carboxylase
- CS:
-
Citrate synthase
- ICDH:
-
Isocitrate dehydrogenase
- MDH:
-
Malate dehydrogenase
- MATE-a:
-
Multidrug and toxic compound extrusion-a
- MATE-b:
-
Multidrug and toxic compound extrusion-b
- MATE-c:
-
Multidrug and toxic compound extrusion-c
- ALS3:
-
Al-sensitive3
- ALMT1:
-
Al-activated malate transporter
- VDAC:
-
Voltage dependent anion channel
- SOD:
-
Superoxide dismutase
- APX:
-
Ascorbate peroxidase
- GPX:
-
Glutathione peroxidase
- CAT:
-
Catalase
References
Alvim MN, Ramos FT, Oliveira DC, Isaias RMS, Franca MGC (2012) Aluminium localization and toxicity symptoms related to root growth inhibition in rice (Oryza sativa L) seedlings. J Biosci 37(1):1079–1088
Anoop VM, Basu U, McCammon MT, McAlister-Henn L, Taylor GJ (2003) Modulation of citrate metabolism alters aluminum tolerance in yeast and transgenic canola overexpressing a mitochondrial citrate synthase. Plant Physiol 132(4):2205–2217
Awasthi JP, Saha B, Panigrahi J, Yanase E, Koyama H, Panda SK (2019) Redox balance, metabolic fingerprint and physiological characterization in contrasting North East Indian rice for Aluminum stress tolerance. Sci Rep 9(1):1–21
Barcelo J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48(1):75–92
Barnhisel R, Bertsch PM (1983) Aluminum. In: Methods of soil analysis: part 2 chemical and microbiological properties, vol 19, pp 275–300
Boscolo PR, Menossi M, Jorge RA (2003) Aluminum-induced oxidative stress in maize. Phytochem 62(2):181–189
Darko E, Ambrus H, Stefanovits-Bányai É, Fodor J, Bakos F, Barnabás B (2004) Aluminium toxicity, Al tolerance and oxidative stress in an Al-sensitive wheat genotype and in Al-tolerant lines developed by in vitro microspore selection. Plant Sci 166(3):583–591
Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H (2004) Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc Natl Acad Sci 101(42):15249–15254
Delhaize E, Ma JF, Ryan PR (2012) Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17(6):341–348
Desai MK, Mishra RN, Verma D, Nair S, Sopory SK, Reddy MK (2006) Structural and functional analysis of a salt stress inducible gene encoding voltage dependent anion channel (VDAC) from pearl millet (Pennisetum glaucum). Plant Physiol Biochem 44(7-9):483–493. https://doi.org/10.1016/j.plaphy.2006.08.008
Dorneles AOS, Pereira AS, Rossato LV, Possebom G, Sasso VM, Bernardy K, Sandri RDQ, Nicoloso FT, Ferreira PAA, Tabaldi LA (2016) Silicon reduces aluminum content in tissues and ameliorates its toxic effects on potato plant growth. Cienc Rural 46(3):506–512
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 6(5):e19379
Ermolayev V, Weschke W, Manteuffel R (2003) Comparison of Al-induced gene expression in sensitive and tolerant soybean cultivars. J Exp Bot 54(393):2745–2756
Erskine W, Rihawi S, Capper BS (1990) Variation in lentil straw quality. Anim Feed Sci Technol 28(1-2):61–69
Eticha D, Zahn M, Bremer M, Yang Z, Rangel AF, Rao IM, Horst WJ (2010) Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Ann Bot 105(7):1119–1128
Fontecha G, Silva-Navas J, Benito C, Mestres MA, Espino FJ, Hernández-Riquer MV, Gallego FJ (2007) Candidate gene identification of an aluminum-activated organic acid transporter gene at the Alt4 locus for aluminum tolerance in rye (Secale cereale L). Theor Appl Genet 114(2):249–260
Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48(8):1081–1091
Gabrielson KM, Cancel JD, Morua LF, Larsen PB (2006) Identification of dominant mutations that confer increased aluminium tolerance through mutagenesis of the Al-sensitive Arabidopsis mutant, als3-1. J Exp Bot 57(4):943–951
Giannakoula A, Moustakas M, Syros T, Yupsanis T (2010) Aluminum stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Environ Exp Bot 67(3):487–494
Guo P, Bai G, Carve B, Li R, Bernardo A, Baum M (2007) Transcriptional analysis between two wheat near-isogenic lines contrasting in aluminum tolerance under aluminum stress. Mol Genet Genomics 277(1):1–12
Han Y, Zhang W, Zhang B, Zhang S, Wang W, Ming F (2009) One novel mitochondrial citrate synthase from Oryza sativa L can enhance aluminum tolerance in transgenic tobacco. Mol Biotechnol 42(3):299–305
Hasanuzzaman M, Bhuyan MHM, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9(8):681
Haug A (1985) Aluminum toxicity in plant: the role of root plasma membrane and calmodulin. In: Frontiers of Membrane Research in Agriculture: Beltsville Symposium, vol 9. Rowman & Allanheld, Totowa, pp 359–381
Hirano Y, Pannatier EG, Zimmermann S, Brunner I (2004) Induction of callose in roots of Norway spruce seedlings after short-term exposure to aluminum. Tree Physiol 24(11):1279–1283
Horst WJ, Wang Y, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot 106(1):185–197
Jolliffe IT (2002) Principal components in regression analysis. In: Principal component analysis. Springer, Amsterdam, pp 167–198. https://doi.org/10.1007/0-387-22440-8_8
Jones DL, Blancaflor EB, Kochian LV, Gilroy S (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ 29(7):1309–1318
Klug B, Specht A, Horst WJ (2011) Aluminium localization in root tips of the aluminium-accumulating plant species buckwheat (Fagopyrum esculentum Moench). J Exp Bot 62(15):5453–5462
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Biol 46(1):237–260
Kochian LV, Pineros MA, Liu J, Magalhaes JV (2015) Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66:571–598
Larsen PB, Geisler MJ, Jones CA, Williams KM, Cancel JD (2005) ALS3 encodes a phloem localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J 41(3):353–363
Li Y, Yang G, Luo L, Ke T, Zhang J, Li K, He G (2008) Aluminium sensitivity and tolerance in model and elite wheat varieties. Cereal Res Commun 36(2):257–267
Li ZY, Xu ZS, He GY, Yang GX, Chen M, Li LC, Ma Y (2013) The voltage-dependent anion channel 1 (AtVDAC1) negatively regulates plant cold responses during germination and seedling development in Arabidopsis and interacts with calcium sensor CBL1. Int J Mol Sci 14(1):701–713
Li N, Meng H, Xing H, Liang L, Zhao X, Luo K (2017) Genome-wide analysis of MATE transporters and molecular characterization of aluminum resistance in Populus. J Exp Bot 68(20):5669–5683
Loskutov IG, Kosareva IA, Melnikova SV, Blinova EV, Bagmet LV (2017) Genetic diversity in tolerance of wild Avena species to aluminium (Al). Genet Resour Crop Evol 64(5):955–965
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6(6):273–278
Ma B, Gao L, Zhang H, Cui J, Shen Z (2012) Aluminum-induced oxidative stress and changes in antioxidant defenses in the roots of rice varieties differing in Al tolerance. Plant Cell Rep 31(4):687–696
Magalhaes JV, Liu J, Guimaraes CT, Lana UG, Alves VM, Wang YH, Schaffert RE, Hoekenga OA, Pineros MA, Shaff JE, Klein PE (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39(9):1156–1161
Mandal KG, Misra AK, Hati KM, Bandyopadhyay KK, Ghosh PK, Mohanty M (2004) Rice residue-management options and effects on soil properties and crop productivity. J Food Agric Environ 2:224–231
Manrique G, Rao I, Beebe S (2006) Identification of aluminum resistant common bean genotypes using a hydroponic screening method. In: 18th world congress of soil science, Philadelphia, USA
Massot N, Llugany M, Poschenrieder C, Barceló J (1999) Callose production as indicator of aluminum toxicity in bean cultivars. J Plant Nutr 22(1):1–0
Pavan S, Bardaro N, Fanelli V, Marcotrigiano AR, Mangini G, Taranto F, Catalano D, Montemurro C, De Giovanni C, Lotti C, Ricciardi L (2019) Genotyping by sequencing of cultivated lentil (Lens culinaris Medik.) highlights population structure in the Mediterranean gene pool associated with geographic patterns and phenotypic variables. Front Genet 10:872
Pellet DM, Grunes DL, Kochian LV (1995) Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.). Planta 196:788–795
Pereira JF, Zhou G, Delhaize E, Richardson T, Zhou M, Ryan PR (2010) Engineering greater aluminium resistance in wheat by over-expressing TaALMT1. Ann Bot 106(1):205–214
Piñeros MA, Kochian LV (2001) A patch-clamp study on the physiology of aluminum toxicity and aluminum tolerance in maize. Identification and characterization of Al3+-induced anion channels. Plant Physiol 125:292–305
Polanco C, de Miera LES, Bett K, de la Vega MP (2018) A genome-wide identification and comparative analysis of the lentil MLO genes. PloSOne 13(3):e0194945
Ramesh T, Bolan NS, Kirkham MB, Wijesekara H, Kanchikerimath M, Rao CS, Wang H (2019) Soil organic carbon dynamics: impact of land use changes and management practices: a review. Adv Agron 156:1–107
Rangel AF, Rao IM, Braun HP, Horst WJ (2010) Aluminum resistance in common bean (Phaseolus vulgaris) involves induction and maintenance of citrate exudation from root apices. Physiol Plant 8(2):176–190
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum activated malate transporter. Plant J 37(5):645–653
Silva S (2012) Aluminium toxicity targets in plants. J Bot 4(7):592–597. https://doi.org/10.1155/2012/219462
Singh D, Singh NP, Chauhan SK, Singh P (2011) Developing aluminium-tolerant crop plants using biotechnological tools. Curr Sci 100(12):1807–1814
Singh D, Pal M, Singh CK, Taunk J, Jain P, Chaturvedi AK, Maurya S, Karwa S, Singh R, Tomar RSS, Nongthombam R (2016) Molecular scanning and morpho-physiological dissection of component mechanism in Lens species in response to aluminium stress. PLoS One 11(7):e0160073
Singh CK, Singh D, Tomar RSS, Karwa S, Upadhyaya KC, Pal M (2018) Molecular mapping of aluminium resistance loci based on root re-growth and Al-induced fluorescent signals (callose accumulation) in lentil (Lens culinaris Medikus). Mol Biol Rep 45(6):2103–2113
Sinha R, Sharma TR, Singh AK (2019) Validation of reference genes for qRT-PCR data normalisation in lentil (Lens culinaris) under leaf developmental stages and abiotic stresses. Physiol Mol Biol Plants 25(1):123–134
Smith E, Naik D, Cumming JR (2011) Genotypic variation in aluminum resistance, cellular aluminum fractions, callose and pectin formation and organic acid accumulation in roots of Populus hybrids. Environ Exp Bot 72(2):182–193
Sun G, Zhu H, Wen S, Liu L, Gou L, Guo Z (2020) Citrate synthesis and exudation confer Al resistance in alfalfa (Medicago sativa L). Plant Soil 449:319–329
Yang ZM, Nian H, Sivaguru M, Tanakamaru S, Matsumoto H (2001) Characterization of aluminium induced citrate secretion in aluminium tolerant soybean (Glycine max) plants. Physiol Plant 113(1):64–71
Yang ZM, Yang H, Wang J, Wang YS (2004) Aluminum regulation of citrate metabolism for Al-induced citrate efflux in the roots of Cassia tora L. Plant Sci 166(6):1589–1594
Zhang WH, Ryan PR, Tyerman SD (2001) Malate-permeable channels and cation channels activated by aluminum in the apical cells of wheat roots. Plant Physiol 125(3):1459–1472
Zhang M, Takano T, Liu S, Zhang X (2015) Arabidopsis mitochondrial voltage-dependent anion channel 3 (AtVDAC3) protein interacts with thioredoxin m2. FEBS Lett 589(11):1207–1213
Zhao Z, Ma JF, Sato K, Takeda K (2003) Differential Al resistance and citrate secretion in barley (Hordeum vulgare L). Planta 217(5):794–800
Zheng SJ, Ma JF, Matsumoto H (1998) High aluminum resistance in buckwheat: I Al-induced specific secretion of oxalic acid from root tips. Plant Physiol 117(3):745–751
Zhou G, Pereira JF, Delhaize E, Zhou M, Magalhaes JV, Ryan PR (2014) Enhancing the aluminium tolerance of barley by expressing the citrate transporter genes SbMATE and FRD3. J Exp Bot 65(9):2381–2390
Acknowledgements
Authors thank Director, Joint Director (Research), ICAR-Indian Agricultural Research Institute (IARI), New Delhi; Head, Division of Genetics and Incharge, National Phytotron Facility, IARI, New Delhi, for their support provided to accomplish the research activities.
Funding
This work has been financially supported by grant provided by ICAR-Indian Agricultural Research Institute, New Delhi (Project no- JAN 09 / 16) and DBT (No. BT/PR25565/NER/95/1254/2017) The funding bodies had no role in the design of the study, data analysis interpretation and writing the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: DS, KCU, MP, and SC. Performed the experiments: CKS, SS and DPS. Analyzed the data: DS and CKS. Contributed reagents/materials/analysis tools: AK and MP. Wrote the paper: DS, CKS, and JT. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Néstor Carrillo
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, C.K., Singh, D., Sharma, S. et al. Morpho-physiological characterization coupled with expressional accord of exclusion mechanism in wild and cultivated lentil under aluminum stress. Protoplasma 258, 1029–1045 (2021). https://doi.org/10.1007/s00709-021-01619-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-021-01619-z