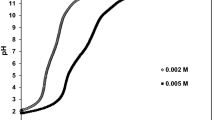
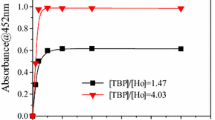
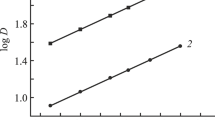
Abstract
The results of an investigation on the interactions between phytate ion (myo-inositol hexaphosphate, Phy) and some lanthanoid cations (La3+, Nd3+, Sm3+, Dy3+, and Yb3+) are reported. The stability constants of various LnH j Phy species (Ln = generic lanthanoid) were determined by potentiometry (ISE-H+ glass electrode) in NaClaq at I = 0.15 mol dm−3 and t = 25 °C, and the corresponding formation enthalpies by calorimetric titration. The thermodynamic data obtained were used to provide a speciation scheme for the lanthanoid(III)–phytate systems at different temperatures. The sequestering ability of this ligand toward Ln3+ was also evaluated by calculation of pL50 values (the total concentration of ligand necessary to bind 50% of a cation present in trace amounts) under different conditions, and equations were formulated to model their dependence on temperature and pH.
Graphical abstract









Similar content being viewed by others
References
De Stefano C, Lando G, Milea D, Pettignano A, Sammartano S (2010) J Solut Chem 39:179
Crea F, De Stefano C, Milea D, Sammartano S (2008) Coord Chem Rev 252:1108
Raboy V (2009) Plant Sci 177:281
Raboy V (2003) Phytochemistry 64:1033
Shamsuddin AM (2002) Int J Food Sci Technol 37:769
Shears SB (2001) Cell Sign 13:151
Reddy NR, Sathe SK (2001) Food Phytates. CRC Press, Boca Raton
Oatway L, Vasanthan T, Helm JH (2001) Food Rev Int 17:419
Paterson-Beedle M, Macaskie LE, Readman JE, Hriljac JA (2009) Adv Mat Res 621
Knox AS, Brigmon RL, Kaplan DI, Paller MH (2008) Sci Total Environ 395:63
Iemma F, Cirillo G, Spizzirri UG, Puoci F, Parisi OI, Picci N (2008) Eur Polym J 44:1183
Grases Freixedas F, Perello Bestard J, Isern Amengual B, Costa Bauza A (2007) PCT Int Appl (Universitat de Les Illes Balears, Spain) Wo, 21 pp
Ulusoy U, Simsek S (2005) J Hazard Mat 127:163
Nash KL, Jensen MP, Schmidt MA (1998) J Alloys Compd 271–273:257
Laveuf C, Cornu S (2009) Geoderma 154:1
Di Bernardo P, Zanonato PL, Melchior A, Portanova R, Tolazzi M, Choppin GR, Wang Z (2008) Inorg Chem 47:1155
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Environ Chem Lett 6:189
Storr T, Thompson KH, Orvig C (2006) Chem Soc Rev 35:534
Fricker SP (2006) Chem Soc Rev 35:524
Choppin GR, Thakur P, Mathur JN (2006) Coord Chem Rev 250:936
Choppin GR, Peterman DR (1998) Coord Chem Rev 174:283
Tyler G (2004) Plant Soil 267:191
Misra SN, Gagnani MA, Devi IM, Shukla RS (2004) Bioinorg Chem Appl 2:155
Shibasaki M, Yoshikawa N (2002) Chem Rev 102:2187
Goering PL, Fisher BR, Fowler BA (1991) In: Merian E (ed) Metals and their compounds in the environment: occurrence, analysis, and biological relevance. VCH, Weinheim, 959 pp
Li N, Wahlberg O (1989) Acta Chem Scand 43:401
Li N, Wahlberg O, Puigdomenech I (1989) Chem Scr 29:91
De Stefano C, Milea D, Porcino N, Sammartano S (2006) Anal Bioanal Chem 386:346
De Stefano C, Milea D, Sammartano S (2003) J Chem Eng Data 48:114
Baes CF, Mesmer RE (1976) The hydrolysis of cations. Wiley, New York
Crea P, De Stefano C, Milea D, Porcino N, Sammartano S (2007) Biophys Chem 128:176
De Stefano C, Milea D, Porcino N, Sammartano S (2006) J Agric Food Chem 54:1459
Crea F, De Stefano C, Milea D, Sammartano S (2009) J Solut Chem 38:115
De Stefano C, Milea D, Sammartano S (2005) Biophys Chem 116:111
Delgado R, do Carmo Figueira M, Quintino S (1997) Talanta 45:451
Anderegg G (1977) Critical survey of stability constants of EDTA complexes. Pergamon Press, Oxford
Sillén LG, Martell AE (1964) Stability constants of metal ion complexes. Special Publ 17. The Chemical Society. Wiley, London
Sillén LG, Martell AE (1964) Stability constants of metal ion complexes. Supplement Special Publ 25. The Chemical Society. Wiley, London
Pettit D, Powell K (1997) IUPAC stability constants database academic software, Otley, UK
May PM, Murray K (2001) Chem Eng Data 46:1035
Martell AE, Smith RM, Motekaitis RJ (2004) NIST Standard Reference Database 46, vers 8, Gaithersburg
Klungness GD, Byrne RH (2000) Polyhedron 19:99
De Stefano C, Milea D, Sammartano S (2004) Thermochim Acta 423:63
De Robertis A, De Stefano C, Rigano C (1986) Thermochim Acta 138:141
Clarke ECW, Glew DN (1966) Trans Faraday Soc 62:539
Fidelis I, Siekierski S (1966) J Inorg Nucl Chem 28:185
Fidelis I, Siekierski S (1971) J Inorg Nucl Chem 33:3191
Siekierski S, Fidelis I (1972) J Inorg Nucl Chem 34:2225
Peppard DF, Mason GW, Lewey S (1969) J Inorg Nucl Chem 31:2271
Peppard DF, Bloomquist CAA, Horwitz EP, Lewey S, Mason GW (1970) J Inorg Nucl Chem 32:339
Casale A, De Stefano C, Manfredi G, Milea D, Sammartano S (2009) Bioinorg Chem Appl 2009:ID 219818
Berto S, Crea F, Daniele PG, De Stefano C, Prenesti E, Sammartano S (2009) J Solut Chem 38:1343
Crea F, Foti C, Sammartano S (2008) Talanta 75:775
Meloun M, Militky J, Forina M (1994) Chemometrics for analytical chemistry, vol 2: PC-aided regression and related methods. Ellis Horwood Limited, New York
Siddiqi KS, Shah SA, Aqra FMAM, Tabassum S, Zaidi SAA, Benlian D (1993) Ind J Chem A 32:421
De Stefano C, Milea D, Pettignano A, Sammartano S (2003) Anal Bioanal Chem 376:1030
Crea F, Crea P, De Stefano C, Milea D, Sammartano S (2008) J Mol Liquids 138:76
Flaschka HA (1959) EDTA titration. Pergamon Press, London
De Stefano C, Princi P, Rigano C, Sammartano S (1987) Ann Chim (Rome) 77:643
De Stefano C, Mineo P, Rigano C, Sammartano S (1993) Ann Chim (Rome) 83:243
De Stefano C, Foti C, Giuffrè O, Mineo P, Rigano C, Sammartano S (1996) Ann Chim (Rome) 86:257
Gans P, Sabatini A, Vacca A (2008) http://www.hyperquad.co.uk/HQ2008.htm
De Stefano C, Sammartano S, Mineo P, Rigano C (1997) In: Gianguzza A, Pelizzetti E, Sammartano S (eds) Marine chemistry––an environmental analytical chemistry approach. Kluwer Academic Publishers, Amsterdam, 71 pp
Acknowledgments
We thank University of Messina (PRA) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Speciation of phytate ion in aqueous solution. Last contribution to this series: Ref. [1].
Rights and permissions
About this article
Cite this article
Crea, F., De Stefano, C., Milea, D. et al. Thermodynamic data for lanthanoid(III) sequestration by phytate at different temperatures. Monatsh Chem 141, 511–520 (2010). https://doi.org/10.1007/s00706-010-0303-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0303-7