Abstract
Porcine deltacoronavirus (PDCoV) and porcine epidemic diarrhea virus (PEDV) have often been detected simultaneously in piglets with coronavirus diarrhea. However, the intestinal immune response to the interaction between circulating PDCoV and PEDV is unknown. Therefore, this study was conducted to investigate the intestinal immunity of neonatal piglets that were exposed first to PDCoV and then to PEDV. The amounts and distribution of CD3+ T lymphocytes, B lymphocytes, and goblet cells (GCs) in the small intestine were analyzed by immunohistochemistry and periodic acid–Schiff staining, respectively. The expression levels of pattern recognition receptors and downstream mediator cytokines were analyzed by qPCR and ELISA. The results showed that the numbers of GCs, CD3+ T lymphocytes, and B lymphocytes in the duodenum and jejunum of the PDCoV + PEDV coinoculated piglets were increased compared with those of piglets inoculated with PEDV alone. The piglets in the PDCoV + PEDV group had significantly upregulated IFN-α and IFN-λ1 compared with the PEDV single-inoculated piglets. These results suggest that PDCoV + PEDV-coinfected piglets can activate intestinal antiviral immunity more strongly than piglets infected with PEDV alone, which provides new insight into the pathogenesis mechanism of swine enteric coronavirus coinfection that may be used for vaccination in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viral porcine diarrhea, mainly caused by coronaviruses such as porcine epidemic diarrhea virus (PEDV) and porcine deltacoronavirus (PDCoV), has continued to spread and causes serious economic losses on pig farms [1]. PEDV and PDCoV were first reported in the United Kingdom [2] and in Hong Kong [3], respectively, and were subsequently detected in pigs in other countries. PDCoV and PEDV are enveloped, single-stranded, positive-sense RNA viruses that belong to the family Coronaviridae [4]. These two viruses can be transmitted through the fecal-oral route and primarily infect small-intestinal epithelial cells [4]. They produce indistinguishable clinical signs characterized by severe diarrhea, anorexia, dehydration, and weight loss [5]. However, the mortality rate is lower and intestinal lesions are milder in PDCoV-infected piglets than in PEDV-infected piglets [6, 7].
Previous studies have shown that coinfection with PEDV occurs in up to 50% of PDCoV-infected diarrheal pigs [7, 8]. Some studies have shown that simultaneous infection with PDCoV and PEDV enhances disease severity in piglets [4, 5]. However, one study showed no increase in disease severity when pigs were first inoculated with the virulent PEDV strain PC21A and then inoculated 16 h later with PDCoV strain OH-FD22 or when they were inoculated simultaneously with both strains [9]. A similar result was also obtained in our previous study, which showed that infection with PDCoV followed by PEDV did not exacerbate disease severity compared to a single infection with either virus [10]. Thus, the possible synergistic effects of PDCoV and PEDV coinfection remain controversial, and the detailed mechanism by which coinfection affects intestinal immunity remains unclear.
The intestinal mucosa often acts as the first line of defense against pathogenic microorganisms [11]. Intraepithelial and lamina propria lymphocytes play a key role in either maintaining or restoring intestinal immune homeostasis [12]. The intestinal mucosal surface is covered with a mucus layer, which mainly consists of mucins secreted by goblet cells (GCs) for both lubrication and maintenance of the barrier function of the intestinal epithelium against enteric pathogens [13, 14]. Toll-like receptors (TLRs) are pattern recognition receptors (PRRs) that can recognize various pathogens and mediate the host innate immune response [15]. Upon virus infection, PRRs recognize viruses and induce production of interferons (IFNs), which are the main antiviral cytokines involved in innate immunity [16]. IFNs are divided into three types (types I, II, and III) based on their different receptors. IFN-α and IFN-λ1 play important roles in antiviral immunity [17]. Notably, one study showed that PDCoV infection significantly induced IFN-α gene expression in the small intestine of piglets, while another study showed that PEDV infection suppressed IFN-α production in the small intestine of piglets [18, 19]. It has also been reported that the inhibitory effect of IFN-λ1 on PEDV infection in porcine intestinal epithelial cells is stronger than that of IFN-α [20].
To further study the effect of intestinal immunity, we coinfected piglets with PDCoV + PEDV. We investigated the number and distribution of immune cells by immunohistochemistry (IHC) and periodic acid–Schiff (PAS) staining in the small intestine. In addition, the gene expression profiles of pattern recognition receptors and downstream mediator cytokines were analyzed by RT-qPCR and ELISA. Our results provide information on the effect on intestinal immunity in piglets coinfected with PDCoV + PEDV.
Materials and methods
Virus strains and piglets
PDCoV strain CHN-HG-2017 (accession number MF095123) and PEDV strain JS-A (accession number MH748550) were used in this study. These two viruses were obtained from Dr. Qigai He and Dr. Zili Li, respectively, of the State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University. Twelve neonatal non-colostrum-fed piglets were selected from a PEDV-, TGEV-, PRCV- and PDCoV-negative herd in Wuhan.
Experimental design
Twelve piglets were divided randomly into four groups (three animals per group): PEDV, PDCoV, PDCoV + PEDV (coinfection), and uninfected control (mock). The piglets were housed in separate rooms according to treatment group with strict biosecurity and biocontainment measures, and they were fed milk substitute (Anyou, China) every 4 h. They were acclimated for 48 h before inoculation. At hour 0 postinoculation (hpi), each piglet in the PDCoV and PDCoV + PEDV groups was inoculated orally with 5 ml of 1 × 105 TCID50/ml PDCoV strain CHN-HG-2017, while those in the PEDV and uninfected control groups were given 5 ml of maintenance medium. At 48 hpi, each piglet in the PEDV and PDCoV + PEDV groups was inoculated orally with 4 ml of 1 × 105 TCID50/mL of PEDV strain JS-A, while those in the PDCoV and uninfected control groups were inoculated orally with an equal volume of maintenance medium. Piglets were euthanized for pathologic examination 72 h after the second exposure as described previously [10]. One part of the tissue samples of the proximal duodenum, middle jejunum, and distal ileum was fixed with 10% formalin for 48 h and then dehydrated, paraffin-embedded, and made into 4-µm serial slices. The other part of the middle jejunum tissue samples was stored at − 80°C for molecular biological analysis.
Periodic acid–Schiff (PAS) staining
To evaluate and quantify goblet cells, sections were stained strictly according to the instructions of the Periodic Acid–Schiff Kit (L/N. G1008-100ML, Servicebio). Six fields were selected randomly from each tissue section for microscopic observation, and the GCs in the villous epithelium were counted using Image-Pro Plus.
Immunohistochemistry (IHC)
For immunohistochemistry, the slides were deparaffinized, rehydrated, and treated with 3% H2O2. Tissue slides were immersed in antigen retrieval buffer containing 5% goat serum for thermal antigen retrieval and sealed at room temperature. An appropriate volume of primary antibody solution was used to cover the tissue at 4 ℃ overnight. Information about the buffers and primary antibodies is given in Table 1. After rinsing with PBS, the slides were incubated with HRP-conjugated goat anti-rat/rabbit IgG (GK600711A, Gene Tech, China) at room temperature for 30 min and then visualized using a DAB Detection Kit (GK500710, Gene Tech, China). The slides were viewed under a microscope to detect positive signals caused by formation of a brown precipitate in the cells. The sections were stained with hematoxylin. Images were obtained using an optical microscope.
Real-time fluorescent quantitative PCR
Total RNA was extracted from the jejunum tissues of each piglet using RNA-easy™ Isolation Reagent (L/N.7E371G9, Vazyme, China), cDNA was synthesized from the extracted RNA by reverse transcription using a HiScript II Q RT Super Mix Kit (L/N.7E421E0, Vazyme, China), and real-time RT-qPCR was performed using Chamq Universal SYBR Master Mix (L/N.7E472E0, Vazyme, China). The levels of TLR2, TLR3, MAVS, IRF3, MDA5, IFN-α, IFN-λ1, and NF-κB were measured by real-time PCR with the primers listed in Table 2. The cycling parameters were 95 °C for 10 min, followed by 40 cycles of 94 °C for 30 s and 60 °C for 1 min. Primers specific for pig genes were designed using the NCBI web server and synthesized by Sangon Biotech Corporation (Wuhan, China).
Enzyme-linked immunosorbent assay
The concentrations of IFN-α and IFN-λ1 in the jejunal tissue supernatant were determined using a Porcine IFN-α ELISA Kit (L/N. YX-E21320, Wenren, China) and a Porcine IFN-λ1 ELISA Kit (L/N.MM-7798602, Meimian, China), respectively, strictly following the instructions. The OD value at 450 nm was measured using an ELISA reader (Multimode Plate Reader EnVision, PerkinElmer, USA) within 15 min.
Statistical analysis
All PAS- and IHC-strained sections of small intestine tissue were examined using a Nikon 80i microscope (Nikon, Japan) equipped with a Nikon 80i camera and Image Scope x64 software (Leica, Germany). Data are presented as the mean ± SD. All data were analyzed using Prism 8.0 (GraphPad Software Inc). One-way ANOVA was used to determine the significance of differences among the different groups. P < 0.05 was considered statistically significant.
Results
Analysis of CD3+ T lymphocytes in the small intestines of piglets
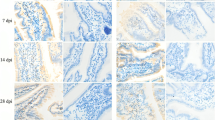
Immunohistochemistry demonstrated that CD3 antigen was positively expressed in villi lamina propria, crypts, and Peyer's patches in the small intestine (Fig. 1a-l). Image analysis demonstrated that CD3 antigen positivity in the small intestines of all virus-infected piglet groups was lower than that in the uninfected control group, with significant differences in the duodenum (P < 0.001, Fig. 1m) and jejunum (P < 0.001, Fig. 1n), but the difference was not significant in the ileum (P > 0.05, Fig. 1o). Image analysis demonstrated that CD3 antigen positivity in the duodenum (P < 0.05, Fig. 1m) of the piglets in the PEDV group was significantly lower than in the piglets in the PDCoV and PDCoV + PEDV groups. In contrast, the piglets in the PDCoV and PDCoV + PEDV groups had lower CD3 antigen positivity in the ileum (P > 0.05, Fig. 1o) than the piglets in the PEDV group. The piglets in the PEDV group had significantly lower CD3 antigen positivity in the jejunum than the piglets in the PDCoV + PEDV group (P < 0.01, Fig. 1n) but did not have significantly lower CD3 antigen positivity in the jejunum than the piglets in the PDCoV group (P > 0.05, Fig. 1n). Image analysis demonstrated that the CD3 antigen positivity of the piglets in the PDCoV group was lower than that in piglets coinfected with PDCoV + PEDV in the jejunum (P < 0.05, Fig. 1n) and ileum (P > 0.05, Fig. 1o) but was slightly higher in the duodenum (P > 0.05, Fig. 1m).
Changes in CD3+ T lymphocyte numbers in the small intestine. (a-l) CD3+ T lymphocytes in the small intestines were detected using immunohistochemistry. (m-o) Integrated optical density of CD3+ T lymphocytes in the intestine (m, duodenum; n, jejunum; o, ileum). Data are presented as the mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (a-c) control group, (d-f) PEDV-infected, (g-i) PDCoV-infected, (j-l) PDCoV + PEDV-infected
Analysis of B lymphocytes in the small intestines of piglets
CD20 is a specific membrane antigen of B lymphocytes [21]. Immunohistochemistry showed that CD20 antigen positivity was mainly present in the intestinal villus lamina propria, submucosa, and Peyer's patches of the small intestine (Fig. 2a-l). Image analysis demonstrated that CD20 antigen positivity in the ileum of all virus-infected piglet groups was lower than that in the uninfected control group (P < 0.001, Fig. 2o) but was slightly higher in the jejunum (P > 0.05, Fig. 2n). Image analysis also demonstrated that CD20 antigen positivity in the duodenum of all uninfected control piglets was lower than in the piglets in the PEDV (P > 0.05, Fig. 2m) and PDCoV + PEDV (P < 0.01, Fig. 2m) groups but was slightly higher than in the PDCoV group (P > 0.05, Fig. 2m). Image analysis demonstrated that CD20 antigen positivity in the duodenum (P < 0.05 or P < 0.001, Fig. 2m) and jejunum (P > 0.05, Fig. 2n) of the piglets in the PDCoV + PEDV group was higher than in the piglets in the PDCoV and PEDV groups. In contrast, the piglets in the PDCoV + PEDV group had lower CD20 antigen positivity in the ileum than the piglets in the PEDV and PDCoV groups (P > 0.05 or P < 0.01, Fig. 2o). Image analysis demonstrated that the CD20 antigen positivity of the piglets in the PDCoV group was slightly lower than in PEDV single-infected piglets in the duodenum (P > 0.05, Fig. 2m) and jejunum (P > 0.05, Fig. 2n) but was significantly higher in the ileum (P < 0.05, Fig. 2o).
Changes in B lymphocyte numbers in the small intestine. (a-l) B lymphocytes in the small intestines were detected using immunohistochemistry. These B lymphocytes were stained using anti-CD20-HRP and counterstained with hematoxylin. (m-o) Integrated optical density of B lymphocyte in the intestine (m, duodenum; n, jejunum; o, ileum). Data are presented as the mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (a-c) control group, (d-f) PEDV-infected, (g-i) PDCoV-infected, (j-l) PDCoV + PEDV-infected
Analysis of goblet cells in the small intestines of piglets
Goblet cells were stained purplish red by PAS staining and were mainly distributed in villi and crypts of the small intestine (Fig. 3a-l). Image analysis demonstrated that the numbers of GCs in the small intestines of all virus-infected piglet groups were lower than in the uninfected control group (P > 0.05, P < 0.01, or P < 0.001, Fig. 3m-o). Image analysis demonstrated that the numbers of GCs in the duodenum (P < 0.001, Fig. 3m) and jejunum (P < 0.001 or P > 0.05, Fig. 3n) of the piglets in the PDCoV and PDCoV + PEDV groups were higher than in the PEDV-single-infected piglets but lower than that in the ileum (P < 0.001 or P > 0.05, Fig. 3o). Image analysis demonstrated that the numbers of GCs in the duodenum (P > 0.05, Fig. 3m) and jejunum (P < 0.01, Fig. 3n) of the piglets in the PDCoV group were higher than in the ileum (P < 0.001, Fig. 3o).
Changes in goblet cell numbers in the small intestine. (a-l) Goblet cells located in the villi and crypt of the intestine. These goblet cells were stained with periodic acid–Schiff stain. (m-o) Changes in the number of goblet cells (m, duodenum; n, jejunum; o, ileum). Data are presented as the mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (a-c) control group, (d-f) PEDV-infected, (g-i) PDCoV-infected, (j-l) PDCoV + PEDV-infected
Expression of cytokines related to intestinal pattern recognition receptors
PDCoV + PEDV-infected piglets were found to have significantly higher TLR2, TLR3, MAVS, and IFN-α mRNA levels than piglets in the PEDV, PDCoV, and uninfected control groups (P < 0.01 or P < 0.001, Fig. 4). PDCoV-infected piglets showed significantly higher TLR3, IRF3, and MAVS mRNA levels than piglets in the PEDV and uninfected control groups (P < 0.001, Fig. 4). PDCoV + PEDV-infected piglets had significantly higher IRF3 mRNA levels than piglets in the PEDV and uninfected control groups (P < 0.001, Fig. 4). PDCoV-infected piglets had significantly lower MDA-5, IFN-λ1, and IFN-α mRNA levels than piglets in the uninfected control group (P < 0.01 or P < 0.001, Fig. 4). PDCoV-infected piglets and PEDV-infected piglets had significantly lower IFN-λ1 mRNA levels than piglets in the PDCoV + PEDV group (P < 0.05 or P < 0.001, Fig. 4). PEDV-infected piglets had significantly lower NF-κB mRNA levels than piglets in the uninfected control and PDCoV + PEDV groups (P < 0.001, Fig. 4). PDCoV-infected piglets had significantly lower NF-κB mRNA levels than piglets in the PDCoV + PEDV group (P < 0.05, Fig. 4). Piglets in the uninfected control group had significantly higher IFN-λ1 mRNA levels than piglets in the PEDV group (P < 0.001, Fig. 4).
Changes in levels of mRNA encoding PRR-pathway-related cytokines in the intestine. To investigate the effect of PDCoV + PEDV coinfection on intestinal PRRs of piglets, the expression of PRR genes and their downstream pathway genes were analyzed by RT-qPCR. Mock, control group (black); PEDV, PEDV-infected (red); PDCoV, PDCoV-infected (green); PDCoV + PEDV, PDCoV + PEDV-infected (blue)
Coinoculation with PDCoV + PEDV upregulates IFN‑α and IFN-λ1
The mean concentrations of IFN-λ1 in all virus-infected piglet groups were lower than that in the uninfected control group (P < 0.05 or P < 0.001, Fig. 5A). PDCoV + PEDV-infected piglets had significantly higher IFN-λ1 concentrations than piglets in the PEDV and PDCoV groups (P < 0.001, Fig. 5A). PDCoV-infected piglets had significantly lower IFN-α concentrations than piglets in the uninfected control group (P < 0.05, Fig. 5B). PDCoV + PEDV-infected piglets had significantly higher IFN‑α concentrations than piglets in the PEDV and PDCoV groups (P < 0.05 or P < 0.01, Fig. 5B).
Discussion
In a previous study, the pathogenicity of PEDV and PDCoV was analyzed. Five-day-old piglets were inoculated orally with 10 ml of 1 × 106 TCID50/ml PDCoV strain CHN-HG-2017 or 3 ml of 1 × 105 TCID50/ml PEDV strain JS-A, and the results were consistent with those of this study [22, 23]. Our previous reports also showed that an animal model of PDCoV + PEDV coinfection was successfully established in neonatal non-colostrum-fed piglets. It was demonstrated that coinfection with PDCoV and PEDV can alter PEDV tropism from epithelial cells of the small intestine to gastric epithelial cells and macrophages in Peyer’s patches in the ileum, and coinfection with PDCoV and PEDV resulted in significantly lower viral loads in the small intestine compared to a single infection with either virus [10]. However, these experimental results need to be verified to determine whether they are related to immune cells in the intestine.
The intestinal mucosal immune system acts as the first line of defense against pathogenic microorganisms [11]. Most intestinal intraepithelial lymphocytes are CD3+ T cells and are distributed in the intestinal epithelium and subjacent superficial lamina propria and thus are positioned at the border of the microbial and dietary environment [24]. B lymphocytes are also major immune cells found in the intestinal mucosa, and these mainly produce IgA antibodies [25]. Intestinal immune cells or secreted cytokines are involved in either maintaining or regulating immune homeostasis [11]. A study showed that the numbers of IgA-positive cells and CD3+ T cells decreased in the small intestine of TGEV-infected piglets [24]. In this study, all infected piglets had decreased numbers of CD3+ T lymphocytes in the small intestine compared with all uninfected control piglets. At the same time, we observed that the numbers of CD3+ T lymphocytes in the PDCoV + PEDV-coinfected piglets were higher than those in the piglets in the PEDV group, especially in the duodenum and jejunum. However, all infected piglets had increased numbers of B lymphocytes, except in the ileum, when compared with uninfected control piglets. To a certain extent, these results showed that viruses may activate antiviral immunity in the small intestines of infected piglets and that B lymphocytes may migrate from the ileal mucosa to the duodenal and jejunal mucosa to secrete antibodies, especially in piglets coinfected with PDCoV + PEDV.
The mucus layer in the small intestine is mainly composed of mucin produced and secreted by GCs, which is an important part of the intestinal mucosal immune system [13]. Previous research has shown that, during the early stages of PEDV infection of piglets, the numbers of GCs in the small intestine are often significantly reduced [14]. As a result, the intestinal mucosal immune barrier of piglets is destroyed, and secondary infections with other enteric pathogens may lead to increased mortality [14, 26]. In this study, all infected piglets had decreased numbers of GCs in the small intestine and significantly decreased numbers in the duodenum and jejunum compared with uninfected control piglets. This result showed that viral infection may decrease the amount of mucins secreted by GCs in the small intestine, possibly leading to an impaired mucus layer. At the same time, we observed that the level of damage to the mucus layer in the piglets in the PDCoV + PEDV coinfection group was lower than that in the piglets in the PEDV group, especially in the duodenum.
Interferons can induce the expression of an extensive range of antiviral effectors that help the host to resist viral infections [27]. In this study, piglets in the PDCoV and PEDV groups exhibited suppressed innate immunity in the intestine, which is consistent with previous reports [4, 18, 28]. Meanwhile, we found that the expression levels of IFN-α and IFN-λ1 in the PDCoV + PEDV-infected piglets were significantly higher than those in piglets inoculated with PDCoV or PEDV alone, and this was confirmed by analysis of the TLR signaling pathway in the small intestine. These results demonstrated that piglets infected first with PDCoV and then with PEDV showed higher levels of intestinal antiviral immunity than those inoculated with PEDV alone. This may partly explain why our previous results showed that piglets coinfected with PDCoV + PEDV showed a significantly lower PDCoV or PEDV viral load in the small intestine than piglets infected with PDCoV or PEDV alone [10]. A similar study showed possible inhibition of PDCoV replication in the gastrointestinal tracts of animals that were first inoculated with the virulent PEDV strain PC21A and then with PDCoV strain OH-FD22 [9]. Another study also yielded a similar result; IFN‑α was significantly upregulated in 4-day-old piglets coinoculated simultaneously with PEDV and PDCoV compared with piglets inoculated with PEDV or PDCoV alone [4].
Taken together, these results show that PEDV may activate the antiviral response of similar immune cells in the small intestine after PDCoV infection, which makes it difficult for piglets to be reinfected with PEDV. This may indicate that inoculation of piglets with an inactivated PDCoV vaccine would also interfere with PEDV infection, but this hypothesis needs to be further evaluated.
In conclusion, this is the first report of the effects of sequential infection with PDCoV followed by PEDV on mucosal immunity in neonatal piglets. Our results suggest that PDCoV + PEDV-coinoculated piglets had increased numbers of B lymphocytes, CD3+ T lymphocytes, and goblet cells in the duodenum and jejunum compared with piglets inoculated with PEDV alone. In addition, IFN‑α and IFN-λ1 were significantly upregulated in PDCoV + PEDV-coinoculated piglets compared with those inoculated with PEDV alone. These results suggest that coinfection with PDCoV + PEDV activates intestinal antiviral immunity more strongly than infection with PEDV alone.
References
Jia S, Feng B, Wang Z, Ma Y, Gao X, Jiang Y, Cui W, Qiao X, Tang L, Li Y, Wang L, Xu Y (2019) Dual priming oligonucleotide (DPO)-based real-time RT-PCR assay for accurate differentiation of four major viruses causing porcine viral diarrhea. Mol Cell Probes 47:101435. https://doi.org/10.1016/j.mcp.2019.101435
Wood EN (1977) An apparently new syndrome of porcine epidemic diarrhoea. Vet Rec 100(12):243–244. https://doi.org/10.1136/vr.100.12.243
Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, Zheng BJ, Chan KH, Yuen KY (2012) Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol 86(7):3995–4008. https://doi.org/10.1128/JVI.06540-11
Saeng-Chuto K, Madapong A, Kaeoket K, Piñeyro PE, Tantituvanont A, Nilubol D (2021) Coinfection of porcine deltacoronavirus and porcine epidemic diarrhea virus increases disease severity, cell trophism and earlier upregulation of IFN-α and IL12. Sci Rep 11(1):3040. https://doi.org/10.1038/s41598-021-82738-8
Zhang H, Han F, Shu X, Li Q, Ding Q, Hao C, Yan X, Xu M, Hu H (2021) Co-infection of porcine epidemic diarrhoea virus and porcine deltacoronavirus enhances the disease severity in piglets. Transbound Emerg Dis. https://doi.org/10.1111/tbed.14144(advance online publication)
Dong N, Fang L, Yang H, Liu H, Du T, Fang P, Wang D, Chen H, Xiao S (2016) Isolation, genomic characterization, and pathogenicity of a Chinese porcine deltacoronavirus strain CHN-HN-2014. Vet Microbiol 196:98–106. https://doi.org/10.1016/j.vetmic.2016.10.022
Zhang H, Liang Q, Li B, Cui X, Wei X, Ding Q, Wang Y, Hu H (2019) Prevalence, phylogenetic and evolutionary analysis of porcine deltacoronavirus in Henan province, China. Prev Vet Med 166:8–15. https://doi.org/10.1016/j.prevetmed.2019.02.017
Feng Y, Xu Z, Zhu L (2020) Prevalence and phylogenetic analysis of porcine deltacoronavirus in Sichuan province, China. Arch Virol 165(12):2883–2889. https://doi.org/10.1007/s00705-020-04796-z
Jung K, Saif LJ (2021) Replication of porcine deltacoronavirus is limited in the gastrointestinal tract of neonatal piglets co-infected simultaneously or 16 hours prior with virulent porcine epidemic diarrhea virus. Vet Microbiol 261:109206. https://doi.org/10.1016/j.vetmic.2021.109206
Jiao Z, Liang J, Yang Y, Li Y, Yan Z, Hu G, Gu C, Hu X, Cheng G, Peng G, Zhang W (2021) Coinfection of porcine deltacoronavirus and porcine epidemic diarrhea virus altered viral tropism in gastrointestinal tract in a piglet model. Virology 558:119–125. https://doi.org/10.1016/j.virol.2021.03.006
Nowarski R, Jackson R, Flavell RA (2017) The stromal intervention: regulation of immunity and inflammation at the epithelial-mesenchymal barrier. Cell 168(3):362–375. https://doi.org/10.1016/j.cell.2016.11.040
Mahlakõiv T, Hernandez P, Gronke K, Diefenbach A, Staeheli P (2015) Leukocyte-derived IFN-α/β and epithelial IFN-λ constitute a compartmentalized mucosal defense system that restricts enteric virus infections. PLoS Pathog 11(4):e1004782. https://doi.org/10.1371/journal.ppat.1004782
McCauley HA, Guasch G (2015) Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med 21(8):492–503. https://doi.org/10.1016/j.molmed.2015.06.003
Jung K, Saif LJ (2017) Goblet cell depletion in small intestinal villous and crypt epithelium of conventional nursing and weaned pigs infected with porcine epidemic diarrhea virus. Res Vet Sci 110:12–15. https://doi.org/10.1016/j.rvsc.2016.10.009
Li H, Pan P, Su X, Liu S, Zhang L, Wu D, Li H, Dai M, Li Y, Hu C, Chen J (2017) Neutrophil Extracellular Traps Are Pathogenic in Ventilator-Induced Lung Injury and Partially Dependent on TLR4. BioMed research international, 2017, 8272504. https://doi.org/10.1155/2017/8272504
Newton AH, Cardani A, Braciale TJ (2016) The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol 38(4):471–482. https://doi.org/10.1007/s00281-016-0558-0
Ingle H, Peterson ST, Baldridge MT (2018) Distinct effects of type I and III interferons on enteric viruses. Viruses 10(1):46. https://doi.org/10.3390/v10010046
Temeeyasen G, Sinha A, Gimenez-Lirola LG, Zhang JQ, Piñeyro PE (2018) Differential gene modulation of pattern-recognition receptor TLR and RIG-I-like and downstream mediators on intestinal mucosa of pigs infected with PEDV non S-INDEL and PEDV S-INDEL strains. Virology 517:188–198. https://doi.org/10.1016/j.virol.2017.11.024
Xu Z, Zhong H, Huang S, Zhou Q, Du Y, Chen L, Xue C, Cao Y (2019) Porcine deltacoronavirus induces TLR3, IL-12, IFN-α, IFN-β and PKR mRNA expression in infected Peyer's patches in vivo. Vet Microbiol 228:226–233. https://doi.org/10.1016/j.vetmic.2018.12.012
Li L, Fu F, Xue M, Chen W, Liu J, Shi H, Chen J, Bu Z, Feng L, Liu P (2017) IFN-lambda preferably inhibits PEDV infection of porcine intestinal epithelial cells compared with IFN-alpha. Antivir Res 140:76–82. https://doi.org/10.1016/j.antiviral.2017.01.012
Tang A, Li C, Chen Z, Li T (2017) Anti-CD20 monoclonal antibody combined with adenovirus vector-mediated IL-10 regulates spleen CD4+/CD8 + T cells and T-bet/GATA-3 expression in NOD mice. Mol Med Rep 16(4):3974–3982. https://doi.org/10.3892/mmr.2017.7111
Zhang MJ, Liu DJ, Liu XL, Ge XY, Jongkaewwattana A, He QG, Luo R (2019) Genomic characterization and pathogenicity of porcine deltacoronavirus strain CHN-HG-2017 from China. Arch Virol 164(2):413–425. https://doi.org/10.1007/s00705-018-4081-6
Qian S, Zhang W, Jia X, Sun Z, Zhang Y, Xiao Y, Li Z (2019) Isolation and identification of porcine epidemic diarrhea virus and its effect on host natural immune response. Front Microbiol 10:2272. https://doi.org/10.3389/fmicb.2019.02272
Xia L, Yang Y, Wang J, Jing Y, Yang Q (2018) Impact of TGEV infection on the pig small intestine. Virol J 15(1):102. https://doi.org/10.1186/s12985-018-1012-9
Berin MC (2012) Mucosal antibodies in the regulation of tolerance and allergy to foods. Semin Immunopathol 34(5):633–642. https://doi.org/10.1007/s00281-012-0325-9
Worliczek HL, Buggelsheim M, Saalmüller A, Joachim A (2007) Porcine isosporosis: infection dynamics, pathophysiology and immunology of experimental infections. Wiener Klinische Wochenschrift 119(Suppl 3):19–20. https://doi.org/10.1007/s00508-007-0859-3
Li S, Yang J, Zhu Z, Zheng H (2020) Porcine epidemic diarrhea virus and the host innate immune response. Pathogens (Basel Switzerland) 9(5):367. https://doi.org/10.3390/pathogens9050367
Zhang Q, Yoo D (2016) Immune evasion of porcine enteric coronaviruses and viral modulation of antiviral innate signaling. Virus Res 226:128–141. https://doi.org/10.1016/j.virusres.2016.05.015
Funding
This work was supported by Da Bei Nong Group Promoted Project for Young Scholar of HZAU (Grant No. 2017DBN004).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jixiang Liang, Yang Li, Zhe Jiao, and Zhishan Yan. The first draft of the manuscript was written by Jixiang Liang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All piglets used in the present study were treated humanely during the experiment and euthanized at the end of the experiment. Animal care and use protocols were reviewed and approved by the experimental animal monitoring committee of Huazhong Agricultural University.
Additional information
Handling Editor: Pablo Pineyro.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, J., Li, Y., Yan, Z. et al. Study of the effect of intestinal immunity in neonatal piglets coinfected with porcine deltacoronavirus and porcine epidemic diarrhea virus. Arch Virol 167, 1649–1657 (2022). https://doi.org/10.1007/s00705-022-05461-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-022-05461-3