Abstract
Geminiviruses are a major threat to agriculture in tropical and subtropical regions of the world. Geminiviruses have small genome with limited coding capacity. Despite this limitation, these viruses have mastered hijacking the host cellular metabolism for their survival. To compensate for the small size of their genome, geminiviruses encode multifunctional proteins. In addition, geminiviruses associate themselves with satellite DNA molecules which also encode proteins that support the virus in establishing successful infection. Geminiviral proteins recruit multiple host factors, suppress the host defense, and manipulate host metabolism to establish infection. We have updated the knowledge accumulated about the proteins of geminiviruses and their satellites in the context of pathogenesis in a single review. We also discuss their interactions with host factors to provide a mechanistic understanding of the infection process.


Similar content being viewed by others
References
Zerbini FM, Briddon RW, Idris A et al (2017) ICTV virus taxonomy profile: geminiviridae. J Gen Virol 98:131–133. https://doi.org/10.1099/jgv.0.000738
Rojas MR, Macedo MA, Maliano MR et al (2018) World management of geminiviruses. Annu Rev Phytopathol 56:637–677. https://doi.org/10.1146/annurev-phyto-080615-100327
Devendran R, Kumar M, Ghosh D et al (2021) Capsicum-infecting begomoviruses as global pathogens: host-virus interplay, pathogenesis, and management. Trends Microbiol. https://doi.org/10.1016/j.tim.2021.05.007
Fiallo-Olivé E, Lett J-M, Martin DP et al (2021) ICTV virus taxonomy profile: geminiviridae 2021. J Gen Virol 102:1–2. https://doi.org/10.1099/jgv.0.001696
Varsani A, Navas-Castillo J, Moriones E et al (2014) Establishment of three new genera in the family Geminiviridae: Becurtovirus, Eragrovirus and Turncurtovirus. Arch Virol 159:2193–2203. https://doi.org/10.1007/s00705-014-2050-2
Varsani A, Roumagnac P, Fuchs M et al (2017) Capulavirus and Grablovirus: two new genera in the family Geminiviridae. Arch Virol 162:1819–1831. https://doi.org/10.1007/s00705-017-3268-6
Brown JK, Zerbini FM, Navas-Castillo J et al (2015) Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch Virol 160:1593–1619. https://doi.org/10.1007/s00705-015-2398-y
Roshan P, Kulshreshtha A, Hallan V (2017) Genome organization of begomoviruses. In: Saxena S, Tiwari AK (eds) Begomoviruses: occurrence and management in Asia and Africa. Springer Singapore, Singapore, pp 11–32
Boulton MI, Pallaghy CK, Chatani M et al (1993) Replication of maize streak virus mutants in maize protoplasts: evidence for a movement protein. Virology 192:85–93. https://doi.org/10.1006/viro.1993.1010
Liu H, Boulton MI, Thomas CL et al (1999) Maize streak virus coat protein is karyophyllic and facilitates nuclear transport of viral DNA. Mol Plant Microbe Interact 12:894–900. https://doi.org/10.1094/MPMI.1999.12.10.894
Briddon RW, Pinner MS, Stanley J, Markham PG (1990) Geminivirus coat protein gene replacement alters insect specificity. Virology 177:85–94. https://doi.org/10.1016/0042-6822(90)90462-Z
Briddon RW, Watts J, Markham PG, Stanley J (1989) The coat protein of beet curly top virus is essential for infectivity. Virology 172:628–633. https://doi.org/10.1016/0042-6822(89)90205-5
Padidam M, Beachy RN, Fauquet CM (1996) The role of AV2 ('precoat’) and coat protein in viral replication and movement in tomato leaf curl geminivirus. Virology 224:390–404. https://doi.org/10.1006/viro.1996.0546
Liu L, Saunders K, Thomas CL et al (1999) Bean yellow dwarf virus RepA, but not rep, binds to maize retinoblastoma protein, and the virus tolerates mutations in the consensus binding motif. Virology 256:270–279. https://doi.org/10.1006/viro.1999.9616
Liu H, Lucy AP, Davies JW, Boulton MI (2001) A single amino acid change in the coat protein of Maize streak virus abolishes systemic infection, but not interaction with viral DNA or movement protein. Mol Plant Pathol 2:223–228. https://doi.org/10.1046/j.1464-6722.2001.00068.x
Kunik T, Mizrachy L, Citovsky V, Gafni Y (1999) Characterization of a tomato karyopherin α that interacts with the Tomato Yellow Leaf Curl Virus (TYLCV) capsid protein1. J Exp Bot 50:731–732. https://doi.org/10.1093/jxb/50.334.731
Rojas MR, Jiang H, Salati R et al (2001) Functional analysis of proteins involved in movement of the monopartite begomovirus, Tomato yellow leaf curl virus. Virology 291:110–125. https://doi.org/10.1006/viro.2001.1194
Ingham DJ, Pascal E, Lazarowitz SG (1995) Both bipartite geminivirus movement proteins define viral host range, but only BL1 determines viral pathogenicity. Virology 207:191–204. https://doi.org/10.1006/viro.1995.1066
Qin S, Ward BM, Lazarowitz SG (1998) The bipartite geminivirus coat protein aids BR1 function in viral movement by affecting the accumulation of viral single-stranded DNA. J Virol 72:9247–9256. https://doi.org/10.1128/JVI.72.11.9247-9256.1998
Unseld S, Höhnle M, Ringel M, Frischmuth T (2001) Subcellular targeting of the coat protein of African cassava mosaic geminivirus. Virology 286:373–383. https://doi.org/10.1006/viro.2001.1003
Sharma P, Ikegami M (2009) Characterization of signals that dictate nuclear/nucleolar and cytoplasmic shuttling of the capsid protein of Tomato leaf curl Java virus associated with DNA beta satellite. Virus Res 144:145–153. https://doi.org/10.1016/j.virusres.2009.04.019
Liu H, Boulton MI, Davies JW (1997) Maize streak virus coat protein binds single- and double-stranded DNA in vitro. J Gen Virol 78(Pt 6):1265–1270. https://doi.org/10.1099/0022-1317-78-6-1265
Guerra-Peraza O, Kirk D, Seltzer V et al (2005) Coat proteins of Rice tungro bacilliform virus and Mungbean yellow mosaic virus contain multiple nuclear-localization signals and interact with importin alpha. J Gen Virol 86:1815–1826. https://doi.org/10.1099/vir.0.80920-0
Pooma W, Petty IT (1996) Tomato golden mosaic virus open reading frame AL4 is genetically distinct from its C4 analogue in monopartite geminiviruses. J Gen Virol 77(Pt 8):1947–1951. https://doi.org/10.1099/0022-1317-77-8-1947
Noris E, Vaira AM, Caciagli P et al (1998) Amino acids in the capsid protein of tomato yellow leaf curl virus that are crucial for systemic infection, particle formation, and insect transmission. J Virol 72:10050–10057. https://doi.org/10.1128/JVI.72.12.10050-10057.1998
Caciagli P, Medina Piles V, Marian D et al (2009) Virion stability is important for the circulative transmission of tomato yellow leaf curl sardinia virus by Bemisia tabaci but virion access to salivary glands does not guarantee transmissibility. J Virol 83:5784–5795. https://doi.org/10.1128/JVI.02267-08
Höhnle M, Höfer P, Bedford ID et al (2001) Exchange of three amino acids in the coat protein results in efficient whitefly transmission of a nontransmissible abutilon mosaic virus isolate. Virology 290:164–171. https://doi.org/10.1006/viro.2001.1140
Pan L-L, Chi Y, Liu C et al (2020) Mutations in the coat protein of a begomovirus result in altered transmission by different species of whitefly vectors. Virus Evol. https://doi.org/10.1093/ve/veaa014
Wang X-R, Wang C, Ban F-X et al (2020) Apoptosis in a whitefly vector activated by a begomovirus enhances viral transmission. mSystems 5:e0043320. https://doi.org/10.1128/mSystems.00433-20
Saurav GK, Rana VS, Popli S et al (2019) A thioredoxin-like protein of Bemisia tabaci interacts with coat protein of begomoviruses. Virus Genes 55:356–367. https://doi.org/10.1007/s11262-019-01657-z
Götz M, Popovski S, Kollenberg M et al (2012) Implication of Bemisia tabaci heat shock protein 70 in begomovirus-whitefly interactions. J Virol 86:13241–13252. https://doi.org/10.1128/JVI.00880-12
Ohnesorge S, Bejarano ER (2009) Begomovirus coat protein interacts with a small heat-shock protein of its transmission vector (Bemisia tabaci). Insect Mol Biol 18:693–703. https://doi.org/10.1111/j.1365-2583.2009.00906.x
Rana VS, Popli S, Saurav GK et al (2016) A Bemisia tabaci midgut protein interacts with begomoviruses and plays a role in virus transmission. Cell Microbiol 18:663–678. https://doi.org/10.1111/cmi.12538
Kanakala S, Ghanim M (2016) Implication of the whitefly Bemisia tabaci cyclophilin b protein in the transmission of tomato yellow leaf curl virus. Front Plant Sci 7:1702. https://doi.org/10.3389/fpls.2016.01702
Gottlieb Y, Zchori-Fein E, Mozes-Daube N et al (2010) The transmission efficiency of tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J Virol 84:9310–9317. https://doi.org/10.1128/JVI.00423-10
Zhao J, Guo T, Lei T et al (2020) Proteomic analyses of whitefly-begomovirus interactions reveal the inhibitory role of tumorous imaginal discs in viral retention. Front Immunol 11:1596. https://doi.org/10.3389/fimmu.2020.01596
Chi Y, Pan L-L, Liu S-S et al (2021) Implication of the whitefly protein vps twenty associated 1 (vta1) in the transmission of cotton leaf curl multan virus. Microorganisms 9:304
Pan L-L, Chen Q-F, Zhao J-J et al (2017) Clathrin-mediated endocytosis is involved in Tomato yellow leaf curl virus transport across the midgut barrier of its whitefly vector. Virology 502:152–159. https://doi.org/10.1016/j.virol.2016.12.029
Fan Y-Y, Zhong Y-W, Zhao J et al (2021) Bemisia tabaci vesicle-associated membrane protein 2 interacts with begomoviruses and plays a role in virus acquisition. Cells 10:1700
Chowda-Reddy RV, Achenjang F, Felton C et al (2008) Role of a geminivirus AV2 protein putative protein kinase C motif on subcellular localization and pathogenicity. Virus Res 135:115–124. https://doi.org/10.1016/j.virusres.2008.02.014
Rothenstein D, Krenz B, Selchow O, Jeske H (2007) Tissue and cell tropism of Indian cassava mosaic virus (ICMV) and its AV2 (precoat) gene product. Virology 359:137–145. https://doi.org/10.1016/j.virol.2006.09.014
Zhao W, Wu S, Barton E et al (2020) Tomato yellow leaf curl virus v2 protein plays a critical role in the nuclear export of v1 protein and viral systemic infection. Front Microbiol 11:1243. https://doi.org/10.3389/fmicb.2020.01243
Bar-Ziv A, Levy Y, Citovsky V, Gafni Y (2015) The Tomato yellow leaf curl virus (TYLCV) V2 protein inhibits enzymatic activity of the host papain-like cysteine protease CYP1. Biochem Biophys Res Commun 460:525–529. https://doi.org/10.1016/j.bbrc.2015.03.063
Muangsan N, Beclin C, Vaucheret H, Robertson D (2004) Geminivirus VIGS of endogenous genes requires SGS2/SDE1 and SGS3 and defines a new branch in the genetic pathway for silencing in plants. Plant J 38:1004–1014. https://doi.org/10.1111/j.1365-313X.2004.02103.x
Glick E, Zrachya A, Levy Y et al (2008) Interaction with host SGS3 is required for suppression of RNA silencing by tomato yellow leaf curl virus V2 protein. Proc Natl Acad Sci USA 105:157–161. https://doi.org/10.1073/pnas.0709036105
Wang B, Yang X, Wang Y et al (2018) Tomato yellow leaf curl virus v2 interacts with host histone deacetylase 6 to suppress methylation-mediated transcriptional gene silencing in plants. J Virol 92:e00036-18. https://doi.org/10.1128/jvi.00036-18
Wang L, Ding Y, He L et al (2020) A virus-encoded protein suppresses methylation of the viral genome through its interaction with AGO4 in the Cajal body. Elife 9:e55542. https://doi.org/10.7554/eLife.55542
Wang Y, Wu Y, Gong Q et al (2019) Geminiviral V2 protein suppresses transcriptional gene silencing through interaction with AGO4. J Virol. https://doi.org/10.1128/JVI.01675-18
Zhang J, Dong J, Xu Y, Wu J (2012) V2 protein encoded by Tomato yellow leaf curl China virus is an RNA silencing suppressor. Virus Res 163:51–58. https://doi.org/10.1016/j.virusres.2011.08.009
Basu S, Kumar Kushwaha N, Kumar Singh A et al (2018) Dynamics of a geminivirus-encoded pre-coat protein and host RNA-dependent RNA polymerase 1 in regulating symptom recovery in tobacco. J Exp Bot 69:2085–2102. https://doi.org/10.1093/jxb/ery043
Desbiez C, David C, Mettouchi A et al (1995) Rep protein of tomato yellow leaf curl geminivirus has an ATPase activity required for viral DNA replication. Proc Natl Acad Sci USA 92:5640–5644. https://doi.org/10.1073/pnas.92.12.5640
Orozco BM, Miller AB, Settlage SB, Hanley-Bowdoin L (1997) Functional domains of a geminivirus replication protein. J Biol Chem 272:9840–9846. https://doi.org/10.1074/jbc.272.15.9840
Hanley-Bowdoin L, Settlage SB, Orozco BM et al (2000) Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Crit Rev Biochem Mol Biol 35:105–140
Ruhel R, Chakraborty S (2019) Multifunctional roles of geminivirus encoded replication initiator protein. VirusDisease 30:66–73. https://doi.org/10.1007/s13337-018-0458-0
Rizvi I, Choudhury NR, Tuteja N (2015) Insights into the functional characteristics of geminivirus rolling-circle replication initiator protein and its interaction with host factors affecting viral DNA replication. Arch Virol 160:375–387. https://doi.org/10.1007/s00705-014-2297-7
Lazarowitz SG, Wu LC, Rogers SG, Elmer JS (1992) Sequence-specific interaction with the viral AL1 protein identifies a geminivirus DNA replication origin. Plant Cell 4:799–809. https://doi.org/10.1105/tpc.4.7.799
Argüello-Astorga GR, Guevara-González RG, Herrera-Estrella LR, Rivera-Bustamante RF (1994) Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication. Virology 203:90–100. https://doi.org/10.1006/viro.1994.1458
Fontes EP, Eagle PA, Sipe PS et al (1994) Interaction between a geminivirus replication protein and origin DNA is essential for viral replication. J Biol Chem 269:8459–8465
Akbar Behjatnia SA, Dry IB, Ali Rezaian M (1998) Identification of the replication-associated protein binding domain within the intergenic region of tomato leaf curl geminivirus. Nucleic Acids Res 26:925–931. https://doi.org/10.1093/nar/26.4.925
Laufs J, Traut W, Heyraud F et al (1995) In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of tomato yellow leaf curl virus. Proc Natl Acad Sci USA 92:3879–3883. https://doi.org/10.1073/pnas.92.9.3879
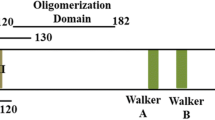
Orozco BM, Kong LJ, Batts LA et al (2000) The multifunctional character of a geminivirus replication protein is reflected by its complex oligomerization properties. J Biol Chem 275:6114–6122. https://doi.org/10.1074/jbc.275.9.6114
Maio F, Arroyo-Mateos M, Bobay BG et al (2019) A lysine residue essential for geminivirus replication also controls nuclear localization of the tomato yellow leaf curl virus rep protein. J Virol. https://doi.org/10.1128/JVI.01910-18
Arroyo-Mateos M, Sabarit B, Maio F et al (2018) Geminivirus replication protein impairs SUMO conjugation of proliferating cellular nuclear antigen at two acceptor sites. J Virol. https://doi.org/10.1128/jvi.00611-18
Clérot D, Bernardi F (2006) DNA helicase activity is associated with the replication initiator protein rep of tomato yellow leaf curl geminivirus. J Virol 80:11322–11330. https://doi.org/10.1128/JVI.00924-06
Choudhury NR, Malik PS, Singh DK et al (2006) The oligomeric Rep protein of Mungbean yellow mosaic India virus (MYMIV) is a likely replicative helicase. Nucleic Acids Res 34:6362–6377. https://doi.org/10.1093/nar/gkl903
George B, Ruhel R, Mazumder M et al (2014) Mutational analysis of the helicase domain of a replication initiator protein reveals critical roles of Lys 272 of the B’ motif and Lys 289 of the β-hairpin loop in geminivirus replication. J Gen Virol 95:1591–1602. https://doi.org/10.1099/vir.0.064923-0
Ruhel R, Mazumder M, Gnanasekaran P et al (2021) Functional implications of residues of the B′ motif of geminivirus replication initiator protein in its helicase activity. FEBS J. https://doi.org/10.1111/febs.16053
Sunter G, Hartitz MD, Bisaro DM (1993) Tomato golden mosaic virus leftward gene expression: autoregulation of geminivirus replication protein. Virology 195:275–280. https://doi.org/10.1006/viro.1993.1374
Hur J, Buckley K, Lee S, Davis K (2007) Transcriptional activator elements for curtovirus C1 expression reside in the 3’ coding region of ORF C1. Mol Cells 23:80–87
Hofer JM, Dekker EL, Reynolds HV et al (1992) Coordinate regulation of replication and virion sense gene expression in wheat dwarf virus. Plant Cell 4:213–223. https://doi.org/10.1105/tpc.4.2.213
Eagle PA, Orozco BM, Hanley-Bowdoin L (1994) A DNA sequence required for geminivirus replication also mediates transcriptional regulation. Plant Cell 6:1157–1170. https://doi.org/10.1105/tpc.6.8.1157
Shung CY, Sunter G (2007) AL1-dependent repression of transcription enhances expression of Tomato golden mosaic virus AL2 and AL3. Virology 364:112–122. https://doi.org/10.1016/j.virol.2007.03.006
Sardo L, Lucioli A, Tavazza M et al (2011) An RGG sequence in the replication-associated protein (Rep) of Tomato yellow leaf curl Sardinia virus is involved in transcriptional repression and severely impacts resistance in Rep-expressing plants. J Gen Virol 92:204–209. https://doi.org/10.1099/vir.0.025817-0
Kushwaha NK, Bhardwaj M, Chakraborty S (2017) The replication initiator protein of a geminivirus interacts with host monoubiquitination machinery and stimulates transcription of the viral genome. PLoS Pathog 13:e1006587. https://doi.org/10.1371/journal.ppat.1006587
Rodríguez-Negrete E, Lozano-Durán R, Piedra-Aguilera A et al (2013) Geminivirus Rep protein interferes with the plant DNA methylation machinery and suppresses transcriptional gene silencing. New Phytol 199:464–475. https://doi.org/10.1111/nph.12286
Li F, Zhang M, Zhang C, Zhou X (2020) Nuclear autophagy degrades a geminivirus nuclear protein to restrict viral infection in solanaceous plants. New Phytol 225:1746–1761. https://doi.org/10.1111/nph.16268
Kushwaha NK, Singh AK et al (2019) Nicotiana benthamiana phosphatidylinositol 4-kinase type II regulates chilli leaf curl virus pathogenesis. Mol Plant Pathol 20:1408–1424. https://doi.org/10.1111/mpp.12846
Wang D, Zhang X, Yao X et al (2020) A 7-amino-acid motif of rep protein essential for virulence is critical for triggering host defense against Sri Lankan cassava mosaic virus. Mol Plant Microbe Interact 33:78–86. https://doi.org/10.1094/MPMI-06-19-0163-FI
Haley A, Zhan X, Richardson K et al (1992) Regulation of the activities of African cassava mosaic virus promoters by the AC1, AC2, and AC3 gene products. Virology 188:905–909. https://doi.org/10.1016/0042-6822(92)90551-y
Sunter G, Bisaro DM (1991) Transactivation in a geminivirus: AL2 gene product is needed for coat protein expression. Virology 180:416–419. https://doi.org/10.1016/0042-6822(91)90049-H
Sunter G, Bisaro DM (1992) Transactivation of geminivirus AR1 and BR1 gene expression by the viral AL2 gene product occurs at the level of transcription. Plant Cell 4:1321–1331. https://doi.org/10.1105/tpc.4.10.1321
Li H, Li F, Zhang M et al (2020) Dynamic subcellular localization, accumulation, and interactions of proteins from tomato yellow leaf curl china virus and its associated betasatellite. Front Plant Sci 11:840. https://doi.org/10.3389/fpls.2020.00840
Hartitz MD, Sunter G, Bisaro DM (1999) The tomato golden mosaic virus transactivator (TrAP) is a single- stranded DNA and zinc-binding phosphoprotein with an acidic activation domain. Virology 263:1–14. https://doi.org/10.1006/viro.1999.9925
Luna AP, Lozano-Durán R (2020) Geminivirus-encoded proteins: not all positional homologs are made equal. Front Microbiol. https://doi.org/10.3389/fmicb.2020.00878
Babu KSD, Manoharan P, Pandi G (2018) Computational studies on Begomoviral AC2/C2 proteins. Bioinformation 14:294–303. https://doi.org/10.6026/97320630014294
Sunter G, Hartitz MD, Hormuzdi SG et al (1990) Genetic analysis of tomato golden mosaic virus: ORF AL2 is required for coat protein accumulation while ORF AL3 is necessary for efficient DNA replication. Virology 179:69–77. https://doi.org/10.1016/0042-6822(90)90275-V
Lacatus G, Sunter G (2009) The Arabidopsis PEAPOD2 transcription factor interacts with geminivirus AL2 protein and the coat protein promoter. Virology 392:196–202. https://doi.org/10.1016/j.virol.2009.07.004
Shivaprasad PV, Akbergenov R, Trinks D et al (2005) Promoters, transcripts, and regulatory proteins of mungbean yellow mosaic geminivirus. J Virol 79:8149–8163. https://doi.org/10.1128/JVI.79.13.8149-8163.2005
Chandran SA, Levy Y, Mett A et al (2012) Mapping of functional region conferring nuclear localization and karyopherin α-binding activity of the C2 protein of bhendi yellow vein mosaic virus. J Gen Virol 93:1367–1374. https://doi.org/10.1099/vir.0.038943-0
Voinnet O, Pinto YM, Baulcombe DC (1999) Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc Natl Acad Sci 96:14147–14152. https://doi.org/10.1073/pnas.96.24.14147
Buchmann RC, Asad S, Wolf JN et al (2009) Geminivirus AL2 and L2 proteins suppress transcriptional gene silencing and cause genome-wide reductions in cytosine methylation. J Virol 83:5005–5013. https://doi.org/10.1128/JVI.01771-08
Yang L-P, Fang Y-Y, An C-P et al (2013) C2-mediated decrease in DNA methylation, accumulation of siRNAs, and increase in expression for genes involved in defense pathways in plants infected with beet severe curly top virus. Plant J 73:910–917. https://doi.org/10.1111/tpj.12081
Sundaresan G, Das SS, Tripathi A et al (2020) Evaluating the strength of RNA silencing suppressor proteins encoded by two geminiviruses using assay based on reversal of GFP silencing. Australas Plant Pathol 49:95–106. https://doi.org/10.1007/s13313-019-00678-4
Wang H, Hao L, Shung C-Y et al (2003) Adenosine kinase is inactivated by geminivirus al2 and l2 proteins. Plant Cell 15:3020–3032. https://doi.org/10.1105/tpc.015180
Wang H, Buckley KJ, Yang X et al (2005) Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins. J Virol 79:7410–7418. https://doi.org/10.1128/jvi.79.12.7410-7418.2005
Hao L, Wang H, Sunter G, Bisaro DM (2003) Geminivirus AL2 and L2 Proteins Interact with and Inactivate SNF1 Kinase. Plant Cell 15:1034–1048. https://doi.org/10.1105/tpc.009530
Trinks D, Rajeswaran R, Shivaprasad PV et al (2005) Suppression of RNA silencing by a geminivirus nuclear protein, AC2, correlates with transactivation of host genes. J Virol 79:2517–2527. https://doi.org/10.1128/JVI.79.4.2517-2527.2005
Tu Y-C, Tsai W-S, Wei J-Y et al (2017) The C2 protein of tomato leaf curl Taiwan virus is a pathogenicity determinant that interferes with expression of host genes encoding chromomethylases. Physiol Plant 161:515–531. https://doi.org/10.1111/ppl.12615
Castillo-González C, Liu X, Huang C et al (2015) Geminivirus-encoded TrAP suppressor inhibits the histone methyltransferase SUVH4/KYP to counter host defense. Elife 4:e06671. https://doi.org/10.7554/eLife.06671
Kumar V, Mishra SK, Rahman J et al (2015) Mungbean yellow mosaic Indian virus encoded AC2 protein suppresses RNA silencing by inhibiting Arabidopsis RDR6 and AGO1 activities. Virology 486:158–172. https://doi.org/10.1016/j.virol.2015.08.015
Zhang Z, Chen H, Huang X et al (2011) BSCTV C2 attenuates the degradation of SAMDC1 to suppress DNA methylation-mediated gene silencing in Arabidopsis. Plant Cell 23:273–288. https://doi.org/10.1105/tpc.110.081695
Matić S, Pegoraro M, Noris E (2016) The C2 protein of tomato yellow leaf curl Sardinia virus acts as a pathogenicity determinant and a 16-amino acid domain is responsible for inducing a hypersensitive response in plants. Virus Res 215:12–19. https://doi.org/10.1016/j.virusres.2016.01.014
Chandran SA, Jeyabharathy C, Usha R (2014) The C2 protein of Bhendi yellow vein mosaic virus plays an important role in symptom determination and virus replication. Virus Genes 48:203–207. https://doi.org/10.1007/s11262-013-0992-1
Hussain M, Mansoor S, Iram S et al (2007) The hypersensitive response to tomato leaf curl New Delhi virus nuclear shuttle protein is inhibited by transcriptional activator protein. Mol Plant Microbe Interact 20:1581–1588. https://doi.org/10.1094/MPMI-20-12-1581
Mubin M, Amin I, Amrao L et al (2010) The hypersensitive response induced by the V2 protein of a monopartite begomovirus is countered by the C2 protein. Mol Plant Pathol 11:245–254. https://doi.org/10.1111/j.1364-3703.2009.00601.x
Soitamo AJ, Jada B, Lehto K (2012) Expression of geminiviral AC2 RNA silencing suppressor changes sugar and jasmonate responsive gene expression in transgenic tobacco plants. BMC Plant Biol 12:204. https://doi.org/10.1186/1471-2229-12-204
Lozano-Durán R, Rosas-Díaz T, Gusmaroli G et al (2011) Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana. Plant Cell 23:1014–1032. https://doi.org/10.1105/tpc.110.080267
Rosas-Díaz T, Macho AP, Beuzón CR et al (2016) The C2 protein from the geminivirus tomato yellow leaf curl sardinia virus decreases sensitivity to jasmonates and suppresses jasmonate-mediated defences. Plants (Basel, Switzerland) 5:8. https://doi.org/10.3390/plants5010008
Li P, Liu C, Deng W-H et al (2019) Plant begomoviruses subvert ubiquitination to suppress plant defenses against insect vectors. PLoS Pathog 15:e1007607. https://doi.org/10.1371/journal.ppat.1007607
Etessami P, Saunders K, Watts J, Stanley J (1991) Mutational analysis of complementary-sense genes of African cassava mosaic virus DNA A. J Gen Virol 72(Pt 5):1005–1012. https://doi.org/10.1099/0022-1317-72-5-1005
Wu M, Wei H, Tan H et al (2021) Plant DNA polymerases α and δ mediate replication of geminiviruses. Nat Commun 12:2780. https://doi.org/10.1038/s41467-021-23013-2
Sunter G, Stenger DC, Bisaro DM (1994) Heterologous complementation by geminivirus AL2 and AL3 genes. Virology 203:203–210. https://doi.org/10.1006/viro.1994.1477
Pasumarthy KK, Choudhury NR, Mukherjee SK (2010) Tomato leaf curl Kerala virus (ToLCKeV) AC3 protein forms a higher order oligomer and enhances ATPase activity of replication initiator protein (Rep/AC1). Virol J. https://doi.org/10.1186/1743-422X-7-128
Settlage SB, Miller AB, Hanley-Bowdoin L (1996) Interactions between geminivirus replication proteins. J Virol 70:6790–6795. https://doi.org/10.1128/JVI.70.10.6790-6795.1996
Settlage SB, Miller AB, Gruissem W, Hanley-Bowdoin L (2001) Dual interaction of a geminivirus replication accessory factor with a viral replication protein and a plant cell cycle regulator. Virology 279:570–576. https://doi.org/10.1006/viro.2000.0719
Settlage SB, See RG, Hanley-Bowdoin L (2005) Geminivirus C3 protein: replication enhancement and protein interactions. J Virol 79:9885–9895. https://doi.org/10.1128/JVI.79.15.9885-9895.2005
Selth LA, Dogra SC, Saif Rasheed M et al (2005) A NAC domain protein interacts with tomato leaf curl virus replication accessory protein and enhances viral replication. Plant Cell 17:311–325. https://doi.org/10.1105/tpc.104.027235
Pasumarthy KK, Mukherjee SK, Choudhury NR (2011) The presence of tomato leaf curl Kerala virus AC3 protein enhances viral DNA replication and modulates virus induced gene-silencing mechanism in tomato plants. Virol J. https://doi.org/10.1186/1743-422X-8-178
Rigden JE, Krake LR, Rezaian MA, Dry IB (1994) ORF C4 of tomato leaf curl geminivirus is a determinant of symptom severity. Virology 204:847–850. https://doi.org/10.1006/viro.1994.1606
Latham JR, Saunders K, Pinner MS, Stanley J (1997) Induction of plant cell division by beet curly top virus gene C4. Plant J 11:1273–1283. https://doi.org/10.1046/j.1365-313X.1997.11061273.x
Zeng R, Liu X, Li H et al (2020) Danger peptide signaling enhances internalization of a geminivirus symptom determinant in plant cells during infection. J Exp Bot 71:2817–2827. https://doi.org/10.1093/jxb/eraa053
Chellappan P, Vanitharani R, Fauquet CM (2005) MicroRNA-binding viral protein interferes with Arabidopsis development. Proc Natl Acad Sci U S A 102:10381–10386. https://doi.org/10.1073/pnas.0504439102
Li H, Zeng R, Chen Z et al (2018) S-acylation of a geminivirus C4 protein is essential for regulating the CLAVATA pathway in symptom determination. J Exp Bot 69:4459–4468. https://doi.org/10.1093/jxb/ery228
Mills-Lujan K, Andrews DL, Chou C, Deom CM (2015) The Roles of phosphorylation and shaggy-like protein kinases in geminivirus c4 protein induced hyperplasia. PLoS One 10:e0122356. https://doi.org/10.1371/journal.pone.0122356
Mills-Lujan K, Deom CM (2010) Geminivirus C4 protein alters Arabidopsis development. Protoplasma 239:95–110. https://doi.org/10.1007/s00709-009-0086-z
Nikitin PA, Luftig MA (2012) The DNA damage response in viral-induced cellular transformation. Br J Cancer 106:429–435. https://doi.org/10.1038/bjc.2011.612
Park J, Hwang H-S, Buckley KJ et al (2010) C4 protein of Beet severe curly top virus is a pathomorphogenetic factor in Arabidopsis. Plant Cell Rep 29:1377–1389. https://doi.org/10.1007/s00299-010-0923-8
Lai J, Chen H, Teng K et al (2009) RKP, a RING finger E3 ligase induced by BSCTV C4 protein, affects geminivirus infection by regulation of the plant cell cycle. Plant J 57:905–917. https://doi.org/10.1111/j.1365-313X.2008.03737.x
Mei Y, Yang X, Huang C et al (2018) Tomato leaf curl Yunnan virus-encoded C4 induces cell division through enhancing stability of Cyclin D 1.1 via impairing NbSKη -mediated phosphorylation in Nicotiana benthamiana. PLOS Pathog 14:e1006789
Vanitharani R, Chellappan P, Pita JS, Fauquet CM (2004) Differential roles of AC2 and AC4 of cassava geminiviruses in mediating synergism and suppression of posttranscriptional gene silencing. J Virol 78:9487–9498. https://doi.org/10.1128/jvi.78.17.9487-9498.2004
Fondong VN, Reddy RV, Lu C et al (2007) The consensus N-myristoylation motif of a geminivirus AC4 protein is required for membrane binding and pathogenicity. Mol Plant Microbe Interact 20:380–391. https://doi.org/10.1094/mpmi-20-4-0380
Rosas-Diaz T, Zhang D, Fan P et al (2018) A virus-targeted plant receptor-like kinase promotes cell-to-cell spread of RNAi. Proc Natl Acad Sci 115:1388–1393. https://doi.org/10.1073/pnas.1715556115
Li Z, Du Z, Tang Y et al (2020) C4, the pathogenic determinant of tomato leaf curl guangdong virus, may suppress post-transcriptional gene silencing by interacting with BAM1 protein. Front Microbiol 11:851. https://doi.org/10.3389/fmicb.2020.00851
Carluccio AV, Prigigallo MI, Rosas-Diaz T et al (2018) S-acylation mediates Mungbean yellow mosaic virus AC4 localization to the plasma membrane and in turns gene silencing suppression. PLoS Pathog 14:e1007207. https://doi.org/10.1371/journal.ppat.1007207
Dogra SC, Eini O, Rezaian MA, Randles JW (2009) A novel shaggy-like kinase interacts with the Tomato leaf curl virus pathogenicity determinant C4 protein. Plant Mol Biol 71:25–38. https://doi.org/10.1007/s11103-009-9506-x
Mei Y, Zhang F, Wang M et al (2020) Divergent symptoms caused by geminivirus-encoded C4 proteins correlate with their ability to bind NbSKη. J Virol. https://doi.org/10.1128/jvi.01307-20
Mei Y, Wang Y, Hu T et al (2018) Nucleocytoplasmic shuttling of geminivirus C4 protein mediated by phosphorylation and myristoylation is critical for viral pathogenicity. Mol Plant 11:1466–1481. https://doi.org/10.1016/j.molp.2018.10.004
Ismayil A, Haxim Y, Wang Y et al (2018) Cotton Leaf Curl Multan virus C4 protein suppresses both transcriptional and post-transcriptional gene silencing by interacting with SAM synthetase. PLoS Pathog 14:e1007282. https://doi.org/10.1371/journal.ppat.1007282
Mei Y, Wang Y, Li F, Zhou X (2020) The C4 protein encoded by tomato leaf curl Yunnan virus reverses transcriptional gene silencing by interacting with NbDRM2 and impairing its DNA-binding ability. PLoS Pathog 16:e1008829. https://doi.org/10.1371/journal.ppat.1008829
Teng K, Chen H, Lai J et al (2010) Involvement of C4 protein of beet severe curly top virus (family Geminiviridae) in virus movement. PLoS One 5:e11280. https://doi.org/10.1371/journal.pone.0011280
Saeed M, Zafar Y, Randles JW, Rezaian MA (2007) A monopartite begomovirus-associated DNA beta satellite substitutes for the DNA B of a bipartite begomovirus to permit systemic infection. J Gen Virol 88:2881–2889. https://doi.org/10.1099/vir.0.83049-0
Mei Y, Ma Z (2020) Geminivirus C4 antagonizes the HIR1-mediated hypersensitive response by inhibiting the HIR1 self-interaction and promoting degradation of the protein. New Phytol 225:1311–1326. https://doi.org/10.1111/nph.16208
Medina-Puche L, Tan H, Dogra V et al (2020) A defense pathway linking plasma membrane and chloroplasts and co-opted by pathogens. Cell 182:1109-1124.e25. https://doi.org/10.1016/j.cell.2020.07.020
Corrales-Gutierrez M, Medina-Puche L, Yu Y et al (2020) The C4 protein from the geminivirus Tomato yellow leaf curl virus confers drought tolerance in Arabidopsis through an ABA-independent mechanism. Plant Biotechnol J 18:1121–1123. https://doi.org/10.1111/pbi.13280
Sharma N, Sahu PP, Prasad A et al (2021) The Sw5a gene confers resistance to ToLCNDV and triggers an HR response after direct AC4 effector recognition. Proc Natl Acad Sci 118:e2101833118. https://doi.org/10.1073/pnas.2101833118
Garnelo Gómez B, Zhang D, Rosas-Díaz T et al (2019) The C4 protein from tomato yellow leaf curl virus can broadly interact with plant receptor-like kinases. Viruses 11:1009
Fontenelle MR, Luz DF, Gomes APS et al (2007) Functional analysis of the naturally recombinant DNA-A of the bipartite begomovirus Tomato chlorotic mottle virus. Virus Res 126:262–267. https://doi.org/10.1016/j.virusres.2007.02.009
Kheyr-Pour A, Bananej K, Dafalla GA et al (2000) Watermelon chlorotic stunt virus from the Sudan and Iran: sequence comparisons and identification of a whitefly-transmission determinant. Phytopathology® 90:629–635. https://doi.org/10.1094/PHYTO.2000.90.6.629
Melgarejo TA, Kon T, Rojas MR et al (2013) Characterization of a new world monopartite begomovirus causing leaf curl disease of tomato in Ecuador and Peru reveals a new direction in geminivirus evolution. J Virol 87:5397–5413. https://doi.org/10.1128/JVI.00234-13
Raghavan V, Malik PS, Choudhury NR, Mukherjee SK (2004) The DNA-A component of a plant geminivirus (Indian mung bean yellow mosaic virus) replicates in budding yeast cells. J Virol 78:2405–2413. https://doi.org/10.1128/jvi.78.5.2405-2413.2004
McGarry RC, Barron YD, Carvalho MF et al (2003) A novel Arabidopsis acetyltransferase interacts with the geminivirus movement protein NSP. Plant Cell 15:1605–1618. https://doi.org/10.1105/tpc.012120
Noueiry AO, Lucas WJ, Gilbertson RL (1994) Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 76:925–932. https://doi.org/10.1016/0092-8674(94)90366-2
Rojas MR, Noueiry AO, Lucas WJ, Gilbertson RL (1998) Bean dwarf mosaic geminivirus movement proteins recognize DNA in a form- and size-specific manner. Cell 95:105–113. https://doi.org/10.1016/s0092-8674(00)81786-9
Pascal E, Sanderfoot AA, Ward BM et al (1994) The geminivirus BR1 movement protein binds single-stranded DNA and localizes to the cell nucleus. Plant Cell 6:995–1006. https://doi.org/10.1105/tpc.6.7.995
Zhou Y, Rojas MR, Park M-R et al (2011) Histone H3 interacts and colocalizes with the nuclear shuttle protein and the movement protein of a geminivirus. J Virol 85:11821–11832. https://doi.org/10.1128/JVI.00082-11
Ward BM, Lazarowitz SG (1999) Nuclear export in plants. Use of geminivirus movement proteins for a cell-based export assay. Plant Cell 11:1267–1276. https://doi.org/10.1105/tpc.11.7.1267
Carvalho CM, Machado JPB, Zerbini FM, Fontes EPB (2008) NSP-Interacting GTPase. Plant Signal Behav 3:752–754. https://doi.org/10.4161/psb.3.9.6641
Carvalho CM, Fontenelle MR, Florentino LH et al (2008) A novel nucleocytoplasmic traffic GTPase identified as a functional target of the bipartite geminivirus nuclear shuttle protein. Plant J 55:869–880. https://doi.org/10.1111/j.1365-313X.2008.03556.x
Gouveia-Mageste BC, Martins LGC, Dal-Bianco M et al (2021) A plant-specific syntaxin-6 protein contributes to the intracytoplasmic route for the begomovirus CabLCV. Plant Physiol 187:158–173. https://doi.org/10.1093/plphys/kiab252
Ye J, Yang J, Sun Y et al (2015) Geminivirus activates ASYMMETRIC LEAVES 2 to accelerate cytoplasmic DCP2-mediated mRNA turnover and weakens RNA silencing in Arabidopsis. PLoS Pathog 11:e1005196. https://doi.org/10.1371/journal.ppat.1005196
Li R, Weldegergis BT, Li J et al (2014) Virulence factors of geminivirus interact with myc2 to subvert plant resistance and promote vector performance. Plant Cell 26:4991–5008. https://doi.org/10.1105/tpc.114.133181
Carvalho MF, Lazarowitz SG (2004) Interaction of the movement protein NSP and the ArabidopsisAcetyltransferase AtNSI Is necessary for cabbage leaf curl geminivirus infection and pathogenicity. J Virol 78:11161–11171. https://doi.org/10.1128/jvi.78.20.11161-11171.2004
Carvalho MF, Turgeon R, Lazarowitz SG (2006) The geminivirus nuclear shuttle protein NSP inhibits the activity of AtNSI, a vascular-expressed arabidopsis acetyltransferase regulated with the sink-to-source transition. Plant Physiol 140:1317–1330. https://doi.org/10.1104/pp.105.075556
Fontes EPB, Santos AA, Luz DF et al (2004) The geminivirus nuclear shuttle protein is a virulence factor that suppresses transmembrane receptor kinase activity. Genes Dev 18:2545–2556. https://doi.org/10.1101/gad.1245904
Carvalho CM, Santos AA, Pires SR et al (2008) Regulated nuclear trafficking of rpL10A mediated by NIK1 represents a defense strategy of plant cells against virus. PLoS Pathog 4:e1000247. https://doi.org/10.1371/journal.ppat.1000247
Zorzatto C, Machado JP, Lopes KV et al (2015) NIK1-mediated translation suppression functions as a plant antiviral immunity mechanism. Nature 520:679–682. https://doi.org/10.1038/nature14171
Florentino LH, Santos AA, Fontenelle MR et al (2006) A PERK-like receptor kinase interacts with the geminivirus nuclear shuttle protein and potentiates viral infection. J Virol 80:6648–6656. https://doi.org/10.1128/JVI.00173-06
Hussain M, Mansoor S, Iram S et al (2005) The nuclear shuttle protein of Tomato leaf curl New Delhi virus is a pathogenicity determinant. J Virol 79:4434–4439. https://doi.org/10.1128/JVI.79.7.4434-4439.2005
Zhang SC, Wege C, Jeske H (2001) Movement proteins (BC1 and BV1) of Abutilon mosaic geminivirus are cotransported in and between cells of sink but not of source leaves as detected by green fluorescent protein tagging. Virology 290:249–260. https://doi.org/10.1006/viro.2001.1185
Frischmuth S, Wege C, Hülser D, Jeske H (2007) The movement protein BC1 promotes redirection of the nuclear shuttle protein BV1 of Abutilon mosaic geminivirus to the plasma membrane in fission yeast. Protoplasma 230:117–123. https://doi.org/10.1007/s00709-006-0223-x
Zhan B, Zhao W, Li S et al (2018) Functional scanning of apple geminivirus proteins as symptom determinants and suppressors of posttranscriptional gene silencing. Viruses 10:488. https://doi.org/10.3390/v10090488
Zhang SC, Ghosh R, Jeske H (2002) Subcellular targeting domains of Abutilon mosaic geminivirus movement protein BC1. Arch Virol 147:2349–2363. https://doi.org/10.1007/s00705-002-0880-9
Lewis JD, Lazarowitz SG (2010) Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.0909080107
Martins LGC, Raimundo GAS, Ribeiro NGA et al (2020) A begomovirus nuclear shuttle protein-interacting immune Hub: Hijacking host transport activities and suppressing incompatible functions. Front Plant Sci 11:398
Radhakrishnan GK, Splitter GA, Usha R (2008) DNA recognition properties of the cell-to-cell movement protein (MP) of soybean isolate of Mungbean yellow mosaic India virus (MYMIV-Sb). Virus Res 131:152–159. https://doi.org/10.1016/j.virusres.2007.09.002
Hou YM, Sanders R, Ursin VM, Gilbertson RL (2000) Transgenic plants expressing geminivirus movement proteins: abnormal phenotypes and delayed infection by Tomato mottle virus in transgenic tomatoes expressing the Bean dwarf mosaic virus BV1 or BC1 proteins. Mol Plant Microbe Interact 13:297–308. https://doi.org/10.1094/MPMI.2000.13.3.297
Duan Y-P, Powell CA, Webb SE et al (1997) geminivirus resistance in transgenic tobacco expressing mutated BC1 protein. Mol Plant-Microbe Interact 10:617–623. https://doi.org/10.1094/MPMI.1997.10.5.617
Fiallo-Olivé E, Martínez-Zubiaur Y, Moriones E, Navas-Castillo J (2012) A novel class of DNA satellites associated with New World begomoviruses. Virology 426:1–6. https://doi.org/10.1016/j.virol.2012.01.024
Zhou X (2013) Advances in understanding begomovirus satellites. Annu Rev Phytopathol 51:357–381. https://doi.org/10.1146/annurev-phyto-082712-102234
Vinoth Kumar R, Singh D, Singh AK, Chakraborty S (2017) Molecular diversity, recombination and population structure of alphasatellites associated with begomovirus disease complexes. Infect Genet Evol 49:39–47. https://doi.org/10.1016/j.meegid.2017.01.001
Mar TB, Mendes IR, Lau D et al (2017) Interaction between the New World begomovirus Euphorbia yellow mosaic virus and its associated alphasatellite: effects on infection and transmission by the whitefly Bemisia tabaci. J Gen Virol 98:1552–1562. https://doi.org/10.1099/jgv.0.000814
Abbas Q, Amin I, Mansoor S et al (2019) The Rep proteins encoded by alphasatellites restore expression of a transcriptionally silenced green fluorescent protein transgene in Nicotiana benthamiana. VirusDisease 30:101–105. https://doi.org/10.1007/s13337-017-0413-5
Nawaz-Ul-Rehman MS, Nahid N, Mansoor S et al (2010) Post-transcriptional gene silencing suppressor activity of two non-pathogenic alphasatellites associated with a begomovirus. Virology 405:300–308. https://doi.org/10.1016/j.virol.2010.06.024
Gnanasekaran P, KishoreKumar R, Bhattacharyya D et al (2019) Multifaceted role of geminivirus associated betasatellite in pathogenesis. Mol Plant Pathol 20:1019–1033. https://doi.org/10.1111/mpp.12800
Reddy K, Bhattacharyya D, Chakraborty S (2020) Mutational study of radish leaf curl betasatellite to understand the role of the non-coding region in begomovirus pathogenesis. Physiol Mol Plant Pathol 112:101549. https://doi.org/10.1016/j.pmpp.2020.101549
Li F, Yang X, Bisaro DM, Zhou X (2018) The βC1 protein of geminivirus-betasatellite complexes: a target and repressor of host defenses. Mol Plant 11:1424–1426. https://doi.org/10.1016/j.molp.2018.10.007
Yang JY, Iwasaki M, Machida C et al (2008) betaC1, the pathogenicity factor of TYLCCNV, interacts with AS1 to alter leaf development and suppress selective jasmonic acid responses. Genes Dev 22:2564–2577. https://doi.org/10.1101/gad.1682208
Bhattacharyya D, Gnanasekaran P, Kumar RK et al (2015) A geminivirus betasatellite damages the structural and functional integrity of chloroplasts leading to symptom formation and inhibition of photosynthesis. J Exp Bot 66:5881–5895. https://doi.org/10.1093/jxb/erv299
Gnanasekaran P, Ponnusamy K, Chakraborty S (2019) A geminivirus betasatellite encoded βC1 protein interacts with PsbP and subverts PsbP-mediated antiviral defence in plants. Mol Plant Pathol 20:943–960. https://doi.org/10.1111/mpp.12804
Prabu G, Neha G, Kalaiarasan P et al (2021) Geminivirus betasatellite-encoded βC1 protein exhibits novel ATP hydrolysis activity that influences its DNA-binding activity and viral pathogenesis. J Virol 95:e00475-21. https://doi.org/10.1128/JVI.00475-21
Eini O, Dogra S, Selth LA et al (2009) Interaction with a host ubiquitin-conjugating enzyme is required for the pathogenicity of a geminiviral DNA beta satellite. Mol Plant Microbe Interact 22:737–746. https://doi.org/10.1094/MPMI-22-6-0737
Shen Q, Hu T, Bao M et al (2016) Tobacco RING E3 ligase NtRFP1 mediates ubiquitination and proteasomal degradation of a geminivirus-encoded βC1. Mol Plant 9:911–925. https://doi.org/10.1016/j.molp.2016.03.008
Nair A, Chatterjee KS, Jha V et al (2020) Stability of Begomoviral pathogenicity determinant βC1 is modulated by mutually antagonistic SUMOylation and SIM interactions. BMC Biol 18:110. https://doi.org/10.1186/s12915-020-00843-y
Yang X, Xie Y, Raja P et al (2011) Suppression of methylation-mediated transcriptional gene silencing by βC1-SAHH protein interaction during geminivirus-betasatellite infection. PLoS Pathog 7:e1002329. https://doi.org/10.1371/journal.ppat.1002329
Zhong X, Wang ZQ, Xiao R et al (2017) Mimic phosphorylation of a βC1 protein encoded by TYLCCNB impairs its functions as a viral suppressor of RNA silencing and a symptom determinant. J Virol 91:e00300–e00317. https://doi.org/10.1128/jvi.00300-17
Eini O (2017) A betasatellite-encoded protein regulates key components of gene silencing system in plants. Mol Biol 51:656–663. https://doi.org/10.7868/s002689841703003x
Li F, Huang C, Li Z, Zhou X (2014) Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLOS Pathog 10:e1003921
Shen Q, Liu Z, Song F et al (2011) Tomato SlSnRK1 protein interacts with and phosphorylates βC1, a pathogenesis protein encoded by a geminivirus β-satellite. Plant Physiol 157:1394–1406. https://doi.org/10.1104/pp.111.184648
Li F, Zhao N, Li Z et al (2017) A calmodulin-like protein suppresses RNA silencing and promotes geminivirus infection by degrading SGS3 via the autophagy pathway in Nicotiana benthamiana. PLOS Pathog 13:e1006213
Haxim Y, Ismayil A, Jia Q et al (2017) Autophagy functions as an antiviral mechanism against geminiviruses in plants. Elife. https://doi.org/10.7554/eLife.23897
Hu T, Huang C, He Y et al (2019) βC1 protein encoded in geminivirus satellite concertedly targets MKK2 and MPK4 to counter host defense. PLOS Pathog 15:e1007728
Saunders K, Bedford ID, Briddon RW et al (2000) A unique virus complex causes Ageratum yellow vein disease. Proc Natl Acad Sci USA 97:6890–6895. https://doi.org/10.1073/pnas.97.12.6890
Cui X, Li G, Wang D et al (2005) A Begomovirus DNAbeta-encoded protein binds DNA, functions as a suppressor of RNA silencing, and targets the cell nucleus. J Virol 79:10764–10775. https://doi.org/10.1128/JVI.79.16.10764-10775.2005
Kumar PP, Usha R, Zrachya A et al (2006) Protein–protein interactions and nuclear trafficking of coat protein and βC1 protein associated with Bhendi yellow vein mosaic disease. Virus Res 122:127–136. https://doi.org/10.1016/j.virusres.2006.07.007
Salvaudon L, De Moraes CM, Yang J-Y et al (2013) Effects of the virus satellite gene βC1 on host plant defense signaling and volatile emission. Plant Signal Behav 8:e23317. https://doi.org/10.4161/psb.23317
Zhao P, Yao X, Cai C et al (2019) Viruses mobilize plant immunity to deter nonvector insect herbivores. Sci Adv 5:eaav9801. https://doi.org/10.1126/sciadv.aav9801
Hu T, Song Y, Wang Y, Zhou X (2020) Functional analysis of a novel βV1 gene identified in a geminivirus betasatellite. Sci China Life Sci 63:688–696. https://doi.org/10.1007/s11427-020-1654-x
Gong P, Tan H, Zhao S et al (2021) Geminiviruses encode additional small proteins with specific subcellular localizations and virulence function. Nat Commun 12:4278. https://doi.org/10.1038/s41467-021-24617-4
Gupta N, Reddy K, Bhattacharyya D, Chakraborty S (2021) Plant responses to geminivirus infection: guardians of the plant immunity. Virol J 18:143. https://doi.org/10.1186/s12985-021-01612-1
Ghosh D, Chakraborty S (2021) Molecular interplay between phytohormones and geminiviruses: a saga of a never-ending arms race. J Exp Bot 72:2903–2917. https://doi.org/10.1093/jxb/erab061
Acknowledgements
We gratefully acknowledge the financial support from the Department of Biotechnology, Govt of India, DBT BUILDER (SLS/BUILDER/SC/2021) and DST-FIST II (SLS/FIST-II/SC/2021). We also thank Malavika M for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing financial interests.
Additional information
Handling Editor: Jesús Navas-Castillo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devendran, R., Namgial, T., Reddy, K.K. et al. Insights into the multifunctional roles of geminivirus-encoded proteins in pathogenesis. Arch Virol 167, 307–326 (2022). https://doi.org/10.1007/s00705-021-05338-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-021-05338-x