Abstract
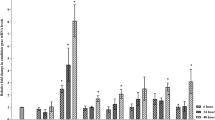
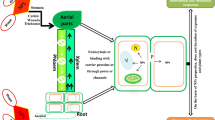
Silver nanoparticles (AgNPs) are a potentially effective tool for preventing viral plant diseases. This study was carried out to evaluate the effectiveness of AgNPs for managing bean yellow mosaic virus (BYMV) disease in faba bean plants from the plant-virus-vector interaction side. AgNPs were evaluated as foliar protective and curative agents. In addition, the effect of AgNPs on virus acquisition and transmission by its vector aphid was investigated. The results indicated that AgNPs exhibited curative viricidal activity and were able to inactivate BYMV when applied 48 hours after virus inoculation. The occurrence of disease was prevented using an AgNP concentration as low as 100 mg L-1, whereas virus infection was completely inhibited when plants were preventatively treated with AgNPs at a concentration of to 200 mg L-1 24 h before virus inoculation. AgNPs proved to be highly bio-reactive, binding to viral particles and suppressing their replication and accumulation within plant tissues. Moreover, AgNPs, at all concentrations tested, were found to upregulate the pathogenesis-related gene PR-1 and induce the production of defense-related oxidizing enzymes in treated plants. Exposure of aphids to AgNPs-treated plants before virus acquisition reduced BYMV acquisition and transmission efficiency by 40.65 to 100% at 24 h post-application, depending on the AgNP dosage. At 10 days after treatment, virus acquisition was reduced by 36.82% and 79.64% upon exposure to AgNPs at a concentration of 250 and 300 mg L-1, respectively. These results suggest that AgNPs have curative viricidal activity due to targeting the virus coat protein and affecting virus-vector interactions. Accordingly, AgNPs may contribute to alleviating the natural disease and virus transmission under field conditions. This is the first report on the activity of nanomaterials against plant virus acquisition and transmission by insects.






Similar content being viewed by others
References
Singh AK, Bharati RC, Manibhushan NC, Pedpati A (2013) An assessment of faba bean (Vicia faba L.) current status and future prospects. Afr J Gric Res 8 (50):6634-6641. https://academicjournals.org/article/article1387443711_Singh%20et%20al.pdf
Robinson GHJ, Balk J, Domoney C (2019) Improving pulse crops as a source of protein, starch and micronutrients. Nutr Bull 44(3):202–215. https://doi.org/10.1111/nbu.12399
Bos L (1970) Bean yellow mosaic virus. CMI/AAB Description of plant viruses, Wageningen: Inst. Phytopathol. Res
ICTVdB, (2006) Bean yellow mosaic virus. In: Büchen-Osmond C (ed) ICTVdB—the Universal virus database, version 4. Columbia University, New York
Sharma PN, Sharma V, Anuradha S, Rajput K, Sharma SK (2015) Identification and molecular characterization of bean yellow mosaic virus infecting French bean in Himachal Pradesh. Virus Dis 26(4):315–318. https://doi.org/10.1007/s13337-015-0270-z
El-Bramawy MAE, El-Beshehy EKF (2011) The resistance of bean yellow mosaic virus (BYMV) in faba bean (Vicia faba L.) with diallel analysis. J Biol Life Sci 2(1):1–15. https://doi.org/10.5296/jbls.v2i1.588
Hampton RO, Jensen A, Hagel GT (2005) Attributes of bean yellow mosaic potyvirus transmission from clover to snap beans by four species of aphids (Homoptera: Aphididae). J Econ Entomol 98(6):1816–1823. https://doi.org/10.1603/0022-0493-98.6.1816
Singh S, Singh BK, Ydava SM, Kupta AK (2014) Applications of nanotechnology in agriculture and their role in disease management. Res J Nanosci Nanotecnol. https://doi.org/10.3923/rjnn.2015.1.5
Prasad R, Bhattacharyya A, Nguyen QD (2017) Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol 8:1014. https://doi.org/10.3389/fmicb.2017.01014
Luca M (2018) Nanotechnology in agriculture: new opportunities and perspectives, new visions in plant science. IntechOpen, London. https://doi.org/10.5772/intechopen.74425
Osuwa JC, Anusionwu PC (2011) Some advances and prospects in nanotechnology: a review. Asian J Inform Technol 10(2):96–100. https://doi.org/10.3923/ajit.2011.96.100
Bakshi M, Singh HB, Abhilash PC (2014) The unseen impact of nanoparticles: more or less? Curr Sci 106:(3)350–352. https://www.jstor.org/stable/24099889
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
Chaloupka K, Malam Y, Seifalian AM (2010) Nanosilver as a new generation of nanoproducts in biomedical applications. Trends Biotechnol 28(11):580–588. https://doi.org/10.1016/j.tibtech.2010.07.006
Elbeshehy EKF, Elazzazy AM, Aggelis G (2015) Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against bean yellow mosaic virus and human pathogens. Front Microbiol 6:453–465. https://doi.org/10.3389/fmicb.2015.00453
Burduşel AC, Gherasim O, Grumezescu AM, Mogoantã L, Ficai A, Andronescu E (2018) Biomedical applications of silver nanoparticles: an up-to-date overview. J Nanomater 8:E681. https://doi.org/10.3390/nano8090681
Jain D, Daima HK, Kachhwaha S, Kothari SL (2009) Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig J Nanom Bios 4 (3):557–653. https://chalcogen.ro/557_Jain.pdf
Mishra S, Singh HB (2015) Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: exploring their scope and potential in agriculture. Appl Microbiol Biotechnol 99:1097–1107
Huang W, Yan M, Duan H, Bi Y, Cheng X, Yu H (2020) Synergistic antifungal activity of green synthesized silver nanoparticles and epoxiconazole against Setosphaeria turcica. J Nanomater. https://doi.org/10.1155/2020/9535432
Noshad A, Mudassar L, Hetherington C, Wahab H (2020) Biogenic AgNPs a nano weapon against bacterial canker of tomato (BCT). Adv Agric 3:1–10. https://doi.org/10.1155/2020/9630785
Jain D, Kothari SL (2014) Green synthesis of silver nanoparticles and their application in plant virus inhibition. Mycol Pl Pathol 44(1):21–24. https://www.researchgate.net/publication/260981626
Yang XX, Li CM, Huang CZ (2016) Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 8:3040–3048. https://doi.org/10.1039/C5NR07918G
Clark MF, Adams AN (1977) Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol 34:475–483. https://doi.org/10.1099/0022-1317-34-3-475
Kuhn CW (1964) Separation of cowpea virus mixtures. Phytopathol 54:739–740
Al-Khalaf M, Kumari SG, Kasem A, Makkouk KM, Shalaby AA, Al-Chaabi SA (2008) Molecular characterization of a Bean yellow mosaic virus isolate from Syria. Phytopathol Mediterr 47:282–285. https://doi.org/10.14601/Phytopathol_Mediterr-2734
Van Dong P, Ha CH, Binh LT, Kaspom J (2012) Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int Nano Lett 2:9. https://doi.org/10.1186/2228-5326-2-9
Richard CM, Braydich-Stolle L, Schrand AM, Schlager J, Hussain SM (2008) Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci 101(2):239–253. https://doi.org/10.1093/toxsci/kfm240
Phanjom DP, Zoremi E, Mazumder J, Saha M, Baruah SB (2012) Green synthesis of silver nanoparticles using leaf extract of Myrica esculenta. Int J Nano-Sci Nanotechnol 7:991–998. https://www.researchgate.net/publication/273762430
Yang X, Liangyi K, Tien P (1996) Resistance of tomato infected with cucumber mosaic virus satellite RNA to potato spindle tuber viroid. Ann Appl Biol 129:543–551. https://doi.org/10.1111/j.1744-7348.1996.tb05775.x
Campos RE, Bejerman N, Nome C, Laguna IG, Pardina PR (2013) Bean yellow mosaic virus in soybean from Argentina. J Phytopathol 162:322–325. https://doi.org/10.1111/jph.12185
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenol oxidase activities during rice leaf senescence. Plant Physiol 57:315–319. https://doi.org/10.1104/pp.57.2.315
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227: 680–685. https://www.nature.com/articles/227680a0
Cheng Y, Zhang H, Yao J, Wang X, Xu J, Han Q, Wei G, Huang L, Kang Z (2012) Characterization of non-host resistance in broad bean to the wheat stripe rust pathogen. BMC Plant Biol 12:96. https://doi.org/10.1186/1471-2229-12-96
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):1–45. https://doi.org/10.1093/nar/29.9.e45
Evans IR, Zettler FW (1970) Aphid and mechanical transmission properties of Bean yellow mosaic virus isolates. Phytopathol 60:1170–1174. https://doi.org/10.1094/Phyto-60-1170
Siliva FDA, Azevedo CA (2009) Principal components analysis in the software assistant-statistical attendance. In: world congress on computers in agriculture, 7, Reno-NV-USA: American Society of Agricultural and Biological Engineers. https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q)
Kumari SG, Makkouk K (2007) Virus diseases of faba bean (Vicia faba L.) in Asia and Africa. Plant Viruses 1(1):93–105. https://www.researchgate.net/publication/272293078_Virus_diseases_of_faba_bean_Vicia_faba_L_in_Asia_and_Africa
Bragard C, Caciagli P, Lemaire O, Lopez-Moya JJ, MacFarlane S, Peters D, Susi P, Torrance L (2013) Status and prospects of plant virus control through interference with vector transmission. Ann Rev Phytopathol. 51:177–201. https://doi.org/10.1146/annurev-phyto-082712-102346
Jones RAC (2014) Trends in plant virus epidemiology: opportunities from new improved technologies. Virus Res 186:3–19. https://doi.org/10.1016/j.virusres.2013.11.003
El-Dougdoug N, Bondok KA (2018) Evaluation of silver nanoparticles as antiviral agent against Tomato mosaic virus (ToMv) and potato virus Y in tomato plants. Mid E J Appl Sci 8(1):100–111. http://www.curresweb.com/mejas/mejas/2018/100-111.pdf
Nakamura S, Sato M, Sato Y, Ando N, Takayama T, Fujita M, Ishihara M (2019) Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int J Mol Sci 20:3620. https://doi.org/10.3390/ijms20153620
Khandelwal N, Kaur G, Kumar N, Tiwari A (2014) Application of silver nanoparticles in viral inhibition: a new hope for antivirals. Dig J Nanomat Bios 9:175–186. https://chalcogen.ro/175_Khandelwal.pdf
Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incoronato N, Russo L, Galdiero S, Galdiero M (2013) Antiviral activity of myco-synthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomed 8:4303–4314
Banerjee V, Das K (2013) Interaction of silver nanoparticles with proteins: a characteristic protein concentration-dependent profile of SPR signal. Colloids Surf Biointerfaces. 11(1):71–79. https://doi.org/10.1016/j.colsurfb.2013.04.052
Ribeiro APC, Anbu S, Alegria ECBA, Fernandes AR, Baptista PV, Mendes R, Pombeiro AJL (2018) Evaluation of cell toxicity and DNA and protein binding of green synthesized silver nanoparticles. Biomed Pharmacother 101:137–144. https://doi.org/10.1016/j.biopha.2018.02.069
Kaveh R, Yue-Shen L, Ranjbar S, Tehrani R, Brueck C, Van Aken B (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ Sci Technol. https://doi.org/10.1021/es402209w
Cvjetko P, Milošić A, Domijan AM, Vinkovic-Vrcek I, Tolic S, PeharecŠtefanic P (2017) Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol Environ Saf 137:18–28. https://doi.org/10.1016/j.ecoenv.2016.11.009
Hawthorne J, Musante C, Sinha SK, White JC (2012) Accumulation and phytotoxicity of engineered nanoparticles to Cucurbita pepo. Int J Phytoremed 14:429–442. https://doi.org/10.1080/15226514.2011.620903
Saha N, Dutta GS (2017) Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips and flower buds of Allium cepa. J Hazard Mater 330:18–28. https://doi.org/10.1016/j.jhazmat.2017.01.021
Mirzajani F, Askari H, Hamzelou S, Schober Y, Rompp A, Ghassempour A, Spengler B (2014) Proteomics study of silver nanoparticles toxicity on Bacillus thuringiensis. Ecotoxicol Environ Saf 100:122–130. https://doi.org/10.1016/j.ecoenv.2013.10.009
Benelli G (2018) Mode of action of nanoparticles against insects. Environ Sci Pollut Res 25(13):1232–1234. https://doi.org/10.1007/s11356-018-1850-4
Chakravarthy A, Chandrashekharaiah K, Kandakoor SB, Bhattacharya A, Dhanabala K, Gurunatha K, Ramesh P (2012) Bioefficacy of inorganic nanoparticles CdS nano-Ag and nano-TiO2 against Spodoptera litura (Lepidoptera: Noctuidae). Curr Biot 6(3):271–281. https://www.researchgate.net/publication/304354666
Nair PMG, Park SY, Lee SW, Choi J (2011) Differential expression of ribosomal protein gene, gonadotrophin-releasing hormone gene and balbiani ring protein gene in silver nanoparticles exposed Chironomus riparius. Aquat Toxicol 101:31–37. https://doi.org/10.1016/j.aquatox.2010.08.013
Deshoux M, Monsion B, Uaest M (2018) Insect cuticular proteins and their role in transmission of phytoviruses. Curr Opin Virol 33:137–143. https://doi.org/10.1016/j.coviro.2018.07.015
Armstrong N, Ramamoorthy M, Lyon D, Jones K, Duttaroy A (2013) Mechanism of silver nanoparticles action on insect pigmentation reveals intervention of copper homeostasis. PLoS ONE 8(1):e53186. https://doi.org/10.1371/journal.pone.0053186
Gadhave KR, Gautam S, Rasmussen DA, Srinivasan R (2020) Aphid transmission of Potyvirus: the largest plant-infecting RNA virus genus. Viruses 12(7):773. https://doi.org/10.3390/v12070773
Whitfield AE, Falk BW, Rotenberg D (2015) Insect vector-mediated transmission of plant viruses. Virol 479–480:278–289. https://doi.org/10.1016/j.virol.2015.03.026
Acknowledgements
The authors would like to thank the Central Laboratory of Nanotechnology & Advanced Materials, Agricultural Research Center (ARC), Giza, Egypt, for providing the laboratory facilities for the characterization of synthesized silver nanoparticles.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Ralf Georg Dietzgen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Gamal, A.Y., Tohamy, M.R., Abou-Zaid, M.I. et al. Silver nanoparticles as a viricidal agent to inhibit plant-infecting viruses and disrupt their acquisition and transmission by their aphid vector. Arch Virol 167, 85–97 (2022). https://doi.org/10.1007/s00705-021-05280-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-021-05280-y