Summary.
A new virus was isolated from tomato plants from the Murcia region in Spain which showed symptoms of ‘torrado disease’ very distinct necrotic, almost burn-like symptoms on leaves of infected plants. The virus particles are isometric with a diameter of approximately 28 nm. The viral genome consists of two (+)ssRNA molecules of 7793 (RNA1) and 5389 nts (RNA2). RNA1 contains one open reading frame (ORF) encoding a predicted polyprotein of 241 kDa that shows conserved regions with motifs typical for a protease-cofactor, a helicase, a protease and an RNA-dependent RNA polymerase. RNA2 contains two, partially overlapping ORFs potentially encoding proteins of 20 and 134 kDa. These viral RNAs are encapsidated by three proteins with estimated sizes of 35, 26 and 23 kDa. Direct protein sequencing mapped these coat proteins to ORF2 on RNA2. Phylogenetic analyses of nucleotide and derived amino acid sequences showed that the virus is related to but distinct from viruses belonging to the genera Sequivirus, Sadwavirus and Cheravirus. This new virus, for which the name tomato torrado virus is proposed, most likely represents a member of a new plant virus genus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past three years, tomato plants with severe necrotic leaf symptoms were observed in the area of Murcia in the South-East of Spain. The symptoms of this new emerging disease consisted initially of necrotic spots, surrounded by a light green or yellow area beginning at the base of leaflets (Fig. 1). This syndrome developed into a severe necrosis of leaves and fruits and overall growth reduction of the plant, resulting in serious economic damage. The disease was named ‘torrado’ by the local farmers, meaning burned or roasted. Primary diagnostics always revealed the presence of pepino mosaic virus (PepMV, genus Potexvirus) [20], but the symptoms could not be attributed to this virus alone. In addition to PepMV, a virus with isometric particles was found in symptomatic samples from the area of Murcia. In this paper, we describe the identification and characterization of this new picorna-like plant virus, which we tentatively named tomato torrado virus (ToTV).
Material and methods
Virus transmission and propagation
ToTV was isolated from a tomato plant showing typical symptoms of ‘torrado’ (i.e. severe leaf necrosis) from the Murcia region, Spain. The isolate was designated PRI-ToTV0301. For mechanical transmission of ToTV to alternative hosts or indicator plants, a standard inoculation buffer (e.g. 0.03 M sodium/potassium phosphate buffer, pH 7.7) proved suitable. The virus was mechanically inoculated to and maintained in Nicotiana glutinosa ‘PRI’ or N. benthamiana.
DAS-ELISA
Double antibody sandwich (DAS)-ELISA was carried out according to standard DAS-ELISA protocols, using antisera to PepMV, tomato aspermy virus (genus Cucumovirus), cucumber mosaic virus (genus Cucumovirus), Andean potato mottle virus (genus Comovirus), Andean potato latent virus (genus Tymovirus), carnation etched ring virus (genus Caulimovirus), potato black ringspot virus (genus Nepovirus), pelargonium flower break virus (genus Carmovirus), pelargonium line pattern virus (genus Carmovirus), melon necrotic spot virus (genus Carmovirus), carnation mottle virus (genus Carmovirus), and carnation ringspot virus (genus Dianthovirus). All antisera were obtained from Prime Diagnostics, Wageningen, The Netherlands.
Virus purification
All centrifugation steps were performed at 6 °C. Infected leaves of N. glutinosa ‘PRI’ or N. benthamiana were harvested approximately 14 days after inoculation of the virus and homogenized in 5 parts (w/v) 0.1 M Tris-HCl, pH 8.0, containing 20 mM Na2SO3, 10 mM Na-DIECA and 5 mM Na-EDTA (homogenization buffer). The homogenate was centrifuged for 30 min at 49,000 g. The supernatant was placed on a sucrose cushion (20% in homogenization buffer) and centrifuged at 70,000 g for 1.5 h. The pellet was resuspended in 2 ml Tris-HCl, pH 8.0, and the suspension was placed onto a sucrose gradient (10–40% in homogenization buffer) and centrifuged at 110,000 g for 2 h. Gradient fractions were tested for the presence of virus by inoculation experiments on N. hesperis ‘67A’. The virus-containing fraction was pipetted from the gradient, placed onto a 10–40% cesium sulfate gradient (in Tris-HCl, pH 8.0) and centrifuged at 125,000 g for 16 h. The virus bands were collected and dialyzed against 0.1 M Tris-HCl, pH 8.0.
Electron microscopy
Virus suspensions were mounted on formvar carbon-coated grids, stained with 2% uranyl acetate and examined in a Philips CM12 electron microscope.
Polyacrylamide gel electrophoresis
Viral proteins were separated by subjecting purified virus particles to 12% denaturing polyacrylamide gel electrophoresis (SDS-PAGE) [10] and visualized by silver staining.
Nucleic acid isolation and evaluation
Purified virus was concentrated by centrifugation (at 115,000 g for 2 h). Pellets were subjected to RNA extraction using a Qiagen RNeasy kit (Qiagen) according to the manufacturer’s instructions. RNA concentration was determined in a Beckmann DU-530 UV-spectrophotometer. Viral RNA integrity and size was checked by running 1 mg of each RNA preparation on a 1% agarose gel using a formaldehyde/formamide/HEPES buffer system. After electrophoresis, the RNA was stained using ortho-toluidine blue.
Protein identification by mass spectrometry
Viral capsid proteins were subjected to mass spectrometry analysis essentially as described by Volpicella et al. [21]. Purified virus particles were separated by SDS-PAGE. After Coomassie Brilliant Blue (CBB R-250, Bio-Rad laboratories) staining, the protein bands of interest were excised from the gel. Prior to in-gel trypsin digestion (Seq. Grade Modified Porcine Trypsin, Promega), the proteins were reduced with dithiothreitol and alkylated with iodoacetamide [16]. After overnight tryptic digestion, the peptides were extracted with 50% acetonitrile, 0.5% formic acid and concentrated in a vacuum centrifuge. The resuspended peptides were then loaded onto a C18 Atlantis column (15 cm × 75 µm ID, Waters). Peptides were eluted by a gradient from 0.5% formic acid in water to 0.5% formic acid in 50% acetonitrile at a speed of approximately 0.2 µl/min. The C18 column was connected to the electro-spray of a Q-Tof-2 mass spectrometer (Waters) by a PicoTip (New Objective). The resulting MS/MS spectra contained the sequence information for a single peptide per spectrum. The ProteinLynx GlobalServer package V2.1 (Waters) was used to process MS/MS data, which were automatically selected for blasting against the protein translation of the ORF2 sequence on RNA2.
cDNA synthesis and cloning
cDNA was synthesized using the SuperScript Choice System for cDNA Synthesis (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA was primed using either oligo(dT) or random hexamer primers. After second-strand synthesis, EcoRI adapters were ligated to facilitate cloning into pBluescript II IR Predigested Vector (Stratagene). The resulting constructs were transformed to TOP10 competent cells (Invitrogen), and insert lengths of recombinant colonies were determined by colony PCR using both T3 and T7 cloning-vector-specific primers. Clones containing inserts over 1500 nucleotides (nts) were used for further sequence analysis. Additional cDNA fragments were obtained by one-tube RT-PCR (Access RT-PCR system, Promega) with ToTV-specific primers derived from earlier obtained sequence data.
The 5′ terminus of the ToTV sequence was determined using the 5′ RACE System for Rapid Amplification of cDNA Ends (LifeTechnologies) using dCTP according to the manufacturer’s instructions.
Nucleotide sequencing and sequence analysis
Cloned cDNA fragments and PCR products were sequenced directly. Sequence analysis was performed using an Applied Biosystems 3100 Genetic Analyser, with a DYEnamic ET Terminator Cycle Sequencing Kit (Amersham) and the primers that had been used for amplification. For additional PCR fragments, ToTV-specific primers were used for primer-walking sequencing.
Nucleotide and amino acid sequence data were analyzed and assembled using the DNASTAR package (Lasergene). Blast searches were carried out using the NCBI Blast server (www.ncbi.nlm.nih.gov) with all available databases. Sequence comparisons with other viruses were performed with programs from the PHYLIP package. Multiple alignments and phylogenies were performed with the CLUSTAL X program after bootstrapping in 1000 replicates. Neighborjoining consensus phylogenies were viewed by the NJplot program [18] and printed using TreeView [15].
Results and discussion
Virus characterisation
Tomato plants showing very typical burn-like necrotic symptoms (‘torrado’) on leaves and fruits were found in the Murcia area in Spain. Initially, infected tomato plants were examined by electron microscopy, which revealed the presence of two distinct virus particles: filamentous particles of approximately 550 nm in length and isometric particles of about 28 nm in diameter. ELISA showed the presence of PepMV in infected plants but no reactions were observed with available antisera to carmovirus, caulimovirus, comovirus, cucumovirus, dianthovirus, nepovirus and tymovirus isolates (results not shown).
Mechanical inoculation experiments onto indicator plants showed that Physalis floridana and Nicotiana glutinosa ‘PRI’ were hosts for the spherical virus, but not for PepMV. These alternative host plants could be used as filter hosts, and a pure isolate of the unknown virus, ToTV, was obtained. Other test plant reactions to ToTV inoculations are presented in Table 1.
To verify that the virus encountered is the causal agent of the ‘torrado’ disease, purified virions were mechanically inoculated onto tomato plants. Necrosis beginning at the base of a leaflet, which is typical for ‘torrado’ disease, emerged two weeks after inoculation. The virus could be re-isolated from the inoculated tomato plants, and its identity was verified by RT-PCR with virus-specific primers (nts 4141-4161 and 4705-4724 on RNA2; results not shown).
Development of a purification protocol allowed further characterization of the virus, however yields were low (15–20 µg/100 g leaf material). The final virus purification step employing Cs2SO4 buoyant density gradient centrifugation yielded two types of particles, identical in size (28 nm) but different in density. The top band in the gradient (T) always contained only a 5.5-kb RNA (RNA2). RNA isolated from the bottom band (B) was always resolved into two RNA bands on a denaturing agarose gel; a 5.5-kb band and a band of about 8 kb (RNA1; see Fig. 2). SDS-PAGE revealed that both particle types were composed of three coat proteins with estimated sizes of 35, 26 and 23 kDa, respectively named Vp35, Vp26 and Vp23 (Fig. 3A).
Denaturating agarose gel electrophoresis of RNAs extracted from ToTV virions and stained with orthotoluidine blue. 1 (M): molecular size standard (Invitrogen 0.24–9.5-kb RNA Ladder); 2 (T): RNA purified from ToTV top component; 3 (B): RNA purified from ToTV bottom component. Arrows indicate positions of the ∼5.5-kb and ∼8-kb RNA bands
A: Silver-stained capsid proteins after denaturing polyacrylamide gelelectrophoresis (SDS-PAGE). 1 and 4 (M): molecular weight markers (Bio-Rad silver stain markers, low range), 2: ToTV-T (top band of purified virions in Cs2SO4 buoyant density gradient centrifugation) and 3: ToTV-B (bottom band of purified virions after Cs2SO4 buoyant density gradient centrifugation). B: Amino acid sequence of RNA2-ORF2. Positions of the three coat proteins (Vp35, Vp26 and Vp23) are indicated by boxes. Putative cleavage site areas are indicated by dotted lines. Peptides found in MS=MS analysis are shaded
Viral RNA analysis
Generally, the band containing the bottom component was more diffuse than the band of the top component. Also, cDNA libraries derived from particles from the bottom fraction of the Cs2SO4 gradient always contained sequences typical for RNA1 and RNA2 and, in addition, were contaminated with plant RNA sequences. This is in contrast to cDNA libraries derived from the top component band, which only contained sequences typical for RNA2 and virtually no plant-specific sequences (results not shown). This indicates that the bottom band after Cs2SO4 buoyant density gradient centrifugation contained both types of particles, each separately encapsidating an RNA1 or RNA2 and, in addition, a minor contamination with plant components.
RNA isolated from each Cs2SO4 gradient component was used to create a cDNA library. cDNA synthesis and cloning of RNA1 from the bottom component and RNA2 from the top component yielded 15 and 14 clones, respectively. Sequence analysis and assembly resulted in two contigs for each of the two RNAs. The missing regions between the contigs were amplified by RT-PCR with ToTV-specific primers derived from the contig sequences already obtained.
The sequence analysis resulted in contigs of ∼7.7 kb and ∼5.2 kb for RNA1 and RNA2, respectively. Determination of the 5′ terminal sequences of the two ToTV RNAs, using a 5′ RACE kit, from 10 individual clones indicated that RNA1 consists of 7793 nt and RNA2 of 5389 nt [both excluding the poly(A) tail]. The genome sequence is A/U rich: A(26.9%), U(28.9%), C(20.5%), G(23.7%).
RNA1
RNA1 [7793 nt, without poly(A) tail] contains one open reading frame (RNA1-ORF1) encoding a predicted polyprotein of 2158 amino acids (aa) with a molecular mass of 241 kDa (Fig. 4). The first inframe AUG is at nt positions 107–109. The ORF has an UGA stop codon at nt positions 6581–6583. The polyprotein contains conserved regions with motifs typical for a protease cofactor, helicase, protease and RNA-dependent RNA polymerase (RdRp).
Genome organization of the tomato torrado virus isolate PRI-ToTV0301. Relative positions of regions containing motifs of protease-cofactor (PRO-co), helicase (HEL), protease (PRO), and RNA-dependent RNA polymerase (RdRp) on RNA1 and of the three coat proteins (Vp35, Vp26 and Vp23) and putative movement protein (MP) on RNA2 are indicated
The closest identities of the complete polyprotein sequence were found with members of the family Sequiviridae and the floating (unassigned) genera Cheravirus and Sadwavirus: rice tungro spherical virus (RTSV, genus Waikavirus; CAA67042), with 29% identity between aa positions 897 and 1647 of the ToTV translated sequence; maize chlorotic dwarf virus (MCDV, genus Waikavirus; AAV86083), with 28% identity between aa positions 905 and 1646; parsnip yellow fleck virus (PYFV, genus Sequivirus; BAA03151.1), with 33% identity between aa positions 1155 and 1655; apple latent spherical virus (ALSV, genus Cheravirus; BAA90870.1), with 32% identity between aa positions 1155 and 1626; strawberry mottle virus (SMoV, genus Sadwavirus; NP_599086), with 30% identity between aa positions 1039 and 1718; and cherry rasp leaf virus (CRLV, genus Cheravirus; CAF21713.1), with 33% identity between aa positions 1155 and 1619.
In the C-terminal part of the RNA1-ORF1, a low level of aa sequence similarity (22%) was observed with a protease co-factor (PRO-co) of patchouli mild mosaic virus (PatMMV; NP_647592.1, a strain of Broad bean wilt virus 2, genus Fabavirus), for the aa positions 106–338.
Typical helicase motifs A (GKS), B (D), C (N) were identified at aa positions 398–400, 444 and 495 of the putative polyprotein. The closest identities in the helicase region were found with RTSV (NP_042507.1; 42% identical between aa positions 381 and 520), MCDV (AAB58882.1; 43% identical between aa positions 383 and 519), SMoV (NP_599086.1; 42% identical between aa positions 386 and 520) and PYFV (NP_619734.1; 42% identical between aa positions 383 and 520). The highest similarity in the protease region (PRO) was found for aa 1000–1100, 25% identity is found with potato virus V (PVV, genus Potyvirus; NP_659008.1; NIa protease between aa positions 1003 and 1088). The RdRp region could be identified between aa 1303 and 1554 by the presence of the typical motifs I (KDE) to VII (FLSR) [9].
RNA2
RNA2 [5389 nt, without poly(A) tail] contains two potential ORFs. The first ORF (RNA2-ORF1) encodes a predicted protein of 187 aa with a molecular mass of 20 kDa (Fig. 4). The first in-frame AUG of RNA2-ORF1 is at nt position 182–184. RNA2-ORF1 has a UGA stop codon at positions 743–745 nt. In Blast searches, the RNA2-ORF1 polyprotein shows no apparent homologies with proteins in sequence databases.
The position of ORF1 on RNA2 of ToTV was verified by sequence analysis of three independently obtained RT-PCR fragments generated from total RNA isolated from three individual ToTV infected host plants. Each RT-PCR fragment (nt pos 140–861 on RNA2) was identical in sequence to the sequences obtained initially from five different cDNA clones. This confirmed the presence of both the ORF1 AUG start codon at position 182–184 and the ORF1 UGA stop codon at position 743–745 as well as the three in-frame AUG start codons of ORF2 at positions 702–710 (see below).
The second ORF (RNA2-ORF2) encodes a predicted protein of 1198 aa with a molecular mass of 134 kDa. The first in-frame AUG of RNA2-ORF2 is at nt positions 702–704, and thus it partly overlaps with RNA2-ORF1. The first AUG is immediately followed by two other AUG start codons in frame. An UAA stop codon is found at nt positions 4296– 4298. The ORF2 polyprotein contains a movement protein (MP) motif and shows low levels of similarity with viral coat proteins (CPs). The N-terminal region of the RNA2-ORF2 polyprotein most likely codes for the putative MP since a motif LRVPML highly similar to the proposed movement protein consensus sequence LxxPxL [13] was found at aa position 262–267. No other sequence homologies were found in the N-terminus of the RNA2-ORF2.
To verify that the three CPs are encoded by the RNA2-ORF2, the three separated CPs were analyzed using a tandem mass spectrometer (MS/MS). This resulted in aa sequences of small peptides, each of which was aligned with the aa sequence deduced from the RNA2 nucleotide sequence. Fragments of the largest CP (Vp35; ∼35 kDa) could be aligned with an area in the RNA2-ORF2 between aa 487–729. Fragments of the Vp26 (∼26 kDa) could be aligned with an area of RNA2-ORF2 between aa 730 and 983, while fragments of the smallest CP (Vp23; ∼23 kDa) could be aligned with the C-terminus of RNA2-ORF2 (aa 983-1195). These results suggested that the coding sequences of the three CPs of ToTV are located in the RNA2-ORF2 and are in the order Vp35, Vp26 and Vp23 (Fig. 3). Thus, ORF2 potentially encodes four proteins which are likely to be cleaved from the polyprotein precursor by proteolytic cleavage. Since detailed alignments did not reveal any homologies with known protease cleavage sites, the exact cleavage site between the putative movement protein and Vp35 remains unknown.
On the basis of the protein sequencing results, the cleavage site between Vp35 and Vp26 is likely to be located in the aa region 730–733 (LRAQ) and that between Vp26 and Vp23 in the aa region 971–982 (KQPQVQVPLRDK) (Fig. 3b). However, no apparent homologies with known polyprotein cleavage sites were identified. Protease recognition sites in plant picorna-like viruses are known to be very diverse, and the exact positions of these cleavage sites remain to be determined experimentally.
For the aa positions 483–981 (Vp35 and Vp26) of RNA2-ORF2, a 21% identity was found with RTSV (AAB17090.1) between aa positions 614 and 930. The Vp35 aa sequence alone showed a 21% identity between aa positions 602 and 704 with human parechovirus (HPeV, genus Parechovirus; BAD05057.1). The closest identities of the Vp26 aa sequence were found with Rhopalosiphum padi virus (genus Cripavirus; NP_046156.1; with 25% identity between aa positions 750 and 917), avian encephalomyelitis virus (genus Hepatovirus; NP_653151.1; with 33% between aa positions 760 and 833), black queen cell virus (unassigned; AAF72338.1; with 43% identity between aa positions 786 and 822), and Solenopsis invicta virus (unassigned; AAU85376.1; with 30% identity between aa positions 772 and 822). For the Vp23 aa sequence, no significant homologies were found.
5′- and 3′-untranslated regions (UTRs)
The 5′-UTR sizes of RNA1 and RNA2 are 106 nt and 181 nt, respectively. There is 31% overall sequence identity in the first 106 nucleotides. For the first 17 nt, both RNAs share a sequence identity of 82%.
The 3′-UTRs of RNA1 and RNA2 are 1210 nt and 1092 nt, respectively, in length and have an overall level of identity of 90.2%. Interestingly, however, the 988 most 3′-terminal nucleotides of both RNAs are almost perfectly conserved (98% identity). To confirm that the 3′-regions of the 3′-UTRs of both RNAs are nearly identical and to exclude cloning artifacts, RT-PCRs were performed on total viral RNA isolated from purified virions using one reverse primer derived from the identical 3′-UTR region (RNA1 at nt 7109–7128 and RNA2 at nt 4705–4724) and two forward primers located upstream of the 3′-UTR region and specific for either RNA1 (nts 6515-6534) or RNA2 (nts 4141-4161) . This resulted in PCR fragments of the expected sizes. Sequence analysis of these RT-PCR fragments confirmed the presence of RNA1- or RNA2-specific sequences at their 5′-end, followed by nearly identical regions in the 3′-part of both 3′-UTRs.
Taxonomic position of ToTV
ToTV shares virion characteristics and sequence similarities with viruses of the genera Sequivirus and Waikavirus (family Sequiviridae) and the unassigned genera Cheravirus and Sadwavirus. Viruses assigned to these genera are distinguished on the basis of the number of genomic RNAs (members of the Sequiviridae have a monopartite genome, whereas those of chera- and sadwaviruses are bipartite) and the number of CPs (two in sadwaviruses vs. three CPs in sequi- and cheraviruses). The virus characterized in this study possesses icosahedral virions measuring 28 nm in diameter and containing three CPs, which encapsidate two single-stranded, positive-sense, polyadenylated RNAs. These data suggest that ToTV shares most structural characteristics with viruses of the genus Cheravirus [11].
The latest ICTV report [4] states: ‘Cheraviruses were previously considered as atypical but tentative members of the genus Nepovirus (family Comoviridae), but were distinguished on the basis of their genomic organization, in particular the number of CP species, as well as sequence homologies and, for some of them, natural transmission by insects’. Both cheraviruses and sadwaviruses are now considered members of unassigned genera with affinities to members of the family Sequiviridae. So far, the genus Cheravirus contains two assigned species, CRLV [7, 8, 19] and ALSV [12], while a third virus (stocky prune virus; StPV) has recently been proposed as a possible new member of this genus [3].
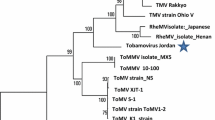
The aa region between the CG protease motif [2] and the GDD RdRp active site [1] in the RNA1-ORF1 is proposed to be a good taxonomic predictor for classifying picorna-like viruses [6]. Because ToTV showed similarities to viruses of the picornavirus ‘superfamily’, this region of ToTV was used in a phylogenetic analysis including comparable regions of viruses from the genera Sadwavirus, Cheravirus and the families Sequiviridae, Comoviridae, Dicistroviridae and Picornaviridae. The resulting dendrogram (Fig. 5A) shows that ToTV does not cluster with cheraviruses. A similar phylogenetic analysis on the basis of the helicase region between the motifs A and C [5] (aa 382–495) confirms the separate taxonomic position of ToTV from the cheraviruses (Fig. 5B).
Phylogenetic analysis of ToTV and related viruses based on the alignment of A) the region between the protease CG motif and GDD RdRp motif (aa 1041-1498 of RNA1-ORF1) and B) the helicase region between motifs A and C (aa 382–495 of RNA1-ORF1). Sequences included in the analysis are those of (with virus acronyms, genus and accession numbers in parentheses): acute bee paralysis virus (ABPV; unassigned species in the family Dicistroviridae; NP_066241), apple latent spherical virus (ALSV; Cheravirus NP_620568), avian encephalomyelitis virus (AEV; Hepatovirus; NP_653151), beet ringspot virus (BRSV; Nepovirus; NP_620112), blackcurrant reversion virus (BRV; Nepovirus; NP_612604), broad bean wilt virus-2 (BBWV-2; Fabavirus; AAK27841), cherry rasp leaf virus (CRLV; Cheravirus; YP_081444), cowpea mosaic virus (CPMV; {urComovirus}; NP_613283), grapevine fanleaf virus (GFLV; Nepovirus; NP_619689), hepatitis A virus (HAV; Hepatovirus; NP_041008), maize chlorotic dwarf virus (MCDV; Waikavirus; NP_619716), navel orange infectious mottling virus (NIMV; Sadwavirus; BAA74537), parsnip yellow fleck virus (PYFV; Sequivirus; NP_619734), rice tungro spherical virus (RTSV; Waikavirus; NP_042507), satsuma dwarf virus (SDV; Sadwavirus; NP_620566), Solenopsis invicta virus (SinV; unassigned species in the family Dicistroviridae; YP_164440), strawberry mottle virus (SMoV; Sadwavirus; NP_599086). strawberry latent ringspot virus (SLRSV; Sadwavirus; NC_006764), stocky prune virus (StPV; Cheravirus; DQ143874). Potato virus Y (PVY; Potyvirus; ABA28320) was used as an outgroup sequence in the analyses. The numbers at each node are the bootstrap values for 1000 replicates. The scale bar represents the number of residue substitutions per site
Interestingly, the 3′-UTRs of ToTV (1210 and 1092 nt) are much longer than those of the cheraviruses ALSV and CRLV and the proposed cheravirus StPV (246–145 nt). Moreover, the largest capsid protein of ToTV (Vp35) is significantly larger than that of cheraviruses (∼25 KDa). StPV and CRLV are likely to be transmitted in a soil-borne fashion, most likely by nematodes [3, 14, 17]. The presence of ToTV in the field suggested an association with an insect vector, and preliminary experiments indicated the involvement of whiteflies in ToTV transmission (results not shown).
The data presented in this paper identify ToTV as a new picorna-like virus naturally infecting tomato. Although it shares the same number of genomic RNA molecules (i.e. two) and capsid proteins (i.e. three) with viruses from the genus Cheravirus, its sequence characteristics clearly separate it from members of this and other plant virus genera. Therefore, ToTV most likely represents a member of a new plant virus genus.
References
P Argos (1988) ArticleTitleA sequence motif in many polymerases Nucleic Acids Res 16 9909–9916 10.1093/nar/16.21.9909 Occurrence Handle10.1093/nar/16.21.9909 Occurrence Handle1:CAS:528:DyaL1MXltlKqtg%3D%3D Occurrence Handle2461550
JF Bazan RJ Fletterick (1988) ArticleTitleViral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications Proc Natl Acad Sci USA 85 7872–7876 10.1073/pnas.85.21.7872 Occurrence Handle10.1073/pnas.85.21.7872 Occurrence Handle1:CAS:528:DyaL1MXhs12iu7k%3D Occurrence Handle3186696
T Candresse L Svanella-Dumas O Le Gall (2006) ArticleTitleCharacterization and partial genome sequence of stocky prune virus, a new member of the genus Cheravirus Arch Virol 151 1179–1188 10.1007/s00705-005-0682-y Occurrence Handle10.1007/s00705-005-0682-y Occurrence Handle1:CAS:528:DC%2BD28XkvFOis70%3D Occurrence Handle16380812
Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (eds) (2005) Virus Taxonomy, Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press
AE Gorbalenya EV Koonin YI Wolf (1990) ArticleTitleA new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses FEBS Lett 262 145–148 10.1016/0014-5793(90)80175-I Occurrence Handle10.1016/0014-5793(90)80175-I Occurrence Handle1:CAS:528:DyaK3cXitFalu7w%3D Occurrence Handle2156730
Ikegami M, Iwanami T, Jones AT, Karasev AV, Le Gall O, Lehto K, Sanfaçon H, Wellink J, Wetzel T (2002) Taxonomy of recognized and putative species in the family Comoviridae. XIIth IUMS Virology Meeting Paris, France, 27th July–12th August 2002
D James C Upton (2002) ArticleTitleNucleotide sequence analysis of RNA-2 of a flat apple isolate of Cherry rasp leaf virus with regions showing greater identity to animal picornaviruses than to related plant viruses Arch Virol 147 1631–1641 10.1007/s00705-002-0833-3 Occurrence Handle10.1007/s00705-002-0833-3 Occurrence Handle1:CAS:528:DC%2BD38XovVamu70%3D Occurrence Handle12181681
D James C Upton (2005) ArticleTitleGenome segment RNA-1 of a flat apple isolate of Cherry rasp leaf virus: nucleotide sequence analysis and RT-PCR detection Arch Virol 150 1469–1476 10.1007/s00705-005-0503-3 Occurrence Handle10.1007/s00705-005-0503-3 Occurrence Handle1:CAS:528:DC%2BD2MXltl2mtr8%3D Occurrence Handle15789268
EV Koonin (1991) ArticleTitleThe phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses J Gen Virol 72 2197 10.1099/0022-1317-72-9-2197 Occurrence Handle10.1099/0022-1317-72-9-2197 Occurrence Handle1895057
UK Laemmli (1970) ArticleTitleCleavage of structural proteins during assembly of the head of bacteriophage T4 Nature 277 680–685 10.1038/227680a0 Occurrence Handle10.1038/227680a0
Le Gall O, Iwanami T, Karasev AV, Jones T, Lehto K, Sanfaçon H, Wellink J, Wetzel T, Yoshikawa N (2005) Genus cheravirus. In: VIIIth Report of the ICTV on Taxonomy of Viruses. Elsevier Academic Press, San Diego
C Li N Yoshikawa T Takahashi T Ito K Yoshida H Koganezawa (2000) ArticleTitleNucleotide sequence and genome organization of Apple latent spherical virus: a new virus classified into the family Comoviridae J Gen Virol 81 541–547 Occurrence Handle1:CAS:528:DC%2BD3cXhtVymu7k%3D Occurrence Handle10644854
AR Mushegian (1994) ArticleTitleThe putative movement domain encoded by nepovirus RNA-2 is conserved in all sequenced nepoviruses Arch Virol 135 437–441 10.1007/BF01310028 Occurrence Handle10.1007/BF01310028 Occurrence Handle1:CAS:528:DyaK2MXhsVehuw%3D%3D Occurrence Handle7979979
G Nyland BF Lownsbery SK Lowe JF Mitchell (1969) ArticleTitleThe transmission of cherry rasp leaf virus by Xiphinema americanum Phytopathology 59 1111–1112
RD Page (1996) ArticleTitleTreeView: an application to display phylogenetic trees on personal computers Comput Biosci 12 357–358 Occurrence Handle1:STN:280:DyaK2s%2FlvVSgtg%3D%3D
A Shevchenko M Wilm O Vorm M Mann (1996) ArticleTitleMass spectrometric sequencing of proteins from silver-stained polyacrylamide gels Anal Chem 68 850–858 10.1021/ac950914h Occurrence Handle10.1021/ac950914h Occurrence Handle1:CAS:528:DyaK28XntlygtA%3D%3D Occurrence Handle8779443
Stace-Smith R, Hansen AJ (1976) Cherry rasp leaf virus. CMI/AAB description of plant viruses No. 159
JD Thompson F Plewniak F Jeanmougin TJ Gibson DG Higgins (1997) ArticleTitleThe CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools Nucleic Acids Res 25 4876–4882 10.1093/nar/25.24.4876 Occurrence Handle10.1093/nar/25.24.4876 Occurrence Handle1:CAS:528:DyaK1cXntFyntQ%3D%3D Occurrence Handle9396791
JR Thompson KL Perry W De Jong (2004) ArticleTitleA new potato virus in a new lineage of picorna-like viruses Arch Virol 149 2141 10.1007/s00705-004-0362-3 Occurrence Handle10.1007/s00705-004-0362-3 Occurrence Handle1:CAS:528:DC%2BD2cXoslGitrg%3D Occurrence Handle15503203
RAA van der Vlugt C Cuperus J Vink ICCM Stijger D-E Lesemann JTJ Verhoeven JW Roenhorst (2002) ArticleTitleIdentification and characterization of Pepino mosaic potexvirus in tomato Bull OEPP/EPPO 32 503–508
M Volpicella LR Ceci J Cordewener T America R Gallerani W Bode MA Jongsma J Beekwilder (2003) ArticleTitleProperties of purified gut trypsin from Helicoverpa zea, adapted to proteinase inhibitors Eur J Biochem 270 10–19 10.1046/j.1432-1033.2003.03368.x Occurrence Handle10.1046/j.1432-1033.2003.03368.x Occurrence Handle1:CAS:528:DC%2BD3sXktFGqsA%3D%3D Occurrence Handle12492470
Acknowledgement
We thank Dr A. H. P. America and Dr J. H. G. Cordewener (Plant Research International, Wageningen, The Netherlands) for the analysis of the coat proteins to determine partial amino acid sequences.
Author information
Authors and Affiliations
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Verbeek, M., Dullemans, A., van den Heuvel, J. et al. Identification and characterisation of tomato torrado virus, a new plant picorna-like virus from tomato. Arch Virol 152, 881–890 (2007). https://doi.org/10.1007/s00705-006-0917-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-006-0917-6