Abstract
Using midbrain cultures, we previously demonstrated that the noble gas xenon is robustly protective for dopamine (DA) neurons exposed to l-trans-pyrrolidine-2,4-dicarboxylate (PDC), an inhibitor of glutamate uptake used to generate sustained, low-level excitotoxic insults. DA cell rescue was observed in conditions where the control atmosphere for cell culture was substituted with a gas mix, comprising the same amount of oxygen (20%) and carbon dioxide (5%) but 75% of xenon instead of nitrogen. In the present study, we first aimed to determine whether DA cell rescue against PDC remains detectable when concentrations of xenon are progressively reduced in the cell culture atmosphere. Besides, we also sought to compare the effect of xenon to that of other noble gases, including helium, neon and krypton. Our results show that the protective effect of xenon for DA neurons was concentration-dependent with an IC50 estimated at about 44%. We also established that none of the other noble gases tested in this study protected DA neurons from PDC-mediated insults. Xenon’s effectiveness was most probably due to its unique capacity to block NMDA glutamate receptors. Besides, mathematical modeling of gas diffusion in the culture medium revealed that the concentration reached by xenon at the cell layer level is the highest of all noble gases when neurodegeneration is underway. Altogether, our data suggest that xenon may be of potential therapeutic value in Parkinson disease, a chronic neurodegenerative condition where DA neurons appear vulnerable to slow excitotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Noble gases are monatomic gases with very low chemical reactivity, which explains why they are referred as inert gases (Selig et al. 1964; Khriachtchev et al. 2000). Their chemical inertness is due to a filled valence shell that prevents covalent bonds with other molecules under standard temperature and pressure conditions (Spaggiari et al. 2013). Despite that, xenon and also other noble gases exhibit interesting biological properties (Deng et al. 2014; Winkler et al. 2016), probably because of interactions with cell components through noncovalent forces (Liu et al. 2010; Sauguet et al. 2016).
Xenon is without doubt the noble gas whose biological effects are the most robust and best documented. Xenon can operate as an anesthetic (Jordan and Wright 2010; Winkler et al. 2016) and it possesses analgesic properties (Esencan et al. 2013). Xenon also exerts rapid antidepressant-like effects (Dandekar et al. 2018) and ameliorates l-dopa-induced dyskinesia in experimental Parkinsonism (Baufreton et al. 2018). It has also the capacity to reduce lesions after brain ischemic damage (Thoresen et al. 2009; Lobo et al. 2013) and to provide organ protection before transplantation (Zhao et al. 2017).
Some of the other noble gases also exert interesting biological effects. Argon has demonstrated anti-apoptotic, organoprotective and neuroprotective effects in a number of in vitro and in vivo studies (Broad et al. 2016; David et al. 2012; Spaggiari et al. 2013; Zhao et al. 2017). Helium is used as substitute for nitrogen to prevent nitrogen narcosis in diving (Berganza and Zhang 2013). Besides, helium was reported to protect myocardial tissue (Oei et al. 2012) and brain neurons (Pan et al. 2007) in preclinical models of ischemia reperfusion. Krypton and neon have not been described as having significant biological effects until now (Jawad et al. 2009; Spaggiari et al. 2013), even if krypton has been found to induce anesthesia under hyperbaric pressure (Cullen and Gross 1951).
We reported previously that xenon promotes neuronal rescue not only against acute ischemic insults as initially demonstrated, but also in experimental situations where neurodegeneration is more slowly progressing (Lavaur et al. 2016a, b). In particular, xenon was found to protect dopamine (DA) neurons in a culture setting where these neurons undergo sustained, low-level excitotoxicity mediated by glutamate (Lavaur et al. 2017), i.e., experimental conditions that model Parkinson disease (PD) neurodegeneration (Nafia et al. 2008). Indeed, an excessive excitatory drive from subthalamic nucleus glutamatergic neurons is thought to contribute to the vulnerability of substantia nigra DA neurons in this disorder (Wallace et al. 2007; Ambrosi et al. 2014).
Specifically, this slow excitotoxic process was mimicked by treating midbrain dopaminergic cultures with l-trans-pyrrolidine-2,4-dicarboxylate (PDC), a synthetic analog of glutamate that operates as a transportable inhibitor of inward glutamate transport (Blitzblau et al. 1996; Grewer et al. 2014). In such paradigm, DA neurons were protected when 75% nitrogen contained in the control atmosphere was substituted with 75% xenon (Lavaur et al. 2017) but we did not explore the neuroprotective potential of lower concentrations of xenon in this setting. Furthermore, even if we reported previously that argon was inactive against DA cell death induced by PDC, we have no information about the effects of other noble gases in the same experimental context. Our two present aims were therefore (i) to determine whether DA cell rescue remains observable when concentrations of xenon are progressively reduced in the cell culture atmosphere and (ii) to study whether helium, neon or krypton can reproduce xenon’s neuroprotective effects.
Materials and methods
Pharmacological reagents and pure gases
The synthetic analog of glutamate, PDC (#0298/10) and the blocker of N-methyl-d-aspartate (NMDA) receptors, memantine (#0773/50) were both from Tocris Biotechne (Lille, France). Compressed gas cylinders of individual pure gases were provided by Air Liquide (France).
Midbrain cell cultures
Animal care and experiments were conducted in accordance with recommendations of the European Union Council Directives (2010/63/EU). Experimental procedures for cell cultures were authorized under No. 0037 by the ethical committee on animal experiments Charles Darwin No. 5.
Cultures were prepared using gestational day 15.5 embryos from Wistar female rats (Janvier LABS, Le Genest St Isle, France) that had been deeply anesthetized with sodium pentobarbital and killed by cervical dislocation. Once dissected, ventral midbrains were processed to obtain dissociated midbrain cultures following procedures described previously in detail (Toulorge et al. 2011; Lavaur et al. 2017). Midbrain cells were then plated at a density of 1.2–1.5 × 105 cells/cm2 onto Nunc 48 well multidish plates (ThermoFisher Scientific, Roskilde, Denmark) pre-coated with 1 mg/ml polyethylenimine diluted in borate buffer, pH 8.3 as described (Sepulveda-Diaz et al. 2016). These cultures which contained about 2–3% of tyrosine hydroxylase (TH) positive neurons, at the time of plating, were maintained in Neurobasal medium (Gibco, Saint Aubin, France) supplemented with a B27 cocktail minus antioxidants (Gibco), a N2 mix (Gibco), 2 mM glutamine and 100 IU/ml penicillin/streptomycin (Nafia et al. 2008; Lavaur et al. 2017).
Treatment procedures
Pharmacological treatments
Treatments with the NMDA receptor blocker memantine and the inhibitor of glutamate transport PDC were initiated just before starting the incubations with the gaseous atmospheres at 12 days in vitro (DIV) and terminated at 16 DIV. The culture medium was never changed during the entire culture time. These conditions were previously reported to allow for optimal development and maintenance of DA neurons in control culture conditions (Lavaur et al. 2017).
Exposure to gas atmospheres
After application of pharmacological treatments, culture plates were positioned within plexiglas incubation chambers (CR1601, EnzyScreen, The Netherlands) filled with humidified gas combinations just above atmospheric pressure. Specific gas concentrations were produced with a gas mixing system (GasMix, Alytech, Juvisy/Orge, France) supplied by compressed gas cylinders of individual pure gases. The description of the technical procedure used to fill up incubation chambers with gas atmospheres has been previously described in detail (Lavaur et al. 2016a, 2017). The control cell culture atmosphere contained 20% O2, 5% CO2 and 75% N2 and test atmospheres adequate percentages of noble gases in replacement of an equivalent amount of nitrogen. The incubation chambers were kept at 37 °C within conventional cell culture incubators for the duration of the treatments. When using helium, the gas atmosphere was replenished daily and silicone grease was applied to renew the gas tight seal of the incubation chamber because helium has a greater tendency to leak.
Detection of dopamine neurons by immunofluorescence
After fixation with 4% formaldehyde in Dulbecco’s phosphate buffered saline (PBS), midbrain cultures were washed twice with PBS before an incubation step at 4 °C for 96 h with a rabbit polyclonal anti-TH antibody (T-9237-13; US Biologicals, Salem, MA, USA) diluted 1:1000 in PBS containing 0.2% Triton X-100 to favor cell membrane permeability. The primary antibody was detected with an Alexa Fluor-555 conjugate of an anti-rabbit antibody (1:1000) obtained from Life Technologies (Saint Aubin, France). All TH+ neurons detected in this type of cultures were reported to have a dopaminergic phenotype (Traver et al. 2006). Cultures were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to enable automated focus during the cell counting procedure.
Counting of TH+ neurons
Individual digitized images of DAPI+ nuclei (blue) and TH+ cells (red) were acquired under a square format using an Arrayscan XTi automated fluorescence microscope fitted with a 10× objective (ThermoScientific, Courtaboeuf, France). After acquisition of about 64% of the total surface area of each culture well (i.e., the square inscribed in the perimeter of the culture well), a merged composite image was generated for quantification of TH+ neurons with the HCStudio software (ThermoScientific, Courtaboeuf, France). Only TH+ cell bodies with undamaged DAPI+ nuclei and at least one neuritic extension were counted as surviving DA neurons.
Mathematical modeling of gas diffusion in culture wells
We also performed calculations to estimate theoretical concentrations of noble gases achievable in culture wells, at the level of cell layers. Precisely, a concentration of 50% was chosen for modeling one-dimensional gas diffusion and the depth of the liquid column corresponding to 500 µl of culture medium in culture wells was estimated to be 6 mm.
The mathematical model and experimental parameters used to perform calculations have been described in detail in one of our previous papers (Katz et al. 2016). In brief, the transport of the Noble gas through the liquid medium to the cells is modeled as a one-dimensional diffusion problem:
where C is the concentration in the liquid and D is the diffusion coefficient in the liquid medium (we assume the D for water). The boundary conditions are no flux at the bottom of the well at y = 0 and saturated at the surface at y = L:
The initial condition is: \( C\left( {y,0} \right) = 0 \) except at the water surface y = L, where it is assumed to be saturated, C = Csat. The solution to this differential equation in the form of a Fourier series is given by
where Fo is the Fourier number for nondimensional time: Fo= Dt/L2.
For calculations, we used diffusion and solubility constants of noble gases in water, at 37 °C (Table 1). Precisely, diffusion data were from Langø et al. (1996) and Nepal and Adhikari (2017). Solubility coefficients were from Wilhelm et al. (1977) and we used interpolated values between 35 and 40 °C. We also chose to provide estimated values, at 2 h of PDC treatment when neurodegeneration is not truly engaged and at 8 h when first neurodegenerative changes are visually observed.
Statistical analysis
Data expressed as mean ± SEM were analyzed using the SigmaPlot 12.5 software (Systat Software Inc, San Jose, CA, USA). Statistical analysis was performed with a one-way ANOVA followed by a Student–Newman–Keuls post hoc test to perform all pairwise comparisons. Estimation of the IC50 value was made with a four-parameter logistic regression model.
Results
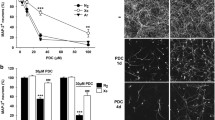
We used the synthetic analog of glutamate PDC to generate, in vitro, low-level excitotoxicity as it may occur in PD (Wallace et al. 2007; Ambrosi et al. 2014; Michel et al. 2016). More precisely, midbrain cultures maintained initially for 12 DIV in serum-free conditions, were then exposed for 4 consecutive days to 100 µM PDC to induce the death of DA (TH+) neurons as described previously (Lavaur et al. 2017). In PDC-treated cultures maintained under a control atmosphere consisting of 75% nitrogen, 20% O2 and 5% CO2, the survival rate of TH+ neurons was decreased by about 90% at 16 DIV (Fig. 1a). When nitrogen was substituted with 75% xenon, DA cell death was virtually absent from the cultures (Fig. 1a, b). Of interest, the survival of TH+ neurons was also significantly improved under an atmosphere where the concentration of xenon was set at 50%. The survival rate of TH+ neurons in these conditions was 86% of that of control (PDC-free) cultures maintained under 75% nitrogen (Fig. 1a, b). The rescuing effect of xenon was progressively reduced at lower concentrations but remained significant with 35% and 25% of the noble gas in the cell culture atmosphere. This set of data allowed us to estimate at 44.4% the concentration of xenon reducing by half (IC50) the death of DA neurons. Note that we also quantified the survival rate of TH+ neurons in PDC-free cultures exposed to various concentrations of xenon (25–75%). There was no significant change in TH+ cell numbers in these conditions, regardless of the concentration of xenon applied to the cultures (Fig. 1a).
Xenon provides concentration-dependent protection against PDC-induced DA cell death in midbrain cultures. a Survival rate of DA neurons (TH+ cells) in 16 DIV midbrain cultures previously exposed or not for 4 consecutive days to PDC (100 µM) under cell culture atmospheres containing 75% N2 or 25–75% Xe. Error bars indicate mean ± SEM (n = 2–8). ***p < 0.001 relative to control cultures maintained under 75% N2. #p < 0.05 and ###p < 0.001 relative to PDC-treated cultures maintained under 75% N2. Application of a 4-parameter logistic regression model to experimental data values gave an IC50 of 44.4% for Xe. b Inverted fluorescence images illustrating the neuroprotective effects provided by 50% and 75% Xe in midbrain cultures exposed for 4 days to 100 µM of PDC. Scale bar 55 µm
We then compared the effect of xenon to that of other noble gases, including helium, neon and krypton. More specifically, we estimated the survival of TH+ neurons in PDC-treated cultures maintained for 4 days under gas atmospheres where nitrogen was substituted with 75% of each of these gases. Xenon was used as reference gas in this context. As shown previously, a treatment of midbrain cultures with 100 µM PDC caused a profound loss of TH+ neurons in cultures maintained under a control atmosphere containing 75% nitrogen. As expected, this loss was prevented when nitrogen was substituted with 75% xenon but the other atmospheres containing 75% of helium, neon or krypton in their composition were totally ineffective (Fig. 2). Note that the blocker of NMDA receptors, memantine (10 µM) used as a non-gaseous reference neuroprotective treatment for DA neurons, provided robust but partial protection against PDC under 75% nitrogen (Fig. 2).
Among noble gases, only xenon protects DA neurons against PDC-induced degeneration in midbrain cultures. Survival rate of TH+ cells in midbrain cultures exposed to PDC (100 µM) for 4 days under a control culture atmosphere containing 75% N2 or other atmospheres enriched with 75% of He, Ne, Kr or Xe. Comparison with a non-gaseous treatment by memantine (MEM) (10 µM) performed under 75% N2. Error bars indicate mean ± SEM (n = 3–8). ***p < 0.001 relative to control cultures maintained under 75% N2. ###p < 0.001 relative to PDC-treated cultures maintained under 75% N2
Finally, a theoretical estimation made with gaseous atmospheres containing noble gases at 50% in their composition, revealed that xenon was the slowest to saturate the culture medium at the level of the cell layer (Table 2). Xenon together with krypton yielded, however, the highest concentrations of all noble gases, after 2 h of PDC treatment (Table 2). Of interest, the concentration of xenon was well above that of any of the other noble gases including krypton after 8 h of PDC exposure, i.e., at a stage where first neurodegenerative changes are visually observable in our culture set-up. In particular, a number of swollen neuronal cell bodies were detectable at this time-point by phase-contrast microscopic examination of living cultures. Note that helium was the noble gas yielding the lowest concentrations after 2 and 8 h of PDC exposure. Argon and neon generated concentrations that were intermediary at both time points.
Discussion
The present work allowed us to further characterize the protective effects of xenon for midbrain DA neurons in culture and to compare its effects to that of other noble gases in the same experimental setting. Using the synthetic analog of glutamate PDC to generate sustained low-level excitotoxicity as it may occur in PD (Wallace et al. 2007; Assous et al. 2014), we confirmed the strong protective potential of xenon for DA neurons. Importantly, we also showed that the effects of xenon were concentration-dependent with optimal effects observed at concentrations comprised between 50 and 75%. Note that we noticed that the atmosphere containing 75% xenon was comparatively more effective in protecting DA neurons in the present study than in a research work we conducted earlier with the same culture system (Lavaur et al. 2017). The experimental set-up and culture conditions being identical in these two studies, we have no clear explanation for such a difference.
By performing non-linear regression analysis of current data values, we estimated at 44.4%, the IC50 for neuroprotection by xenon. The IC50 established by us appears higher than IC50 values reported previously for xenon-mediated neuroprotection (Wilhelm et al. 2002). More specifically, in cortical cultures submitted to acute exposures to NMDA and glutamate, IC50 values for xenon were estimated at 19% and 28%, respectively. Such a difference may relate to the fact that we induced excitotoxic stress by means of a treatment with PDC, a toxin that does not act as a direct agonist at glutamate receptors but operates indirectly by causing a small but permanent rise of extracellular glutamate (Maki et al. 1994; Lavaur et al. 2017). Furthermore, a prolonged exposure to PDC for 4 days might have made xenon less efficacious during the course of treatment. This explanation appears, however, pertinent only when considering the lowest concentrations of xenon (i.e., 25% and 35%) and their limited efficacy to rescue DA neurons from PDC exposure in our paradigm.
At concentrations of 50% or above, xenon was reported to be highly effective in reducing NMDA receptor-mediated currents (Dickinson et al. 2007; Haseneder et al. 2009), which probably explains why this noble gas did provide robust protection under these conditions. Consistent with this view, DA cell death induced by PDC was also efficiently reduced by antagonizing NMDA receptors with memantine, in cultures maintained under 75% nitrogen. Note that the site to which xenon binds on NMDA receptors appears distinct from that of memantine (Kotermanski and Johnson 2009; Liu et al. 2010) and unrelated to the glycine binding site of the receptor in the present setting (Lavaur et al. 2016a). Indeed, glycine is inherently present in Neurobasal culture medium at a concentration of 400 μM, which is sufficient to saturate the NMDA receptor glycine-binding site (Dickinson et al. 2007).
We also found that in cultures not treated with PDC, TH+ cell numbers were unaffected by xenon regardless of the gas concentration used for treatment. This result indirectly demonstrates the lack of toxicity of xenon in present culture conditions. It also shows that xenon did not mimic the effects of some synthetic compounds and biological molecules reported to induce the expression of the TH enzyme in dormant neurons not initially committed to this neurotransmitter phenotype (Stull et al. 2001; Traver et al. 2006). This indicates that xenon operated as a true neuroprotectant in the present paradigm.
We also tested whether other noble gases, including helium, neon and krypton had the potential to rescue DA neurons in the same experimental paradigm. Based on the literature, the rational for testing neon and krypton is relatively limited. Helium, however, has been reported to provide protection to brain neurons in preclinical models of ischemia reperfusion (Pan et al. 2007) and traumatic brain injury (Coburn et al. 2008). Our results show that neither helium, neon nor krypton had the capacity to protect PDC-treated DA neurons from degeneration when the concentration of these gases in the cell culture atmosphere was set at 75%. Note that despite a number of studies reporting on the protective effects of argon in preclinical models of acute ischemic/anoxic stress (Coburn et al. 2012; Zhao et al. 2017), we have previously established that argon was also ineffective to protect DA neurons from PDC-induced degeneration (Lavaur et al. 2017). This suggests that among noble gases, only xenon has the potential to provide protection when neurodegeneration is sustained and slowly progressing.
One may assume that this property is related to the fact that xenon possesses the highest atomic number and therefore the largest atomic radius of all noble gases (The International Union of Pure and Applied Chemistry (IUPAC) 2018; Zhang and Xu 1995), a feature that may be correlated to the capacity of xenon to block NMDA receptors. Coherent with this view, argon, krypton, neon and helium, all failed to interfere with NMDA receptor activity (Harris et al. 2013).
Note that we cannot exclude, however, that favorable PK properties also contributed to the efficacy of xenon. In particular, in this in vitro experimental set-up, the concentrations of xenon at the level of the cell layer and the time necessary to reach these concentrations may be important factors for neuroprotection (Katz et al. 2016). Theoretical calculations made with gaseous atmospheres containing noble gases at a concentration of 50%, revealed that xenon was the slowest of all these gases to saturate the culture medium. Despite of that, xenon yielded the highest theoretical concentrations in the culture medium, at the level of the cell layer. This was particularly true after 8 h of PDC exposure, i.e., when first neurodegenerative changes are observable in the cultures. Therefore, our data suggest that xenon protective effects may relate not only to specific pharmacological properties but also to its availability for neuronal cells. However, it is clear that these in vitro PK results do not consider the clinically relevant physiological transport to and location of the cellular targets in vivo (e.g., blood flow to the brain, partition coefficient and lipophilicity of the gas molecule…).
Overall, we have demonstrated that xenon is particularly effective in the present experimental setting that models the slow excitotoxic component of DA cell death in PD. It remains to be demonstrated, however, whether such properties are clinically relevant.
References
Ambrosi G, Cerri S, Blandini F (2014) A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J Neural Transm (Vienna) 121:849–859. https://doi.org/10.1007/s00702-013-1149-z
Assous M, Had-Aissouni L, Gubellini P, Melon C, Nafia I, Salin P, Kerkerian-Le-Goff L, Kachidian P (2014) Progressive Parkinsonism by acute dysfunction of excitatory amino acid transporters in the rat substantia nigra. Neurobiol Dis 65:69–81. https://doi.org/10.1016/j.nbd.2014.01.011
Baufreton J, Milekovic T, Li Q, McGuire S, Moraud EM, Porras G, Sun S, Ko WKD, Chazalon M, Morin S, Normand E, Farjot G, Milet A, Pype J, Pioli E, Courtine G, Bessière B, Bezard E (2018) Inhaling xenon ameliorates l-dopa-induced dyskinesia in experimental Parkinsonism. Mov Disord 33(10):1632–1642. https://doi.org/10.1002/mds.27404
Berganza CJ, Zhang JH (2013) The role of helium gas in medicine. Med Gas Res 3:18. https://doi.org/10.1186/2045-9912-3-18
Blitzblau R, Gupta S, Djali S, Robinson MB, Rosenberg PA (1996) The glutamate transport inhibitor l-trans-pyrrolidine-2,4-dicarboxylate indirectly evokes NMDA receptor mediated neurotoxicity in rat cortical cultures. Eur J Neurosci 8:1840–1852. https://doi.org/10.1111/j.1460-9568.1996.tb01328.x
Broad KD, Fierens I, Fleiss B, Rocha-Ferreira E, Ezzati M, Hassell J, Alonso-Alconada D, Bainbridge A, Kawano G, Ma D, Tachtsidis I, Gressens P, Golay X, Sanders RD, Robertson NJ (2016) Inhaled 45–50% argon augments hypothermic brain protection in a piglet model of perinatal asphyxia. Neurobiol Dis 87:29–38. https://doi.org/10.1016/j.nbd.2015.12.001
Coburn M, Maze M, Franks NP (2008) The neuroprotective effects of xenon and helium in an in vitro model of traumatic brain injury. Crit Care Med 36:588–595. https://doi.org/10.1097/01.CCM.0B013E3181611F8A6
Coburn M, Sanders RD, Ma D, Fries M, Rex S, Magalon G, Rossaint R (2012) Argon: the ‘lazy’ noble gas with organoprotective properties. Eur J Anaesthesiol 29:549–551. https://doi.org/10.1097/EJA.0b013e328357bfdd
Cullen SC, Gross EG (1951) The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science 113:580–582. https://doi.org/10.1126/science.113.2942.580
Dandekar MP, Peng T, McPherson DD, Quevedo J, Soares JC, Huang SL (2018) Intravenous infusion of xenon-containing liposomes generates rapid antidepressant-like effects. Prog Neuropsychopharmacol Biol Psychiatry 86:140–149. https://doi.org/10.1016/j.pnpbp.2018.03.011
David HN, Haelewyn B, Degoulet M, Colomb DG Jr, Risso JJ, Abraini JH (2012) Ex vivo and in vivo neuroprotection induced by argon when given after an excitotoxic or ischemic insult. PLoS One 7(2):e30934. https://doi.org/10.1371/journal.pone.0030934
Deng J, Lei C, Chen Y, Fang Z, Yang Q, Zhang H, Cai M, Shi L, Dong H, Xiong L (2014) Neuroprotective gases: fantasy or reality for clinical use? Prog Neurobiol 115:210–245. https://doi.org/10.1016/j.pneurobio.2014.01.001
Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP (2007) Competitive inhibition at the glycine site of the N-methyl-d-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology 107(5):756–767. https://doi.org/10.1097/01.anes.0000287061.77674.71
Esencan E, Yuksel S, Tosun YB, Robinot A, Solaroglu I, Zhang JH (2013) Xenon in medical area: emphasis on neuroprotection in hypoxia and anesthesia. Med Gas Res 3:4. https://doi.org/10.1186/2045-9912-3-4
Grewer C, Gameiro A, Rauen T (2014) SLC1 glutamate transporters. Pflugers Arch 466:3–24. https://doi.org/10.1007/s00424-013-1397-7
Harris K, Armstrong SP, Campos-Pires R, Kiru L, Franks NP, Dickinson R (2013) Neuroprotection against traumatic brain injury by xenon, but not argon, is mediated by inhibition at the N-methyl-d-aspartate receptor glycine site. Anesthesiology 119:1137–1148. https://doi.org/10.1097/ALN.0b013e3182a2a265
Haseneder R, Kratzer S, Kochs E, Höfelmann D, Auberson Y, Eder M, Rammes G (2009) The xenon-mediated antagonism against the NMDA receptor is non-selective for receptors containing either NR2A or NR2B subunits in the mouse amygdala. Eur J Pharmacol 619:33–37. https://doi.org/10.1016/j.ejphar.2009.08.011
Jawad N, Rizvi M, Gu J, Adeyi O, Tao G, Maze M, Ma D (2009) Neuroprotection (and lack of neuroprotection) afforded by a series of noble gases in an in vitro model of neuronal injury. Neurosci Lett 460:232–236. https://doi.org/10.1016/j.neulet.2009.05.069
Jordan BD, Wright EL (2010) Xenon as an anesthetic agent. AANA J 78(5):387–392
Katz I, Palgen M, Murdock J, Martin AR, Farjot G, Caillibotte G (2016) Gas transport during in vitro and in vivo preclinical testing of inert gas therapies. Med Gas Res 6(1):14–19. https://doi.org/10.4103/2045-9912.179342(eCollection 2016)
Khriachtchev L, Pettersson M, Runeberg N, Lundell J, Rasanen M (2000) A stable argon compound. Nature 406:874–876. https://doi.org/10.1038/35022551
Kotermanski SE, Johnson JW (2009) Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci 29:2774–2779. https://doi.org/10.1523/jneurosci.3703-08.2009
Langø T, Mørland T, Brubakk AO (1996) Diffusion coefficients and solubility coefficients for gases in biological fluids and tissues: a review. Undersea Hyperb Med 23(4):247–272
Lavaur J, Lemaire M, Pype J, Le Nogue D, Hirsch EC, Michel PP (2016a) Xenon-mediated neuroprotection in response to sustained, low-level excitotoxic stress. Cell Death Discov 2:16018. https://doi.org/10.1038/cddiscovery.2016.18
Lavaur J, Lemaire M, Pype J, Le Nogue D, Hirsch EC, Michel PP (2016b) Neuroprotective and neurorestorative potential of xenon. Cell Death Dis 7:e2182. https://doi.org/10.1038/cddis.2016.86
Lavaur J, Le Nogue D, Lemaire M, Pype J, Farjot G, Hirsch EC, Michel PP (2017) The noble gas xenon provides protection and trophic stimulation to midbrain dopamine neurons. J Neurochem 142(1):14–28. https://doi.org/10.1111/jnc.14041
Liu LT, Xu Y, Tang P (2010) Mechanistic insights into xenon inhibition of NMDA receptors from MD simulations. J Phys Chem B 114:9010–9016. https://doi.org/10.1021/jp101687j
Lobo N, Yang B, Rizvi M, Ma D (2013) Hypothermia and xenon: novel noble guardians in hypoxic-ischemic encephalopathy? J Neurosci Res 91:473–478. https://doi.org/10.1002/jnr.23178
Maki R, Robinson MB, Dichter MA (1994) The glutamate uptake inhibitor L-trans-pyrrolidine-2,4-dicarboxylate depresses excitatory synaptic transmission via a presynaptic mechanism in cultured hippocampal neurons. J Neurosci 14(11 Pt 1):6754–6762. https://doi.org/10.1523/jneurosci.14-11-06754.1994
Michel PP, Hirsch EC, Hunot S (2016) Understanding dopaminergic cell death pathways in Parkinson disease. Neuron 90:675–691. https://doi.org/10.1016/j.neuron.2016.03.038
Nafia I, Re DB, Masmejean F, Melon C, Kachidian P, Kerkerian-Le Goff L, Nieoullon A, Had-Aissouni L (2008) Preferential vulnerability of mesencephalic dopamine neurons to glutamate transporter dysfunction. J Neurochem 105:484–496. https://doi.org/10.1111/j.1471-4159.2007.05146.x
Nepal NK, Adhikari NP (2017) Molecular dynamics study of diffusion of xenon in water at different temperatures. Sci Bruneiana 16(2):12–22
Oei GT, Huhn R, Heinen A, Hollmann MW, Schlack WS, Preckel B, Weber NC (2012) Helium-induced cardioprotection of healthy and hypertensive rat myocardium in vivo. Eur J Pharmacol 684:125–131. https://doi.org/10.1016/j.ejphar.2012.03.045
Pan Y, Zhang H, VanDeripe DR, Cruz-Flores S, Panneton WM (2007) Heliox and oxygen reduce infarct volume in a rat model of focal ischemia. Exp Neurol 205:587–590. https://doi.org/10.1016/j.expneurol.2007.03.023
Sauguet L, Fourati Z, Prangé T, Delarue M, Colloc’h N (2016) Structural basis for xenon inhibition in a cationic pentameric ligand-gated ion channel. PLoS One 11(2):e0149795. https://doi.org/10.1371/journal.pone.0149795
Selig H, Malm JG, Claassen HN (1964) The chemistry of noble gases. Sci Am 210:66–77
Sepulveda-Diaz JE, Ouidja MO, Socias SB, Hamadat S, Guerreiro S, Raisman-Vozari R, Michel PP (2016) A simplified approach for efficient isolation of functional microglial cells: application for modeling neuroinflammatory responses in vitro. Glia 64:1912–1924. https://doi.org/10.1002/glia.23032
Spaggiari S, Kepp O, Rello-Varona S, Chaba K, Adjemian S, Pype J, Galluzzi L, Lemaire M, Kroemer G (2013) Antiapoptotic activity of argon and xenon. Cell Cycle 12:2636–2642. https://doi.org/10.4161/cc.25650
Stull ND, Jung JW, Iacovitti L (2001) Induction of a dopaminergic phenotype in cultured striatal neurons by bone morphogenetic proteins. Brain Res Dev Brain Res 130:91–98. https://doi.org/10.1016/S0165-3806(01)00216-4
The International Union of Pure and Applied Chemistry (IUPAC) (2018) Periodic table of elements, version dated 1 December 2018. https://iupac.org/what-we-do/periodic-table-of-elements/
Thoresen M, Hobbs CE, Wood T, Chakkarapani E, Dingley J (2009) Cooling combined with immediate or delayed xenon inhalation provides equivalent long-term neuroprotection after neonatal hypoxia-ischemia. J Cereb Blood Flow Metab 29:707–714. https://doi.org/10.1038/jcbfm.2008.163
Toulorge D, Guerreiro S, Hild A, Maskos U, Hirsch EC, Michel PP (2011) Neuroprotection of midbrain dopamine neurons by nicotine is gated by cytoplasmic Ca2+. FASEB J 25(8):2563–2573. https://doi.org/10.1096/fj.11-182824
Traver S, Marien M, Martin E, Hirsch EC, Michel PP (2006) The phenotypic differentiation of locus ceruleus noradrenergic neurons mediated by brain-derived neurotrophic factor is enhanced by corticotropin releasing factor through the activation of a cAMP-dependent signaling pathway. Mol Pharmacol 70:30–40. https://doi.org/10.1124/mol.106.022715
Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, Benabid AL (2007) Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain 130(Pt8):2129–2145. https://doi.org/10.1093/brain/awm137
Wilhelm E, Battino R, Wilcock RJ (1977) Low-pressure solubility of gases in liquid water. Chem Rev 77(2):219–262. https://doi.org/10.1021/cr60306a003
Wilhelm S, Ma D, Maze M, Franks NP (2002) Effects of xenon on in vitro and in vivo models of neuronal injury. Anesthesiology 96(6):1485–1491. https://doi.org/10.1097/00000542-200206000-00031
Winkler DA, Thornton A, Farjot G, Katz I (2016) The diverse biological properties of the chemically inert noble gases. Pharmacol Ther 160:44–64. https://doi.org/10.1016/j.pharmthera.2016.02.002
Zhang Y, Xu Z (1995) Atomic radii of noble gas elements in condensed phases. Am Miner 80:670–675. https://doi.org/10.2138/am-1995-7-807
Zhao H, Rossaint R, Coburn M, Ma D, Argon Organo-Protective Network (AON) (2017) The renoprotective properties of xenon and argon in kidney transplantation. Eur J Anaesthesiol 34:637–640. https://doi.org/10.1097/EJA.0000000000000632
Acknowledgements
This study was supported by a grant from Air Liquide Santé International. The research leading to these results also received support from the programs “Investissements d’Avenir” ANR-10-IAIHU-06 and ANR-11-INBS-0011-NeurATRIS: Translational Research Infrastructure for Biotherapies in Neurosciences. DLN was supported by a CIFRE doctoral fellowship co-funded by Association Nationale de la Recherche et de la Technologie (ANRT, 2014/0589) and Air Liquide Santé International. We wish to mention the contribution of Matthieu Chalopin for technical advice and we thank François Gilles for technical support with gas delivery systems. This work benefited from equipment and services from the Celis cell culture facility, at ICM. More specifically, we thank David Akbar regarding assistance for quantification with automated fluorescence microscopy.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DLN, IK and PPM. The first draft of the manuscript was written by PPM and DLN. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
AM, GF, IK, ML and J-FR are Air Liquide employees. Air Liquide has filed patent applications for the use of xenon as therapeutic agent for Parkinson’s disease and related disorders.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Le Nogue, D., Lavaur, J., Milet, A. et al. Neuroprotection of dopamine neurons by xenon against low-level excitotoxic insults is not reproduced by other noble gases. J Neural Transm 127, 27–34 (2020). https://doi.org/10.1007/s00702-019-02112-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02112-x