Abstract
Sleep and circadian rhythm disruption (SCRD) and schizophrenia are often co-morbid. Here, we propose that the co-morbidity of these disorders stems from the involvement of common brain mechanisms. We summarise recent clinical evidence that supports this hypothesis, including the observation that the treatment of SCRD leads to improvements in both the sleep quality and psychiatric symptoms of schizophrenia patients. Moreover, many SCRD-associated pathologies, such as impaired cognitive performance, are routinely observed in schizophrenia. We suggest that these associations can be explored at a mechanistic level by using animal models. Specifically, we predict that SCRD should be observed in schizophrenia-relevant mouse models. There is a rapidly accumulating body of evidence which supports this prediction, as summarised in this review. In light of these emerging data, we highlight other models which warrant investigation, and address the potential challenges associated with modelling schizophrenia and SCRD in rodents. Our view is that an understanding of the mechanistic overlap between SCRD and schizophrenia will ultimately lead to novel treatment approaches, which will not only ameliorate SCRD in schizophrenia patients, but also will improve their broader health problems and overall quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Generation of circadian rhythms and sleep
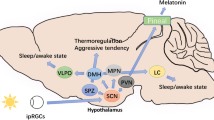
Sleep and circadian rhythms are not synonymous. Circadian rhythms are endogenous 24-h oscillations in physiology and behaviour that enable an organism to anticipate and adapt to the changing temporal demands of the environment. An internal clock acts to coordinate the 24-h rhythms of multiple cellular and organ systems within an individual so that different aspects of physiology and behaviour are appropriately synchronised to each other. These rhythms arise from a sub-cellular transcriptional–translational feedback loop (Fig. 1a), involving a number of core clock genes (Lowrey and Takahashi 2011; Reppert and Weaver 2002). In mammals, light is the primary time cue (zeitgeber), which entrains the internal clock to the external light environment. Light information is relayed from the eyes to the primary circadian pacemaker located in the suprachiasmatic nuclei (SCN) of the hypothalamus (Moore 1973; Moore and Klein 1974), which in turn regulates physiology and behaviour. Additional oscillators are found in tissues throughout the body, regulating local physiology (Dibner et al. 2010).
Schematic illustration of the mechanisms underlying circadian rhythm generation and sleep regulation. a Light detected by the eye is relayed to the suprachiasmatic nuclei (SCN) in the hypothalamus via the retinohypothalamic tract (RHT), which uses the neurotransmitters glutamate and PACAP. Circadian rhythms are generated by a cell autonomous transcriptional–translational feedback loop (TTFL) involving a set of core clock genes. The molecular clock in the SCN synchronises circadian clocks found in tissues throughout the body, which regulate local physiology. b Sleep is the product of multiple brain regions and neurotransmitters. Abbreviations for brain regions: BF basal forebrain, DR/MR dorsal/medial raphe nucleus, LC locus coeruleus, LDT laterodorsal tegmental nuclei, LH lateral hypothalamus, LPT lateral pontine tegmentum, PPT pedunculopontine tegmental nuclei, SCN suprachiasmatic nuclei, SLD sublaterodorsal nucleus, TMN tuberomammilary nucleus, VLPO ventrolateral preoptic nuclei, vPAG ventral periaqueductal grey, vlPAG ventrolateral periaqueductal grey. Abbreviations for neurotransmitters: 5-HT serotonin, ACh acetylcholine, DA dopamine, GABA γ-aminobutyric acid, Gal galanin, Glut glutamate, His histamine, NA noradrenaline, ORX orexin
Whilst sleep/wake behaviour is perhaps the most obvious output of the circadian system, sleep biology involves much more than the circadian system. In addition to a circadian drive for wakefulness, sleep is under homeostatic regulation, whereby an increased duration of wakefulness leads to an increased need for sleep. The homeostatic drive for sleep is the product of a complex network of brain regions and neurotransmitter pathways (Fig. 1b), none of which are exclusive to the generation of sleep (Tobler 1995). This complexity makes sleep very vulnerable to disruption. Small changes in brain function can have a big impact on sleep, and disrupted sleep leads to multiple health problems (Table 1).
Sleep and circadian rhythm disruption (SCRD) in schizophrenia
The relationship between schizophrenia and abnormal sleep was first described in the late nineteenth century by the German psychiatrist Emil Kraepelin (Manoach and Stickgold 2009). Today, sleep and circadian rhythm disruption (SCRD) is reported in 30–80 % of patients with schizophrenia, and is increasingly recognised as one of the most common features of the disorder (Cohrs 2008). Sleep disturbances in schizophrenia include increases in sleep latency, and reductions in total sleep time, sleep efficiency, REM sleep latency, REM sleep density and slow-wave sleep duration (Cohrs 2008; Manoach and Stickgold 2009).
Schizophrenia is also associated with significant circadian disruption, including the abnormal phasing, instability and fragmentation of rest-activity rhythms (Martin et al. 2001, 2005; Wulff et al. 2006, 2009). Crucially, patients with SCRD score badly on many quality-of-life clinical subscales, highlighting the human cost of SCRD in schizophrenia (Cohrs 2008; Goldman et al. 1996; Hofstetter et al. 2005). To reinforce this, schizophrenia patients often comment that an improvement in sleep is one of their highest priorities during treatment (Auslander and Jeste 2002).
Common brain mechanisms: an explanation for the co-morbidity of SCRD and schizophrenia
Sleep and circadian rhythm disruption in schizophrenia could be viewed as a side effect of antipsychotic medication, given that antipsychotic drugs, particularly typical antipsychotics, have a severe sedative effect when taken at high doses (Miller 2004). This seems unlikely, however, as SCRD affects both medication-naïve (Wulff et al. 2010) and medicated patients (Krystal et al. 2008). Indeed, the emergence of sleep disruption often precedes the diagnosis of schizophrenia, occurring before any drugs have been prescribed (Wulff et al. 2010). Recent data would suggest that, if anything, antipsychotic drug treatment can actually improve sleep quality in schizophrenia (Cohrs 2008; Krystal et al. 2008). More specifically, schizophrenia patients treated with typical antipsychotics show an increase in their sleep efficiency and total sleep time (Cohrs 2008).
Social isolation, and the resulting absence of social constraints, is also routinely suggested as a cause of SCRD in schizophrenia. This hypothesis was recently addressed by comparing the sleep patterns of schizophrenia patients with those of unemployed healthy volunteers (Wulff et al. 2011). Severe SCRD was observed in schizophrenia, but could not be attributed to an absence of routine, as major sleep disruption was observed in schizophrenia patients that followed a fixed routine. Conversely, undisturbed sleep was seen in a number of control participants that did not follow a fixed routine.
A more likely explanation for the co-morbidity of SCRD and schizophrenia is the involvement of common brain mechanisms. As described above, sleep is the product of a complex interaction between multiple brain regions and neurotransmitters. As a consequence, abnormalities in any key neurotransmitter system will impinge upon sleep at multiple levels. Similarly, schizophrenia is a disorder of distributed brain circuits, affecting a range of neurotransmitter systems (Weinberger and Harrison 2011), many of which overlap with those involved in sleep regulation (Wulff et al. 2010). Viewed in this context, it is no surprise that SCRD is common in schizophrenia, or that SCRD will in turn have widespread effects, ranging across many aspects of neural, neuroendocrine and cognitive function (Fig. 2).
The key components in the regulation and maintenance of the sleep/wake cycle and its relationship to mood/cognition and mental health. The sleep/wake cycle is regulated directly by (i) The 24-h body clock/circadian clock located within the suprachiasmatic nuclei (SCN), (ii) wake-dependent homeostatic drivers (e.g. adenosine) that build-up and generate “sleep pressure”, (iii) social behaviours that force a sleep/wake pattern on the individual. The SCN drives wakefulness throughout the day and sleep during the night. This 24-h rhythm interacts with the homeostatic “hourglass oscillator” producing increased sleep pressure during wake and its dissipation during sleep. The circadian system directly regulates multiple neurotransmitter and brain systems that either drive or modulate sleep, including the hypothalamo–pituitary–adrenal axis (HPA) and melatonin from the pineal gland. The SCN also coordinates the 24-h biology of peripheral oscillators, maintaining internal synchrony of 24-h biological timing processes. A mismatch between central and peripheral oscillators (internal desynchrony), as occurs in shift work or jet-lag, is associated with mood imbalance and depression. All of the components within the triangle are modulated by light, which acts to: entrain the circadian pacemaker to the light/dark cycle; alter melatonin production from the pineal gland; modulate the HPA axis; and elevate or suppress levels of mood and cognition. Social behaviours will also change an individual’s exposure to the light/dark cycle (Mistlberger and Skene 2004), and have a major effect on mood/cognition and mental health. The quality of sleep and the stability of the sleep/wake cycle have a direct effect upon mood, cognitive processing and mental health (Table 1)
Sleep and circadian rhythm disruption in schizophrenia could also arise from dysfunction at any point in the circadian axis (i.e. input, oscillator or output). Such defects would impact upon sleep via the circadian drive for wakefulness, as appears to be the case in familial advanced sleep phase syndrome (FASPS), which has previously been linked to mutations in the clock gene Per2 (Toh et al. 2001).
Clinical evidence for common brain mechanisms in SCRD and schizophrenia
Sleep and circadian rhythm disruption is rarely targeted for treatment in schizophrenia, but when it is, patients report improvements in both their sleep quality and psychiatric symptoms (Kantrowitz et al. 2010). In a recent study, insomnia was treated in 15 patients with persistent persecutory delusions and schizophrenia (Myers et al. 2011). Following a cognitive behavioural therapy (CBT) intervention, there were significant reductions in both insomnia and persecutory delusions. At least two-thirds of participants showed a substantial (>25 %) improvement in insomnia, whilst approximately half showed a substantial (>25 %) reduction in persecutory delusions. There were also reductions in levels of hallucinations, anxiety and depression. Although consistent with the existence of common mechanisms in SCRD and schizophrenia, the results of this study should be interpreted with caution, due to a number of methodological limitations; the sample size was small, there was no control group, and 14 of the 15 patients received antipsychotic medication during the CBT intervention.
Significantly, many of the pathologies caused by SCRD are routinely reported as co-morbid with schizophrenia, but are rarely linked to the disruption of sleep. For example, sleep deprivation (Alhola and Polo-Kantola 2007; Chee and Chuah 2008; Horne 1993; Van Dongen et al. 2003) and circadian de-synchronisation (Kyriacou and Hastings 2010) are known to impair cognition in healthy individuals, and cognitive impairment is a core symptom of schizophrenia. Thus, cognitive impairments in schizophrenia could be exacerbated by circadian de-synchronisation and/or disturbed sleep. Consistent with this, associations between sleep and cognitive performance have been reported in medication-naïve (Forest et al. 2007), medicated (Bromundt et al. 2011; Goder et al. 2004, 2008; Wulff and Joyce 2011), and unmedicated schizophrenia patients (Yang and Winkelman 2006). In the latter study, the severity of patients’ cognitive symptoms was inversely related with slow-wave sleep duration and REM sleep density (Yang and Winkelman 2006).
Memory consolidation is just one cognitive function that warrants further investigation. Memory impairment is prevalent in schizophrenia (Aleman et al. 1999), and it has been suggested that sleep makes a crucial contribution to memory consolidation (Stickgold 2005). Reduced overnight consolidation of procedural learning has been demonstrated in schizophrenia patients (Manoach et al. 2004), and more recently, this effect was linked to a reduction in slow-wave sleep duration (Manoach et al. 2010). The negative symptoms of schizophrenia might also be sensitive to SCRD, since circadian de-synchronisation increases negative mood, irritability and affective volatility in healthy volunteers (Kyriacou and Hastings 2010; Murray and Harvey 2010). Consistent with this, improvements in sleep quality are frequently correlated with the amelioration of negative symptoms in schizophrenia patients (Hofstetter et al. 2005; Yamashita et al. 2004).
Using animal models to establish mechanistic links between SCRD and schizophrenia
Animal models provide essential tools to understand the mechanisms underlying neuropathological processes, enabling highly controlled genetic, anatomical, physiological and behavioural studies that are not possible in humans. If, as our central hypothesis proposes, common mechanisms are involved in the pathogenesis of SCRD and schizophrenia, then SCRD should be observed in schizophrenia-relevant animal models. Conversely, animal models of SCRD may display schizophrenia-relevant behavioural and neurobiological abnormalities. There is a rapidly accumulating body of evidence which supports the first of these predictions, while the second prediction has yet to be tested.
SCRD in schizophrenia-relevant mouse models: existing evidence
The hypotheses outlined above apply to genetic, pharmacological and environmental models, and are not species-specific. For the sake of brevity, this paper focuses on schizophrenia-relevant transgenic mouse models. Sleep and circadian function has yet to be investigated in any pharmacological or environmental mouse models, but this represents a promising avenue for future research; it would be particularly interesting to see whether sleep is affected in the PCP, MK-801 and ketamine models of schizophrenia. It should also be noted that SCRD has already been demonstrated in two schizophrenia-relevant transgenic Drosophila models (Sawamura et al. 2008; Zheng and Sehgal 2010).
A number of genes have been linked with schizophrenia, and endophenotypes thereof, through a combination of genetic linkage studies, genome-wide association studies and appraisals of biological plausibility. Those most established in the ‘candidate gene’ literature are Nrg1 (Harrison and Law 2006; Mei and Xiong 2008; Stefansson et al. 2002), Akt1 (Arguello and Gogos 2008), Disc1 (Brandon and Sawa 2011; Johnstone et al. 2011), Grm3 (Harrison et al. 2008), Dao (Chumakov et al. 2002; Verrall et al. 2010), Comt (Tunbridge et al. 2006), Dtnbp1 (Williams et al. 2005) and ErbB4 (Mei and Xiong 2008). More recent additions to the literature include Snap-25 (Corradini et al. 2009), Vipr2 (Vacic et al. 2011), Cckar (Koefoed et al. 2009), Gsk3b (Lipina et al. 2012), Pde4d (Fatemi et al. 2008; Numata et al. 2008; Tomppo et al. 2009), Tcf4 (Brzozka et al. 2010), MIR137 (Ripke et al. 2011) and ZNF804A (O’Donovan et al. 2008). To the best of our knowledge, sleep and circadian function has only been evaluated in knockout or mutant models of four of these genes, namely Snap-25 (Oliver et al. 2012), Vipr2 (Hughes and Piggins 2008), Nrg1 (Johnson et al. 2002) and Cckar (Shimazoe et al. 2008). In all four cases, significant SCRD was observed, as outlined below.
When circadian disruption is observed in a mouse model, it is important to identify the level of the circadian system at which this disturbance arises (Fig. 3). Does the deficit affect inputs to the SCN, the function of the SCN itself, or the physiological outputs of the SCN? Such knowledge is vital, as it will inform the selection and/or development of the most appropriate therapeutic intervention. To date, this approach has only been implemented in the Snap-25 (Oliver et al. 2012) and Vipr2 models (Hughes and Piggins 2008).
Suggested model of the mechanistic links between cognitive and circadian disturbances. We propose the evaluation of both schizophrenia-relevant models and models of circadian disruption, to confirm whether defects at one level are associated with defects at the other. Similarly, therapeutic interventions that correct defects at one level should produce concomitant improvements at the other
How can one determine the level at which circadian disturbances arise? Light input to the SCN can be evaluated by measuring circadian and molecular responses to light (e.g. phase shifting, entrainment, negative masking and clock gene induction) (Albrecht and Foster 2002; Jud et al. 2005), whilst core oscillator function can be assessed at a behavioural level via activity rhythms under constant conditions, and at a cellular level using clock-gene reporter assays (e.g. Per2::Luc) (Savelyev et al. 2011). Clock outputs can be determined from hormonal rhythms, and from gene expression and reporter assays in peripheral tissues. For example, hormones such as corticosterone can be monitored with real-time measurements from faeces (Abraham et al. 2006). Finally, sleep/wake behaviour can be assessed using non-invasive methods such as video tracking (Fisher et al. 2012).
Snap-25 (blind-drunk mutant)
Previous work has shown that the blind-drunk (Bdr) mouse, a model of Snap-25 exocytotic disruption, displays schizophrenia-related endophenotypes that are modulated by environmental stress (Jeans et al. 2007; Oliver and Davies 2009). Bdr mutants also show disturbances in circadian organisation that appear to specifically affect outputs of the SCN (Fig. 4). The rest/activity rhythms of Bdr mice are phase advanced and fragmented under a light/dark cycle. Retinal inputs appear normal in Bdr mice, as light-induced phase shifts, masking, pupil constriction and retinal histology are all unaffected. Similarly, clock gene rhythms within the SCN are normally phased both in vitro and in vivo. However, the 24-h rhythms of arginine vasopressin (Avp) within the SCN and plasma corticosterone are both markedly phase-advanced in Bdr mice. These data suggest that the circadian phenotype of the Bdr mouse arises from a disruption of synaptic connectivity within the SCN that alters critical output signals (Oliver et al. 2012).
Summary of findings in the blind-drunk (Bdr) mouse model of Snap-25 exocytotic disruption. The rest/activity rhythms of Bdr mice are phase advanced and fragmented under a light/dark cycle, reminiscent of the disturbed sleep/wake patterns observed in schizophrenia. Retinal inputs appear normal in mutants, and clock gene rhythms within the suprachiasmatic nuclei (SCN) [e.g. Per2 and vasoactive intestinal peptide (Vip)] are normally phased both in vitro and in vivo. However, the 24-h rhythms of arginine vasopressin (Avp) within the SCN and plasma corticosterone are both markedly phase advanced in Bdr mice. We suggest that the Bdr sleep/wake phenotype arises from a disruption of synaptic connectivity within the SCN that alters critical output signals (Oliver et al. 2012). Abbreviations for brain regions: DMH dorsomedial hypothalamus, sPVZ subparaventricular zone
Vipr2
Recent studies have shown that Vipr2 duplications confer a significant risk for schizophrenia (Vacic et al. 2011). Vasoactive intestinal polypeptide (Vip) and its receptor, Vipr2 (VPAC2), play a critical role within the SCN. Real-time imaging of circadian gene expression in SCN slices has shown that the VPAC2 receptor is required for the maintenance of circadian oscillations within SCN neurons, and for the synchronisation of oscillations between these neurons (Maywood et al. 2006). Vipr2 knockout mice demonstrate circadian abnormalities, including a shortened circadian period of approximately 22 h (Hughes and Piggins 2008). The deficit in these mice must reside in the SCN itself or in its outputs, as light input to the SCN appears relatively normal; exposure to light increases phosphoprotein and immediate early gene expression in the Vipr2 −/− SCN, whilst a subset of Vipr2 −/− mice respond appropriately to nocturnal light pulses, displaying robust phase-shift responses.
Nrg1
Neuregulin 1 (NRG1) is a growth factor involved in neurodevelopment and plasticity, which has been associated with both schizophrenia (Harrison and Law 2006; Mei and Xiong 2008; Stefansson et al. 2002) and schizotypal personality disorder (Lin et al. 2005). There is some evidence that its expression is increased in the brains of schizophrenia patients (Harrison and Law 2006; Hashimoto et al. 2004). Mice heterozygous for a disruption in the Nrg1 gene show disrupted rest/activity rhythms (Johnson et al. 2002), whilst wheel-running activity is inhibited by the long-term infusion of NRG1 into the third ventricle of the hamster brain (Snodgrass-Belt et al. 2005). NRG1 is expressed in the SCN and retinal ganglion cells (Bernstein et al. 2006; Sharif et al. 2009), consistent with its proposed involvement in circadian function.
Cckar
The Cholecystokinin A receptor (CCK-AR) is a G-protein coupled receptor that binds the neuropeptide cholecystokinin (CCK) (Noble et al. 1999). Abnormal levels of CCK mRNA have been observed in the brains of schizophrenia patients (Bachus et al. 1997; Zachrisson et al. 1999), while several studies have reported an association between the Cckar gene and schizophrenia (Koefoed et al. 2009; Tachikawa et al. 2000, 2001; Toirac et al. 2007; Wei and Hemmings 1999). Dopamine may mediate the relationship between CCK and schizophrenia, as CCK-ARs modulate CCK-stimulated dopamine release in the mesolimbic system (Marshall et al. 1991). There is also evidence that CCK plays a role in sleep regulation. The intraperitoneal administration of CCK promotes slow-wave sleep and inhibits locomotor activity in rats (Kapas et al. 1988). CCK may exert its effects through orexin, a neurotransmitter known to influence wakefulness, as CCK activates orexin neurons by binding to CCK-ARs (Tsujino et al. 2005). CCK-ARs are also involved in photoentrainment; light-induced phase shifts are significantly attenuated in Cckar knockout mice, as is light-induced clock gene expression in the SCN (Shimazoe et al. 2008).
SCRD in schizophrenia-relevant mouse models: future studies
The preceding section describes four schizophrenia-relevant mouse models with significant sleep and circadian deficits. The challenge for the future is to identify more of these models and to characterise them fully. Characterisation entails screening for both SCRD and schizophrenia-relevant behaviours; the models mentioned above are only described as ‘schizophrenia-relevant’ by virtue of the fact that the genes in question share a significant association with schizophrenia (or in the case of Snap-25, a plausible biological connection). Which of these mice provides the best model of schizophrenia remains to be seen. The Bdr mutant (Jeans et al. 2007; Oliver and Davies 2009) and NRG1type 1-tg mouse (Deakin et al. 2009) share some features in common with the disorder, while the Vipr2 and Cckar knockout models have not been extensively tested from a behavioural perspective. To redress this balance, a variety of tests could be employed across a range of behavioural domains (Chadman et al. 2009), as described in “Challenges associated with modelling schizophrenia in rodents”. In the paragraphs that follow, we consider four more genes with the potential to link schizophrenia with sleep and circadian function.
ErbB4
NRG1 (see “SCRD in schizophrenia-relevant mouse models: existing evidence”) acts through the same family of tyrosine kinase receptors as TGF-a, a putative inhibitory output signal of the SCN (Kramer et al. 2001). TGF-a acts on ERBB1 receptors, whilst NRG1 acts on ERBB4 receptors. ERBB1 receptors have a known role in locomotor activity and sleep; mice with reduced ERBB1 receptor activity show reduced negative masking by light (Kramer et al. 2001). Future studies should address whether ERBB4 receptors play a similar role. Polymorphisms in ErbB4 have previously been associated with schizophrenia (Mei and Xiong 2008).
Gsk3b
GSK3 is a serine/threonine protein kinase, whose deregulation has been implicated in schizophrenia and bipolar disorder (Lipina et al. 2012). GSK3 activity is inhibited by the putative schizophrenia risk genes Disc1 and Akt1 (Bradshaw and Porteous 2012; Lipina et al. 2012). Significantly, the GSK3 inhibitor TDZD-8 ameliorates hyperactivity and prepulse inhibition (PPI) deficits in the Disc1-L100P mutant, a mouse model of schizophrenia (Lipina et al. 2012). In the Disc1-Q31L mutant mouse, a model of depression, TDZD-8 corrects a PPI deficit, reduces immobility in the forced swim test, and increases social interaction (Lipina et al. 2012). Interestingly, clinicians often prescribe the mood stabiliser lithium (a GSK3 inhibitor) to schizophrenia patients to augment their antipsychotic medication, although the efficacy of this strategy is unclear (Leucht et al. 2004). GSK3b has a known role in circadian function; it phosphorylates clock proteins and regulates several components of the transcriptional–translational feedback loop that generates circadian rhythms (Cross et al. 1995; Iitaka et al. 2005; Martinek et al. 2001; Yin et al. 2006). GSK3b also inhibits CREB DNA-binding activity (Grimes and Jope 2001), which is involved in circadian signal transduction (Lee et al. 2010).
Pde4d
PDE4 is a phosphodiesterase that been associated with schizophrenia in a number of studies (Fatemi et al. 2008; Numata et al. 2008; Tomppo et al. 2009). Like GSK3, PDE4 activity is inhibited by the putative schizophrenia risk gene Disc1 (Bradshaw and Porteous 2012; Lipina et al. 2012). Crucially, the PDE4 inhibitor Rolipram acts as a cognitive enhancer, facilitating long-term potentiation, memory performance and latent inhibition in wildtype rodents (Barad et al. 1998; Davis and Gould 2005; Zhang et al. 2004), and attenuating PPI deficits in the Disc1-L100P mutant model of schizophrenia (Lipina et al. 2012). In addition, Rolipram has antidepressant-like properties, reducing immobility in the forced swim test in wildtype rats (Zhang et al. 2006). From a circadian perspective, PDE4 regulates cAMP signalling, which is involved in circadian signal transduction (O’Neill and Reddy 2012). Intriguingly, a polymorphism in Pde4d has been associated with sleepiness in healthy individuals (Gottlieb et al. 2007).
Tcf4
Several large genome-wide association studies have identified the basic helix-loop-helix (bHLH) transcription factor TCF4 as one of the most significant schizophrenia susceptibility genes (Brzozka et al. 2010). The protein encoded by this gene resembles ID (inhibitor of DNA-binding) proteins, which act as negative regulators of the transcriptional–translational feedback loop that generates intracellular circadian rhythms (Duffield et al. 2009). To date, at least eight different Tcf4 mutants have been produced by ENU mutagenesis, but their circadian profiles have yet to be characterised.
Schizophrenia-relevant abnormalities in mouse models of SCRD
To establish mechanistic links between SCRD and schizophrenia, a parallel approach is to screen mouse models of circadian disruption for schizophrenia-relevant behaviours, such as impaired cognitive function. To our knowledge, the Clock mutant, described below, is the only mouse model of SCRD which has been studied in this way (Roybal et al. 2007). The Clock mutant does not show any schizophrenia-relevant behaviours, but has a striking mania-like phenotype, which can be reversed with the mood stabiliser lithium (Roybal et al. 2007). Future research will reveal whether other mouse models of SCRD have a schizophrenia-relevant phenotype.
Clock (Clock mutant)
The Clock mutant was first identified in 1994 from a circadian screen of ENU mutants (Vitaterna et al. 1994). These animals have an extended circadian period (Vitaterna et al. 1994), and sleep significantly less than wildtypes (Naylor et al. 2000), consistent with the decreased need for sleep observed in patients with mania (Plante and Winkelman 2008). Their behavioural profile is also reminiscent of bipolar patients in the manic state; they show hyperactivity, excessive-reward seeking behaviour [as measured by intracranial self-stimulation (ICSS), cocaine preference and sucrose preference], reduced depression like-behaviour (as measured with the Porsolt forced swim test and learned helplessness test), reduced anxiety-like behaviour (as measured with the open field test and elevated plus maze) (Roybal et al. 2007) and increased exploratory behaviour (Easton et al. 2003).
Therapeutic interventions in mouse models with simultaneous deficits
Once models that display simultaneous circadian and schizophrenia-relevant abnormalities have been identified, therapeutic interventions can be introduced. If schizophrenia and SCRD share a common mechanistic origin, then two further predictions logically follow. The administration of drugs used to treat schizophrenia (e.g. antipsychotics and mood-stabilisers) should produce a concurrent improvement in the animals’ SCRD and schizophrenia-relevant deficits, while therapies that target SCRD should do likewise (Fig. 3). The latter approach could involve either pharmacological (e.g. melatonin administration) or environmental interventions (e.g. modification of the light/dark cycle or scheduled voluntary exercise) (Power et al. 2010). This type of experiment has never been performed in a schizophrenia-relevant animal model, although the pharmacological imposition of sleep has been shown to slow cognitive decline in a transgenic mouse model of Huntington’s disease (Pallier et al. 2007).
Challenges associated with modelling schizophrenia in rodents
Animal studies afford a level of control that is simply not possible in human studies. Human participants differ from each other in all manner of dimensions, including their age, education, medication history, genetic makeup and life experiences. In this context, it is difficult to attribute a specific behavioural observation (e.g. disturbed sleep) to a specific underlying trait (e.g. the possession of a particular risk-conferring gene). In animal studies, there are relatively fewer confounds, so making such attributions is more straightforward. Genetic manipulation is also possible in rodents, enabling the investigator to move beyond correlation and infer causality.
Modelling schizophrenia in animals raises a number of complex theoretical and experimental design issues for the investigator (for reviews, see Arguello and Gogos 2010; Harrison et al. 2012; Nestler and Hyman 2010; Papaleo et al. 2012). The first and perhaps biggest challenge is the process of selecting which gene to manipulate. Despite years of research, no gene has been unequivocally established as conferring increased susceptibility to schizophrenia (Crow 2008). The literature is littered with genes that have yielded positive results in one or two genetic association studies, only for several follow-up studies to draw a blank (Crow 2008). A ‘schizophrenia model’ is only as good as the evidence linking the gene in question with the disorder itself, and from this perspective, the validity of almost any model can be questioned. The likely explanation for these results is that schizophrenia is caused by a large number of genes, each one having a very small effect (Chakravarti 1999). Put simply, no single gene is either necessary or sufficient to cause the disorder. In light of this, the value of single gene models is debatable. Double- or triple-gene models may be more realistic, but these are more complex and costly to produce. A further limitation is that some schizophrenia risk genes (e.g. G72/DAOA) have no known ortholog in rodents (Chumakov et al. 2002).
Besides choosing which gene to manipulate, the investigator must decide how to manipulate it. Knockout models are the simplest and therefore the most common, but again, are unlikely to represent schizophrenia that faithfully. There is no evidence for null mutations in schizophrenia, but there is evidence for the up- or down-regulation of specific genes (Harrison and Weinberger 2005). In this context, transgenic over-expression or heterozygous knockout may prove more suitable tools for modelling schizophrenia. The investigator must also decide whether the genetic manipulation is constitutive or conditional. Most models are constitutive; that is, the manipulation is present throughout the brain and throughout the animal’s lifetime. Given evidence that brain abnormalities in schizophrenia are more pronounced in some brain areas than others, and that they can develop over time (Ellison-Wright et al. 2008), the constitutive approach may not be the most appropriate. Conditional models, in which the timing and location of the manipulation are more tightly regulated, provide a more flexible alternative.
Another complication is the not inconsiderable variation amongst patients labelled with schizophrenia. The disorder is associated with a wide range of symptoms, and two patients with the same diagnosis may present a very different subset of these symptoms (Andreasen 1999). Thus, treating a condition as complex as schizophrenia as a unitary disorder could be a mistake. Perhaps a more appropriate approach would be to model specific symptoms (or endophenotypes) in isolation (Kaffman and Krystal 2012).
In rodents, some schizophrenia-relevant behaviours are easier to model than others. Schizophrenia is characterised by positive symptoms (e.g. hallucinations and delusions), negative symptoms (e.g. avolition and anhedonia) and cognitive symptoms (e.g. impaired working memory, sensorimotor gating and attentional set-shifting) (Andreasen 1995; Elvevag and Goldberg 2000). In humans, hallucinations and delusions are only revealed through patients’ verbal reports, so these symptoms are difficult to investigate in rodents. Nonetheless, hyperlocomotion and stereotypic behaviours are considered by many to reflect the positive symptoms of schizophrenia (Nilsson et al. 1997; Sams-Dodd 1996). Similarly, reduced social interaction in rodents is often presented as a direct analogue of the negative symptoms witnessed in schizophrenia (Lee et al. 2005; Sams-Dodd 1996). Whether these behaviours are conceptually and neurally equivalent seems highly unlikely, however (Bussey et al. 2012; Garner et al. 2006). In contrast, the human and rodent versions of most cognitive tasks are at least superficially similar. PPI, for example, is a measure of sensorimotor gating which can be tested almost identically in humans and rodents (Swerdlow et al. 2008), while the attentional set-shifting task, which measures cognitive flexibility, is modelled directly on its human counterpart, the Wisconsin Card Sorting Test (Bissonette and Powell 2012; Garner et al. 2006). Questions remain as to the suitability of set-shifting tasks for mice, however (Garner et al. 2006). Working memory is also routinely tested in rodents, although again, it is unclear whether the human and rodent versions of these tasks tap the same neural substrates (Sanderson and Bannerman 2010).
Of course, behavioural assessment is not the only way to assess the validity of schizophrenia-relevant models. Investigators can also look for neurophysiological changes reminiscent of those observed in schizophrenia. These include structural changes, such as reduction of cortical thickness and enlargement of the lateral ventricles (Weinberger et al. 1982), and characteristic changes in neurotransmitter systems, such as glutamatergic hypofunction (Konradi and Heckers 2003).
To summarise, it would be a significant overstatement to suggest that schizophrenia can be faithfully modelled in rodents. First, some of the core symptoms of schizophrenia simply cannot be measured in rats or mice, as explained above. Secondly, schizophrenia is a multi-gene disorder, so it seems improbable that a single-gene model could ever demonstrate the full range of symptoms observed in a patient. Finally, there are notable neuroanatomical differences between humans and rodents; the prefrontal cortex, for example, is far more developed in humans than it is in rodents. Despite these limitations, murine models may still yield valuable insights into the roles of schizophrenia risk genes in brain function, such as whether they contribute to sleep and circadian function.
Challenges associated with sleep and circadian phenotyping in rodents
Sleep and circadian rhythms in humans and rodents are not identical, not least as rodents are nocturnal and humans diurnal. In addition, humans typically sleep once every 24 h, whereas rodents tend to alternate between several bouts of sleep and wakefulness (Fisher et al. 2012). As a result, the sleep and circadian phenotype of a rodent model may not translate directly to humans. In addition, environmental light intensity varies continuously in the natural world, whereas most experimental paradigms employ a discrete transition from light to dark and vice versa.
The way we assess the circadian/sleep phenotype of rodents might also cause problems. Crucially, locomotor activity is the dependent variable in most circadian screening paradigms, and locomotor activity is subject to all manner of influences besides circadian function, including basic motor function, anxiety and arousal levels. Therefore, this approach might not be the most appropriate for assessing mouse models with locomotor deficits, such as the schizophrenia-relevant NRG1type 1-tg mouse (Deakin et al. 2009). An obesity phenotype, as seen in Clock mutant mice, could also complicate behavioural phenotyping (Turek et al. 2005). Invasive measures of circadian function (e.g. clock gene rhythms within the SCN) provide a useful alternative, as they are not subject to the same confounds.
Differences in mouse strain must also be taken into consideration. For example, the circadian system of the C57/BL6 mouse is more sensitive to light than that of the C3H mouse. Hence, C57/BL6 mice are able to entrain their circadian behaviour at much lower irradiances (Foster and Helfrich-Forster 2001). There is also evidence that pineal melatonin content varies between mouse strains (Ebihara et al. 1986; Goto et al. 1989). In contrast to wild mice, several inbred strains, including the C57/BL6 mouse, do not have detectable melatonin levels in their pineal glands.
Conclusion and future directions
Sleep and circadian rhythm disruption and schizophrenia are often co-morbid, and this co-morbidity may arise from the involvement of common brain mechanisms. SCRD in schizophrenia is not merely a side effect of antipsychotic medication, nor is it a by-product of an absence of social routine. The treatment of insomnia in schizophrenia patients produces a concomitant improvement in psychiatric symptoms, which provides further support for the hypothesis that common mechanisms are involved. Moreover, many symptoms associated with SCRD, such as impaired cognitive performance, are frequently observed in schizophrenia patients. Animal models provide a means to test our hypothesis of mechanistic overlap. Implicit in our theory are four key predictions that can be tested in rodents:
-
1.
Sleep and circadian rhythm disruption should be observed in schizophrenia-relevant models.
-
2.
Schizophrenia-relevant behavioural abnormalities (e.g. cognitive impairments) may be observed in models of SCRD.
-
3.
Therapies that target SCRD (e.g. melatonin or scheduled voluntary exercise) should ameliorate both SCRD and schizophrenia-relevant behavioural abnormalities in models which display simultaneous deficits.
-
4.
Therapies that target schizophrenia-relevant behavioural abnormalities (e.g. antipsychotic or mood-stabilising drugs) should do likewise.
In this review, we have drawn attention to several schizophrenia-relevant mouse models which show significant sleep and circadian deficits. The challenge for the future is to identify more of these models, characterise them fully, and investigate their responses to therapeutic interventions. Given the continued interest in, and availability of, schizophrenia-relevant mouse models, the evaluation of circadian and sleep physiology and behaviour in these models represents an excellent opportunity to better understand the shared mechanistic basis of SCRD and schizophrenia.
References
Abraham D, Dallmann R, Steinlechner S, Albrecht U, Eichele G, Oster H (2006) Restoration of circadian rhythmicity in circadian clock-deficient mice in constant light. J Biol Rhythms 21:169–176
Acheson A, Richards JB, de Wit H (2007) Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav 91:579–587
Albrecht U, Foster RG (2002) Placing ocular mutants into a functional context: a chronobiological approach. Methods 28:465–477
Aleman A, Hijman R, de Haan EH, Kahn RS (1999) Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry 156:1358–1366
Alhola P, Polo-Kantola P (2007) Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat 3:553–567
Andreasen NC (1995) Symptoms, signs, and diagnosis of schizophrenia. Lancet 346:477–481
Andreasen NC (1999) A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry 56:781–787
Arguello PA, Gogos JA (2008) A signaling pathway AKTing up in schizophrenia. J Clin Invest 118:2018–2021
Arguello PA, Gogos JA (2010) Cognition in mouse models of schizophrenia susceptibility genes. Schizophr Bull 36:289–300
Auslander LA, Jeste DV (2002) Perceptions of problems and needs for service among middle-aged and elderly outpatients with schizophrenia and related psychotic disorders. Community Ment Health J 38:391–402
Bachus SE, Hyde TM, Herman MM, Egan MF, Kleinman JE (1997) Abnormal cholecystokinin mRNA levels in entorhinal cortex of schizophrenics. J Psychiatr Res 31:233–256
Banks S, Dinges DF (2007) Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med 3:519–528
Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E (1998) Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA 95:15020–15025
Baranski JV, Pigeau RA (1997) Self-monitoring cognitive performance during sleep deprivation: effects of modafinil, d-amphetamine and placebo. J Sleep Res 6:84–91
Baranski JV, Thompson MM, Lichacz FM, McCann C, Gil V, Pasto L, Pigeau RA (2007) Effects of sleep loss on team decision making: motivational loss or motivational gain? Hum Factors 49:646–660
Basner M, Glatz C, Griefahn B, Penzel T, Samel A (2008a) Aircraft noise: effects on macro- and microstructure of sleep. Sleep Med 9:382–387
Basner M, Muller U, Elmenhorst EM, Kluge G, Griefahn B (2008b) Aircraft noise effects on sleep: a systematic comparison of EEG awakenings and automatically detected cardiac activations. Physiol Meas 29:1089–1103
Bernstein HG, Lendeckel U, Bertram I, Bukowska A, Kanakis D, Dobrowolny H, Stauch R, Krell D, Mawrin C, Budinger E, Keilhoff G, Bogerts B (2006) Localization of neuregulin-1alpha (heregulin-alpha) and one of its receptors, ErbB-4 tyrosine kinase, in developing and adult human brain. Brain Res Bull 69:546–559
Bissonette GB, Powell EM (2012) Reversal learning and attentional set-shifting in mice. Neuropharmacology 62:1168–1174
Boivin DB, Tremblay GM, James FO (2007) Working on atypical schedules. Sleep Med 8:578–589
Bradshaw NJ, Porteous DJ (2012) DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology 62:1230–1241
Brandon NJ, Sawa A (2011) Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci 12:707–722
Bromundt V, Koster M, Georgiev-Kill A, Opwis K, Wirz-Justice A, Stoppe G, Cajochen C (2011) Sleep-wake cycles and cognitive functioning in schizophrenia. Br J Psychiatry 198:269–276
Brzozka MM, Radyushkin K, Wichert SP, Ehrenreich H, Rossner MJ (2010) Cognitive and sensorimotor gating impairments in transgenic mice overexpressing the schizophrenia susceptibility gene Tcf4 in the brain. Biol Psychiatry 68:33–40
Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM (2012) New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology 62:1191–1203
Chadman KK, Yang M, Crawley JN (2009) Criteria for validating mouse models of psychiatric diseases. Am J Med Genet B Neuropsychiatr Genet 150B:1–11
Chakravarti A (1999) Population genetics—making sense out of sequence. Nat Genet 21:56–60
Chee MW, Chuah LY (2008) Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol 21:417–423
Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H, Bougueleret L, Barry C, Tanaka H, La Rosa P, Puech A, Tahri N, Cohen-Akenine A, Delabrosse S, Lissarrague S, Picard FP, Maurice K, Essioux L, Millasseau P, Grel P, Debailleul V, Simon AM, Caterina D, Dufaure I, Malekzadeh K, Belova M, Luan JJ, Bouillot M, Sambucy JL, Primas G, Saumier M, Boubkiri N, Martin-Saumier S, Nasroune M, Peixoto H, Delaye A, Pinchot V, Bastucci M, Guillou S, Chevillon M, Sainz-Fuertes R, Meguenni S, Aurich-Costa J, Cherif D, Gimalac A, Van Duijn C, Gauvreau D, Ouellette G, Fortier I, Raelson J, Sherbatich T, Riazanskaia N, Rogaev E, Raeymaekers P, Aerssens J, Konings F, Luyten W, Macciardi F, Sham PC, Straub RE, Weinberger DR, Cohen N, Cohen D (2002) Genetic and physiological data implicating the new human gene G72 and the gene for d-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 99:13675–13680
Cohrs S (2008) Sleep disturbances in patients with schizophrenia: impact and effect of antipsychotics. CNS Drugs 22:939–962
Corradini I, Verderio C, Sala M, Wilson MC, Matteoli M (2009) SNAP-25 in neuropsychiatric disorders. Ann N Y Acad Sci 1152:93–99
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789
Crow TJ (2008) The emperors of the schizophrenia polygene have no clothes. Psychol Med 38:1681–1685
Dahl RE, Lewin DS (2002) Pathways to adolescent health sleep regulation and behavior. J Adolesc Health 31:175–184
Davis JA, Gould TJ (2005) Rolipram attenuates MK-801-induced deficits in latent inhibition. Behav Neurosci 119:595–602
Davis S, Mirick DK (2006) Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control 17:539–545
Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ, Bannerman DM (2009) Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. Neuroreport 20:1523–1528
Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549
Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, Aptowicz C, Pack AI (1997) Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 20:267–270
Duffield GE, Watson NP, Mantani A, Peirson SN, Robles-Murguia M, Loros JJ, Israel MA, Dunlap JC (2009) A role for Id2 in regulating photic entrainment of the mammalian circadian system. Curr Biol 19:297–304
Dworak M, Schierl T, Bruns T, Struder HK (2007) Impact of singular excessive computer game and television exposure on sleep patterns and memory performance of school-aged children. Pediatrics 120:978–985
Easton A, Arbuzova J, Turek FW (2003) The circadian clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav 2:11–19
Ebihara S, Marks T, Hudson DJ, Menaker M (1986) Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 231:491–493
Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E (2008) The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry 165:1015–1023
Elvevag B, Goldberg TE (2000) Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol 14:1–21
Fatemi SH, King DP, Reutiman TJ, Folsom TD, Laurence JA, Lee S, Fan YT, Paciga SA, Conti M, Menniti FS (2008) PDE4B polymorphisms and decreased PDE4B expression are associated with schizophrenia. Schizophr Res 101:36–49
Fisher SP, Godinho SI, Pothecary CA, Hankins MW, Foster RG, Peirson SN (2012) Rapid assessment of sleep-wake behavior in mice. J Biol Rhythms 27:48–58
Forest G, Poulin J, Daoust AM, Lussier I, Stip E, Godbout R (2007) Attention and non-REM sleep in neuroleptic-naive persons with schizophrenia and control participants. Psychiatry Res 149:33–40
Foster RG, Helfrich-Forster C (2001) The regulation of circadian clocks by light in fruitflies and mice. Philos Trans R Soc Lond B Biol Sci 356:1779–1789
Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB (2005) Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 28:1289–1296
Garner JP, Thogerson CM, Wurbel H, Murray JD, Mench JA (2006) Animal neuropsychology: validation of the intra-dimensional extra-dimensional set shifting task for mice. Behav Brain Res 173:53–61
Goder R, Boigs M, Braun S, Friege L, Fritzer G, Aldenhoff JB, Hinze-Selch D (2004) Impairment of visuospatial memory is associated with decreased slow wave sleep in schizophrenia. J Psychiatr Res 38:591–599
Goder R, Scharffetter F, Aldenhoff JB, Fritzer G (2007) Visual declarative memory is associated with non-rapid eye movement sleep and sleep cycles in patients with chronic non-restorative sleep. Sleep Med 8:503–508
Goder R, Fritzer G, Gottwald B, Lippmann B, Seeck-Hirschner M, Serafin I, Aldenhoff JB (2008) Effects of olanzapine on slow wave sleep, sleep spindles and sleep-related memory consolidation in schizophrenia. Pharmacopsychiatry 41:92–99
Goldman M, Tandon R, DeQuardo JR, Taylor SF, Goodson J, McGrath M (1996) Biological predictors of 1-year outcome in schizophrenia in males and females. Schizophr Res 21:65–73
Goto M, Oshima I, Tomita T, Ebihara S (1989) Melatonin content of the pineal gland in different mouse strains. J Pineal Res 7:195–204
Gottlieb DJ, O’Connor GT, Wilk JB (2007) Genome-wide association of sleep and circadian phenotypes. BMC Med Genet 8(Suppl 1):S9
Grimes CA, Jope RS (2001) CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem 78:1219–1232
Hansen J (2006) Risk of breast cancer after night- and shift work: current evidence and ongoing studies in Denmark. Cancer Causes Control 17:531–537
Harrison PJ, Weinberger DR (2005) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10:40–68; image 5
Harrison PJ, Law AJ (2006) Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry 60:132–140
Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA (2008) The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol 22:308–322
Harrison PJ, Pritchett D, Stumpenhorst K, Betts JF, Nissen W, Schweimer J, Lane T, Burnet PW, Lamsa KP, Sharp T, Bannerman DM, Tunbridge EM (2012) Genetic mouse models relevant to schizophrenia: taking stock and looking forward. Neuropharmacology 62:1164–1167
Harrison Y, Horne JA (2000) The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl 6:236–249
Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR (2004) Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry 9:299–307
Hofstetter JR, Lysaker PH, Mayeda AR (2005) Quality of sleep in patients with schizophrenia is associated with quality of life and coping. BMC Psychiatry 5:13
Horne JA (1988) Sleep loss and “divergent” thinking ability. Sleep 11:528–536
Horne JA (1993) Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry 162:413–419
Hughes AT, Piggins HD (2008) Behavioral responses of Vipr2−/− mice to light. J Biol Rhythms 23:211–219
Iitaka C, Miyazaki K, Akaike T, Ishida N (2005) A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem 280:29397–29402
Irwin M (2002) Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun 16:503–512
Jeans AF, Oliver PL, Johnson R, Capogna M, Vikman J, Molnar Z, Babbs A, Partridge CJ, Salehi A, Bengtsson M, Eliasson L, Rorsman P, Davies KE (2007) A dominant mutation in Snap25 causes impaired vesicle trafficking, sensorimotor gating, and ataxia in the blind-drunk mouse. Proc Natl Acad Sci USA 104:2431–2436
Johnson EO, Roth T, Breslau N (2006) The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res 40:700–708
Johnson MA, Devay P, Role LW (2002) The cystine-rich domain of the neuregulin-1 gene (crd-NRG-1) is required for survival of a subset of neurons in the suprachiasmatic nucleus (SCN). Society for Neuroscience, Washington DC (online). 2002 Abstract Viewer/Itinerary Planner, 2002
Johnstone M, Thomson PA, Hall J, McIntosh AM, Lawrie SM, Porteous DJ (2011) DISC1 in schizophrenia: genetic mouse models and human genomic imaging. Schizophr Bull 37:14–20
Jones K, Harrison Y (2001) Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev 5:463–475
Jud C, Schmutz I, Hampp G, Oster H, Albrecht U (2005) A guideline for analyzing circadian wheel-running behavior in rodents under different lighting conditions. Biol Proced Online 7:101–116
Kaffman A, Krystal JJ (2012) New frontiers in animal research of psychiatric illness. Methods Mol Biol 829:3–30
Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WD (2007) The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med 8:215–221
Kahol K, Leyba MJ, Deka M, Deka V, Mayes S, Smith M, Ferrara JJ, Panchanathan S (2008) Effect of fatigue on psychomotor and cognitive skills. Am J Surg 195:195–204
Kantrowitz JT, Oakman E, Bickel S, Citrome L, Spielman A, Silipo G, Battaglia J, Javitt DC (2010) The importance of a good night’s sleep: an open-label trial of the sodium salt of gamma-hydroxybutyric acid in insomnia associated with schizophrenia. Schizophr Res 120:225–226
Kapas L, Obal F Jr, Alfoldi P, Rubicsek G, Penke B, Obal F (1988) Effects of nocturnal intraperitoneal administration of cholecystokinin in rats: simultaneous increase in sleep, increase in EEG slow-wave activity, reduction of motor activity, suppression of eating, and decrease in brain temperature. Brain Res 438:155–164
Kelman BB (1999) The sleep needs of adolescents. J Sch Nurs 15:14–19
Killgore WD, Balkin TJ, Wesensten NJ (2006a) Impaired decision making following 49 h of sleep deprivation. J Sleep Res 15:7–13
Killgore WD, McBride SA, Killgore DB, Balkin TJ (2006b) The effects of caffeine, dextroamphetamine, and modafinil on humor appreciation during sleep deprivation. Sleep 29:841–847
Killgore WD, Killgore DB, Day LM, Li C, Kamimori GH, Balkin TJ (2007) The effects of 53 hours of sleep deprivation on moral judgment. Sleep 30:345–352
Killgore WD, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ (2008) Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med 9:517–526
Knutson KL, Spiegel K, Penev P, Van Cauter E (2007) The metabolic consequences of sleep deprivation. Sleep Med Rev 11:163–178
Koefoed P, Hansen TV, Woldbye DP, Werge T, Mors O, Hansen T, Jakobsen KD, Nordentoft M, Wang A, Bolwig TG, Rehfeld JF (2009) An intron 1 polymorphism in the cholecystokinin-A receptor gene associated with schizophrenia in males. Acta Psychiatr Scand 120:281–287
Konradi C, Heckers S (2003) Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther 97:153–179
Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ (2001) Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science 294:2511–2515
Krystal AD, Thakur M, Roth T (2008) Sleep disturbance in psychiatric disorders: effects on function and quality of life in mood disorders, alcoholism, and schizophrenia. Ann Clin Psychiatry 20:39–46
Kundermann B, Krieg JC, Schreiber W, Lautenbacher S (2004) The effect of sleep deprivation on pain. Pain Res Manag 9:25–32
Kyriacou CP, Hastings MH (2010) Circadian clocks: genes, sleep, and cognition. Trends Cogn Sci 14:259–267
Lamond N, Jay SM, Dorrian J, Ferguson SA, Jones C, Dawson D (2007) The dynamics of neurobehavioural recovery following sleep loss. J Sleep Res 16:33–41
Landis CA, Savage MV, Lentz MJ, Brengelmann GL (1998) Sleep deprivation alters body temperature dynamics to mild cooling and heating not sweating threshold in women. Sleep 21:101–108
Laposky AD, Bass J, Kohsaka A, Turek FW (2008) Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett 582:142–151
Lee B, Li A, Hansen KF, Cao R, Yoon JH, Obrietan K (2010) CREB influences timing and entrainment of the SCN circadian clock. J Biol Rhythms 25:410–420
Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI (2005) Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology 30:1883–1894
Leucht S, Kissling W, McGrath J (2004) Lithium for schizophrenia revisited: a systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry 65:177–186
Lin HF, Liu YL, Liu CM, Hung SI, Hwu HG, Chen WJ (2005) Neuregulin 1 gene and variations in perceptual aberration of schizotypal personality in adolescents. Psychol Med 35:1589–1598
Lipina TV, Wang M, Liu F, Roder JC (2012) Synergistic interactions between PDE4B and GSK-3: DISC1 mutant mice. Neuropharmacology 62:1252–1262
Lorton D, Lubahn CL, Estus C, Millar BA, Carter JL, Wood CA, Bellinger DL (2006) Bidirectional communication between the brain and the immune system: implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation 13:357–374
Lowrey PL, Takahashi JS (2011) Genetics of circadian rhythms in mammalian model organisms. Adv Genet 74:175–230
Lucidi F, Russo PM, Mallia L, Devoto A, Lauriola M, Violani C (2006) Sleep-related car crashes: risk perception and decision-making processes in young drivers. Accid Anal Prev 38:302–309
Lynn SJ, Lilienfeld SO, Merckelbach H, Giesbrecht T, van der Kloet D (2012) Dissociation and dissociative disorders. Curr Dir Psychol Sci 21:48–53
Maemura K, Takeda N, Nagai R (2007) Circadian rhythms in the CNS and peripheral clock disorders: role of the biological clock in cardiovascular diseases. J Pharmacol Sci 103:134–138
Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R (2004) A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry 56:951–956
Manoach DS, Stickgold R (2009) Does abnormal sleep impair memory consolidation in schizophrenia? Front Hum Neurosci 3:21
Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Djonlagic I, Vangel MG, Goff DC, Stickgold R (2010) Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res 44:112–120
Marshall FH, Barnes S, Hughes J, Woodruff GN, Hunter JC (1991) Cholecystokinin modulates the release of dopamine from the anterior and posterior nucleus accumbens by two different mechanisms. J Neurochem 56:917–922
Martin J, Jeste DV, Caliguiri MP, Patterson T, Heaton R, Ancoli-Israel S (2001) Actigraphic estimates of circadian rhythms and sleep/wake in older schizophrenia patients. Schizophr Res 47:77–86
Martin JL, Jeste DV, Ancoli-Israel S (2005) Older schizophrenia patients have more disrupted sleep and circadian rhythms than age-matched comparison subjects. J Psychiatr Res 39:251–259
Martinek S, Inonog S, Manoukian AS, Young MW (2001) A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105:769–779
Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH (2006) Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol 16:599–605
McKenna BS, Dicjinson DL, Orff HJ, Drummond SP (2007) The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res 16:245–252
Meerlo P, Sgoifo A, Suchecki D (2008) Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev 12:197–210
Mei L, Xiong WC (2008) Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 9:437–452
Miller DD (2004) Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry 6:3–7
Mistlberger RE, Skene DJ (2004) Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev Camb Philos Soc 79:533–556
Moore RY (1973) Retinohypothalamic projection in mammals: a comparative study. Brain Res 49:403–409
Moore RY, Klein DC (1974) Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res 71:17–33
Muecke S (2005) Effects of rotating night shifts: literature review. J Adv Nurs 50:433–439
Murray G, Harvey A (2010) Circadian rhythms and sleep in bipolar disorder. Bipolar Disord 12:459–472
Myers E, Startup H, Freeman D (2011) Cognitive behavioural treatment of insomnia in individuals with persistent persecutory delusions: a pilot trial. J Behav Ther Exp Psychiatry 42:330–336
Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW (2000) The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci 20:8138–8143
Nestler EJ, Hyman SE (2010) Animal models of neuropsychiatric disorders. Nat Neurosci 13:1161–1169
Nilsson M, Carlsson A, Carlsson ML (1997) Glycine and d-serine decrease MK-801-induced hyperactivity in mice. J Neural Transm 104:1195–1205
Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, Roques BP (1999) International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev 51:745–781
Numata S, Ueno S, Iga J, Song H, Nakataki M, Tayoshi S, Sumitani S, Tomotake M, Itakura M, Sano A, Ohmori T (2008) Positive association of the PDE4B (phosphodiesterase 4B) gene with schizophrenia in the Japanese population. J Psychiatr Res 43:7–12
O’Brien EM, Mindell JA (2005) Sleep and risk-taking behavior in adolescents. Behav Sleep Med 3:113–133
O’Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, Nikolov I, Hamshere M, Carroll L, Georgieva L, Dwyer S, Holmans P, Marchini JL, Spencer CC, Howie B, Leung HT, Hartmann AM, Moller HJ, Morris DW, Shi Y, Feng G, Hoffmann P, Propping P, Vasilescu C, Maier W, Rietschel M, Zammit S, Schumacher J, Quinn EM, Schulze TG, Williams NM, Giegling I, Iwata N, Ikeda M, Darvasi A, Shifman S, He L, Duan J, Sanders AR, Levinson DF, Gejman PV, Cichon S, Nothen MM, Gill M, Corvin A, Rujescu D, Kirov G, Owen MJ, Buccola NG, Mowry BJ, Freedman R, Amin F, Black DW, Silverman JM, Byerley WF, Cloninger CR (2008) Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet 40:1053–1055
O’Neill JS, Reddy AB (2012) The essential role of cAMP/Ca2+ signalling in mammalian circadian timekeeping. Biochem Soc Trans 40:44–50
Oginska H, Pokorski J (2006) Fatigue and mood correlates of sleep length in three age-social groups: school children, students, and employees. Chronobiol Int 23:1317–1328
Oken BS, Salinsky MC, Elsas SM (2006) Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol 117:1885–1901
Oliver PL, Davies KE (2009) Interaction between environmental and genetic factors modulates schizophrenic endophenotypes in the Snap-25 mouse mutant blind-drunk. Hum Mol Genet 18:4576–4589
Oliver PL, Sobczyk MV, Maywood ES, Edwards B, Lee S, Livieratos A, Oster H, Butler R, Godinho SI, Wulff K, Peirson SN, Fisher SP, Chesham JE, Smith JW, Hastings MH, Davies KE, Foster RG (2009) Interaction between environmental and genetic factors modulates schizophrenic endophenotypes in the Snap-25 mouse mutant blind-drunk. Hum Mol Genet 18:4576–4589
Pallier PN, Maywood ES, Zheng Z, Chesham JE, Inyushkin AN, Dyball R, Hastings MH, Morton AJ (2007) Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington’s disease. J Neurosci 27:7869–7878
Papaleo F, Lipska BK, Weinberger DR (2012) Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology 62:1204–1220
Philip P, Akerstedt T (2006) Transport and industrial safety, how are they affected by sleepiness and sleep restriction? Sleep Med Rev 10:347–356
Pilcher JJ, Huffcutt AI (1996) Effects of sleep deprivation on performance: a meta-analysis. Sleep 19:318–326
Pilcher JJ, Lambert BJ, Huffcutt AI (2000) Differential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic review. Sleep 23:155–163
Plante DT, Winkelman JW (2008) Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry 165:830–843
Power A, Hughes AT, Samuels RE, Piggins HD (2010) Rhythm-promoting actions of exercise in mice with deficient neuropeptide signaling. J Biol Rhythms 25:235–246
Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK (1998) Cognitive function following acute sleep restriction in children ages 10–14. Sleep 21:861–868
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941
Riemann D, Voderholzer U (2003) Primary insomnia: a risk factor to develop depression? J Affect Disord 76:255–259
Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin DY, Duan J, Ophoff RA, Andreassen OA, Scolnick E, Cichon S, St Clair D, Corvin A, Gurling H, Werge T, Rujescu D, Blackwood DH, Pato CN, Malhotra AK, Purcell S, Dudbridge F, Neale BM, Rossin L, Visscher PM, Posthuma D, Ruderfer DM, Fanous A, Stefansson H, Steinberg S, Mowry BJ, Golimbet V, De Hert M, Jonsson EG, Bitter I, Pietilainen OP, Collier DA, Tosato S, Agartz I, Albus M, Alexander M, Amdur RL, Amin F, Bass N, Bergen SE, Black DW, Borglum AD, Brown MA, Bruggeman R, Buccola NG, Byerley WF, Cahn W, Cantor RM, Carr VJ, Catts SV, Choudhury K, Cloninger CR, Cormican P, Craddock N, Danoy PA, Datta S, de Haan L, Demontis D, Dikeos D, Djurovic S, Donnelly P, Donohoe G, Duong L, Dwyer S, Fink-Jensen A, Freedman R, Freimer NB, Friedl M, Georgieva L, Giegling I, Gill M, Glenthoj B, Godard S, Hamshere M, Hansen M, Hansen T, Hartmann AM, Henskens FA, Hougaard DM, Hultman CM, Ingason A, Jablensky AV, Jakobsen KD, Jay M, Jurgens G, Kahn RS, Keller MC, Kenis G, Kenny E, Kim Y, Kirov GK, Konnerth H, Konte B, Krabbendam L, Krasucki R, Lasseter VK, Laurent C, Lawrence J, Lencz T, Lerer FB, Liang KY, Lichtenstein P, Lieberman JA, Linszen DH, Lonnqvist J, Loughland CM, Maclean AW, Maher BS, Maier W, Mallet J, Malloy P, Mattheisen M, Mattingsdal M, McGhee KA, McGrath JJ, McIntosh A, McLean DE, McQuillin A, Melle I, Michie PT, Milanova V, Morris DW, Mors O, Mortensen PB, Moskvina V, Muglia P, Myin-Germeys I, Nertney DA, Nestadt G, Nielsen J, Nikolov I, Nordentoft M, Norton N, Nothen MM, O’Dushlaine CT, Olincy A, Olsen L, O’Neill FA, Orntoft TF, Owen MJ, Pantelis C, Papadimitriou G, Pato MT, Peltonen L, Petursson H, Pickard B, Pimm J, Pulver AE, Puri V, Quested D, Quinn EM, Rasmussen HB, Rethelyi JM, Ribble R, Rietschel M, Riley BP, Ruggeri M, Schall U, Schulze TG, Schwab SG, Scott RJ, Shi J, Sigurdsson E, Silverman JM, Spencer CC, Stefansson K, Strange A, Strengman E, Stroup TS, Suvisaari J, Terenius L, Thirumalai S, Thygesen JH, Timm S, Toncheva D, van den Oord E, van Os J, van Winkel R, Veldink J, Walsh D, Wang AG, Wiersma D, Wildenauer DB, Williams HJ, Williams NM, Wormley B, Zammit S, Sullivan PF, O’Donovan MC, Daly MJ, Gejman PV (2011) Genome-wide association study identifies five new schizophrenia loci. Nat Genet 43:969–976
Roehrs T, Roth T (2001a) Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev 5:287–297
Roehrs T, Roth T (2001b) Sleep, sleepiness, and alcohol use. Alcohol Res Health 25:101–109
Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T (2006) Sleep loss and REM sleep loss are hyperalgesic. Sleep 29:145–151
Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA Jr, McClung CA (2007) Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA 104:6406–6411
Sams-Dodd F (1996) Phencyclidine-induced stereotyped behaviour and social isolation in rats: a possible animal model of schizophrenia. Behav Pharmacol 7:3–23
Sanderson DJ, Bannerman DM (2010) The role of habituation in hippocampus-dependent spatial working memory tasks: Evidence from GluA1 AMPA receptor subunit knockout mice. Hippocampus 22:981–994
Savelyev SA, Larsson KC, Johansson AS, Lundkvist GB (2011) Slice preparation, organotypic tissue culturing and luciferase recording of clock gene activity in the suprachiasmatic nucleus. J Vis Exp 48:2439
Sawamura N, Ando T, Maruyama Y, Fujimuro M, Mochizuki H, Honjo K, Shimoda M, Toda H, Sawamura-Yamamoto T, Makuch LA, Hayashi A, Ishizuka K, Cascella NG, Kamiya A, Ishida N, Tomoda T, Hai T, Furukubo-Tokunaga K, Sawa A (2008) Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol Psychiatry 13(1138–1148):1069
Scott JP, McNaughton LR, Polman RC (2006) Effects of sleep deprivation and exercise on cognitive, motor performance and mood. Physiol Behav 87:396–408
Scott LD, Hwang WT, Rogers AE, Nysse T, Dean GE, Dinges DF (2007) The relationship between nurse work schedules, sleep duration, and drowsy driving. Sleep 30:1801–1807
Selvi Y, Gulec M, Agargun MY, Besiroglu L (2007) Mood changes after sleep deprivation in morningness–eveningness chronotypes in healthy individuals. J Sleep Res 16:241–244
Sharif A, Duhem-Tonnelle V, Allet C, Baroncini M, Loyens A, Kerr-Conte J, Collier F, Blond S, Ojeda SR, Junier MP, Prevot V (2009) Differential erbB signaling in astrocytes from the cerebral cortex and the hypothalamus of the human brain. Glia 57:362–379
Sharma V, Mazmanian D (2003) Sleep loss and postpartum psychosis. Bipolar Disord 5:98–105
Shimazoe T, Morita M, Ogiwara S, Kojiya T, Goto J, Kamakura M, Moriya T, Shinohara K, Takiguchi S, Kono A, Miyasaka K, Funakoshi A, Ikeda M (2008) Cholecystokinin-A receptors regulate photic input pathways to the circadian clock. FASEB J 22:1479–1490
Snodgrass-Belt P, Gilbert JL, Davis FC (2005) Central administration of transforming growth factor-alpha and neuregulin-1 suppress active behaviors and cause weight loss in hamsters. Brain Res 1038:171–182
Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K (2002) Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 71:877–892
Stickgold R (2005) Sleep-dependent memory consolidation. Nature 437:1272–1278
Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL (2008) Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 199:331–388
Tachikawa H, Harada S, Kawanishi Y, Okubo T, Shiraishi H (2000) Novel polymorphisms of the human cholecystokinin A receptor gene: an association analysis with schizophrenia. Am J Med Genet 96:141–145
Tachikawa H, Harada S, Kawanishi Y, Okubo T, Suzuki T (2001) Linked polymorphisms (-333G>T and -286A>G) in the promoter region of the CCK-A receptor gene may be associated with schizophrenia. Psychiatry Res 103:147–155
Tobler I (1995) Is sleep fundamentally different between mammalian species? Behav Brain Res 69:35–41
Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH (2001) An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291:1040–1043
Toirac I, Sanjuan J, Aguilar EJ, Gonzalez JC, Artigas F, Rivero O, Najera C, Molto MD, de Frutos R (2007) Association between CCK-AR gene and schizophrenia with auditory hallucinations. Psychiatr Genet 17:47–53
Tomppo L, Hennah W, Lahermo P, Loukola A, Tuulio-Henriksson A, Suvisaari J, Partonen T, Ekelund J, Lonnqvist J, Peltonen L (2009) Association between genes of disrupted in schizophrenia 1 (DISC1) interactors and schizophrenia supports the role of the DISC1 pathway in the etiology of major mental illnesses. Biol Psychiatry 65:1055–1062
Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, Takahashi S, Goto K, Sakurai T (2005) Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci 25:7459–7469
Tunbridge EM, Weinberger DR, Harrison PJ (2006) A novel protein isoform of catechol O-methyltransferase (COMT): brain expression analysis in schizophrenia and bipolar disorder and effect of Val158Met genotype. Mol Psychiatry 11:116–117
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J (2005) Obesity and metabolic syndrome in circadian clock mutant mice. Science 308:1043–1045
Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, Makarov V, Yoon S, Bhandar A, Corominas R, Iakoucheva LM, Krastoshevsky O, Krause V, Larach-Walters V, Welsh DK, Craig D, Kelsoe JR, Gershon ES, Leal SM, DellAquila M, Morris DW, Gill M, Corvin A, Insel PA, McClellan J, King MC, Karayiorgou M, Levy DL, DeLisi LE, Sebat J (2011) Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature 471:499–503
Van Dongen HP, Maislin G, Mullington JM, Dinges DF (2003) The cumulative cost of additional wakefulness: dose–response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26:117–126
Venkatraman V, Chuah YM, Huettel SA, Chee MW (2007) Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep 30:603–609
Verrall L, Burnet PW, Betts JF, Harrison PJ (2010) The neurobiology of d-amino acid oxidase and its involvement in schizophrenia. Mol Psychiatry 15:122–137
Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS (1994) Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science 264:719–725
Wei J, Hemmings GP (1999) The CCK-A receptor gene possibly associated with auditory hallucinations in schizophrenia. Eur Psychiatry 14:67–70
Weinberger DR, DeLisi LE, Perman GP, Targum S, Wyatt RJ (1982) Computed tomography in schizophreniform disorder and other acute psychiatric disorders. Arch Gen Psychiatry 39:778–783
Weinberger DR, Harrison PJ (2011) Schizophrenia, Vol. Wiley-Blackwell, Chichester; Hoboken, NJ
Williams NM, O’Donovan MC, Owen MJ (2005) Is the dysbindin gene (DTNBP1) a susceptibility gene for schizophrenia? Schizophr Bull 31:800–805
Wulff K, Joyce E, Middleton B, Dijk DJ, Foster RG (2006) The suitability of actigraphy, diary data, and urinary melatonin profiles for quantitative assessment of sleep disturbances in schizophrenia: a case report. Chronobiol Int 23:485–495
Wulff K, Porcheret K, Cussans E, Foster RG (2009) Sleep and circadian rhythm disturbances: multiple genes and multiple phenotypes. Curr Opin Genet Dev 19:237–246
Wulff K, Gatti S, Wettstein JG, Foster RG (2010) Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci 11:589–599
Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM (2012) Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry 200:308–316
Wulff K, Joyce E (2011) Circadian rhythms and cognition in schizophrenia. Br J Psychiatry 198:250–252
Yamashita H, Mori K, Nagao M, Okamoto Y, Morinobu S, Yamawaki S (2004) Effects of changing from typical to atypical antipsychotic drugs on subjective sleep quality in patients with schizophrenia in a Japanese population. J Clin Psychiatry 65:1525–1530
Yang C, Winkelman JW (2006) Clinical significance of sleep EEG abnormalities in chronic schizophrenia. Schizophr Res 82:251–260
Yin L, Wang J, Klein PS, Lazar MA (2006) Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science 311:1002–1005
Young ME, Bray MS (2007) Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med 8:656–667
Zachrisson O, de Belleroche J, Wendt KR, Hirsch S, Lindefors N (1999) Cholecystokinin CCK(B) receptor mRNA isoforms: expression in schizophrenic brains. Neuroreport 10:3265–3268
Zhang HT, Zhao Y, Huang Y, Dorairaj NR, Chandler LJ, O’Donnell JM (2004) Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology 29:1432–1439
Zhang HT, Zhao Y, Huang Y, Deng C, Hopper AT, De Vivo M, Rose GM, O’Donnell JM (2006) Antidepressant-like effects of PDE4 inhibitors mediated by the high-affinity rolipram binding state (HARBS) of the phosphodiesterase-4 enzyme (PDE4) in rats. Psychopharmacology (Berl) 186:209–217
Zheng X, Sehgal A (2010) AKT and TOR signaling set the pace of the circadian pacemaker. Curr Biol 20:1203–1208
Acknowledgments
RGF, SNP and DMB are funded by the Wellcome Trust. RGF and KW are funded by the Oxford Biomedical Research Centre. DP holds a University of Oxford Christopher Welch Scholarship. We would like to take the opportunity to thank Guy Goodwin, Daniel Freeman and Emily Holmes for their contribution to this work.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pritchett, D., Wulff, K., Oliver, P.L. et al. Evaluating the links between schizophrenia and sleep and circadian rhythm disruption. J Neural Transm 119, 1061–1075 (2012). https://doi.org/10.1007/s00702-012-0817-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-012-0817-8