Abstract
Both clinical and preclinical studies demonstrate the antidepressant activity of the functional NMDA receptor antagonists. In this study, we assessed the effects of two glycine/NMDA receptor ligands, namely L-701,324 (antagonist) and d-cycloserine (a partial agonist) on the action of antidepressant drugs with different pharmacological profiles in the forced swim test in mice. Swim sessions were conducted by placing mice individually in glass cylinders filled with warmed water for 6 min. The duration of behavioral immobility during the last 4 min of the test was evaluated. The locomotor activity of mice was measured with photoresistor actimeters. L-701,324 and d-cycloserine given with reboxetine (administered in subeffective doses) did not change the behavior of animals in the forced swim test. A potentiating effect was seen when both tested glycine site ligands were given concomitantly with imipramine or fluoxetine in this test. The lesion of noradrenaline nerve terminals produced by DSP-4 neither altered the baseline activity nor influenced the antidepressant-like action of L-701,324 or d-cycloserine. The depletion of serotonin by p-CPA did not alter baseline activity in the forced swim test. However, it completely antagonized the antidepressant-like action produced by L-701,324 and d-cycloserine. Moreover, the antidepressant-like effects of imipramine, fluoxetine and reboxetine were abolished by d-serine, a full agonist of glycine/NMDA receptors. The present study demonstrates that glycine/NMDA receptor functional antagonists enhance the antidepressant-like action of serotonin, but not noradrenaline-based antidepressants and such their activity seems to depend on serotonin rather than noradrenaline pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a chronic recurring illness that affects more than 120 million people worldwide. In industrialized societies, approximately five percent of the population experienced a major depressive episode. It is considered that symptoms of depression result from perturbations in monoaminergic neurotransmission. Thus, drugs currently used for treatment of depression include medications that modulate monoaminergic neurotransmission, primarily serotonin and noradrenaline pathways. Unfortunately, their efficacies are unsatisfactory, they produce multiple unwanted side effects and the mechanism of their antidepressant action remains not entirely elucidated (Hollister and Csernansky 1990). Moreover, these drugs require at least 2–4 weeks to produce a clinically meaningful improvement in depressive symptomatology (Rosenzweig-Lipson et al. 2007); therefore, novel therapeutic strategies are sought.
Over the past 20 years, numerous data demonstrated that N-methyl-d-aspartate (NMDA) receptors may be involved in the mechanism of action of antidepressant treatment (Skolnick et al. 1996; Skolnick 1999; Skolnick et al. 2009). In addition, significantly higher serum and plasma levels of glutamate in patients with depression than those of healthy controls were reported (Kim et al. 1982; Altamura et al. 1993; Mauri et al. 1998; Nowak et al. 2003; Mitani et al. 2006; Siwek et al. 2009). Moreover, increased levels of glutamate in the frontal cortex were demonstrated, implicating abnormality of glutamatergic neurotransmission in the pathophysiological features of major depressive disorder (Sanacora et al. 2004).
Extensive studies have shown that competitive and noncompetitive NMDA receptor antagonists, polyamine site antagonists and inorganic inhibitors of NMDA receptor function, zinc and magnesium, produced antidepressant-like activity in preclinical antidepressant screening procedures (Trullas and Skolnick 1990; Maj et al. 1992a, b; Moryl et al. 1993; Maj et al. 1994; Layer et al. 1995; Kroczka et al. 2000, 2001; Nowak et al. 2003; Poleszak et al. 2004; Nowak et al. 2006; Szewczyk et al. 2008; Siwek et al. 2009). Furthermore, an antagonist of the NMDA receptor complex, ketamine, and memantine are effective in human depression (Berman et al. 2000; Zarate et al. 2006a, b). Moreover, CP-101, 606 (a selective antagonist of NR2B subunit of the NMDA receptor) and zinc (non specific antagonist of NMDA receptor) enhance efficacy of antidepressant therapy in major depression (especially treatment resistant subtype) (Nowak et al. 2003; Preskorn et al. 2008; Siwek et al. 2009). However, it should be mentioned that there is a discrepancy between long-term effects of ketamine in clinical observations and experimental paradigms used to evaluate antidepressant activity (e.g., Maeng et al. 2008; Popik et al. 2008; Bechtholt-Gompf et al. 2011).
Unfortunately, both competitive and noncompetitive NMDA antagonists induce severe side effects (Willetts et al. 1990) that limit their applicability as antidepressants for clinical use in humans. Thus, an alternative strategy to the use of competitive and noncompetitive NMDA antagonist was modulation of the glycine co-agonist site at the NMDA receptor (Kemp and Leeson 1993).
Numerous behavioral studies have shown that antagonists and partial agonists at the glycine site exhibit antidepressant-like activity in experimental screening procedures (Trullas and Skolnick 1990; Przegaliński et al. 1997; Vamvakides 1998). Consequently, in this study, we assessed the effects of two glycine B receptor ligands, namely L-701,324 (antagonist) and d-cycloserine (a partial agonist) on the action of antidepressant drugs with different pharmacological profiles in the forced swim test in mice.
Materials and methods
Animals
The experiments were carried out on adult male Albino Swiss mice (25–30 g) purchased from the licensed breeder (Kołacz, Warsaw, Poland). The animals were kept in cages (up to 10 per cage) on a natural day–night cycle with free access to food and water and they were used after 7 days of acclimatization to laboratory conditions. Each experimental group consisted of 8–12 animals. The experimental protocol was approved by the Local Ethics Committee at the Medical University of Lublin (license number 31/2007), and all the procedures were in strict compliance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Drug administration
L-701,324 [7-chloro-4-hydroxy-3-(3-phenoxy)phenylquinolin-2(1H)-one, Sigma] was suspended in a 1% aqueous solution of Tween 80 and administered intraperitoneally (i.p.) 60 min before the tests. d-cycloserine (DCS, d-4-amino-3-isoxazolidone, Sigma) and antidepressant drugs: imipramine (IMI, Sigma), fluoxetine (FX, kindly provided by Polpharma, Starogard Gdański, Poland) and reboxetine (RB, Sigma) were dissolved in 0.9% saline freshly before use and administered 60 min before the tests. Control animals received an i.p. injection of saline (vehicle). d-serine (Sigma, USA) was administered intracerebroventricularly (i.c.v.) 15 min before the test. The i.c.v. administration was performed according to a modified method described by Lipman and Spencer (1980). Briefly, a 10-μl glass Hamilton microsyringe (type 701) was used. The needle (26 G) was shortened to a length of 7 mm, sharpened and polished to a fine tip. Rigid PVC tubing was put on the needle to limit its penetration to 3 mm. The injection site was approximately 2 mm posterior to an imaginary line intersecting the posterior extent of the orbits of the eyes and 1 mm lateral to the midline. The control animals received an i.p. and i.c.v. injections of saline (vehicle). The volume of vehicles or drug solutions for i.p. and i.c.v. administrations was 10 ml/kg and 5 μl per mouse, respectively. Drug doses and pretreatment schedules were taken from the literature and were confirmed in our previous experiments (Poleszak et al. 2005b; Poleszak 2007; Poleszak et al. 2007b; Szewczyk et al. 2010).
Serotonergic depletion
In a separate series of experiments, in order to investigate the possible contribution of serotonergic system to the effect of DCS or L-701,324 in the FST, mice were treated with p-chlorophenylalanine (p-CPA, Sigma). p-CPA is known to be an inhibitor of serotonin biosynthesis and depletes serotonin from the brain and other tissues by the selective inhibition of tryptophane hydroxylase (Koe and Weissman 1966; Poleszak 2007). In the present experiments, the mice were injected i.p. either with saline or with p-CPA. p-CPA was dissolved in 0.9% saline and administered at a dose of 200 mg/kg once daily for 3 consecutive days. On the fourth day, the mice received DCS, L-701,324 or saline injections 60 min before testing.
Noradrenergic lesion
In a separate series of experiments, in order to investigate the possible contribution of noradrenergic system to the effect DCS or L-701,324 in the FST, mice were pretreated with N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4, Sigma)—a selective noradrenergic neurotoxin (Matsumoto et al. 1995). In the present experiments, the mice were pretreated i.p. with saline or with DSP-4. DSP-4 was dissolved in 0.9% saline and administered at a dose of 50 mg/kg 4 days prior to the test. On the day of testing, the mice received DCS, L-701,324 or saline injections 60 min before testing.
Forced swim test (FST)
The studies were carried out on mice according to the method of Porsolt et al. (1977). Mice were placed individually into glass cylinders (height 25 cm, diameter 10 cm) containing 10 cm of water, maintained at 23–25°C. The animals were left in the cylinder for 6 min. After the first 2 min, the total duration of immobility was measured during a 4-min test with a summing stopwatch. The mouse was judged to be immobile when it remained floating passively in the water, performing slow motion to keep head above the water.
Locomotor activity
The actimeter consists of a cylinder (30 cm diameter, 12 cm high, MultiServ, Lublin, Poland) equipped with two perpendicular infrared light beams located 1.5 cm above the floor. Mice were i.p. pretreated with respective drugs combinations and after a given time period they were placed in the actimeter and locomotor activity (number of interruptions of light beams) was recorded for a period of 10 min after placing the mouse into the actimeter.
Statistical analysis
All experimental results are presented as the mean ± SEM. Comparisons between control and experimental groups were performed by analysis of variance (ANOVA) followed by Student-Neuman-Keuls post hoc test, where appropriate. P < 0.05 was considered as a statistically significant difference.
Results
Forced swim test
Effect of joint administration of L-701,324 and IMI in the FST
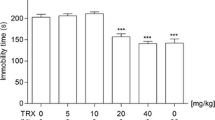
The effects of a combined administration of L-701,324 and IMI on total duration of immobility in mice are shown in Fig. 1a [ANOVA: F(3,30) = 6.670, p = 0.0014]. L-701,324 at a dose of 1 mg/kg had no effect on the immobility time in mice. IMI given alone at a dose of 15 mg/kg was also ineffective. L-701,324 (1 mg/kg) injected in combination with IMI (15 mg/kg) significantly reduced the immobility time in mice.
Effect of joint administration of L-701,324 and a imipramine (IMI), b fluoxetine (FX) or c reboxetine (RB) on the total duration of immobility in the forced swim test in mice. L-701,324 and antidepressant drugs were administered i.p. 60 min before the test. The values represent means ± SEM of 8–9 mice. **p < 0.01, ***p < 0.001 versus control (vehicle-treated animals) (Student–Newman–Keuls post hoc test)
Effect of joint administration of L-701,324 and FX in the FST
The effects of a combined administration of L-701,324 and FX on total duration of immobility in mice are shown in Fig. 1b [ANOVA: F(3,31) = 11.59, p < 0.0001]. L-701,324 at a dose of 1 mg/kg had no effect on the immobility time in mice. FX given alone at a dose of 5 mg/kg was also ineffective. L-701,324 (1 mg/kg) injected in combination with FX (5 mg/kg) significantly reduced the immobility time in mice.
Effect of joint administration of L-701,324 and RB in the FST
The effects of combined administration of L-701,324 and RB on total duration of immobility in mice are shown in Fig. 1c [ANOVA: F(3,29) = 0.2083, p = 0.8898]. L-701,324 at a dose of 1 mg/kg had no effect on the immobility time in mice. RB given alone at a dose of 2.5 mg/kg was also ineffective. L-701,324 (1 mg/kg) injected in combination with RB (2.5 mg/kg) was ineffective in this test.
Effect of pretreatment with p-CPA on the antidepressant-like activity of L-701,324 in the FST
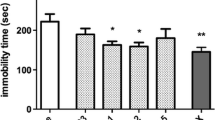
The effects of L-701,324 and p-CPA on the behavior in the FST are shown in Fig. 2a [ANOVA: F(3,33) = 7.629, p = 0.0005]. In controls, L-701,324 (4 mg/kg) significantly reduced the immobility time in comparison with saline. The administration of p-CPA (200 mg/kg i.p. once a day for 3 consecutive days) did not alter baseline behavior during the FST. However, depletion of serotonin completely blocked the decrease of the immobility time elicited by L-701,324.
The effects of pre-treatment of animals with p-CPA and DSP-4 on the antidepressant-like effects of a–b L-701,324 and c–d d-cycloserine (DCS) in the forced swim test in mice. p-CPA (200 mg/kg, i.p.) was administered once daily for 3 consecutive days. DSP-4 was administered at a dose of 50 mg/kg 4 days prior to the test. The values represent means ± SEM of 9–10 mice. **p < 0.01, ***p < 0.001 versus control (vehicle-treated animals); ## p < 0.01, ### p < 0.001 versus, respective glycine/NMDA ligand given alone (Student–Newman–Keuls post hoc test)
Effect of pretreatment with DSP-4 on the antidepressant-like activity of L-701,324 in the FST
The effects of L-701,324 and DSP-4 on the behavior in the FST are shown in Fig. 2b [ANOVA: F(3,35) = 7.833, p = 0.0004]. In controls, L-701,324 (4 mg/kg) significantly reduced the immobility time in comparison with saline. The administration of DSP-4 (50 mg/kg i.p.) did not alter baseline behavior during the FST and did not change the reduction of the immobility time elicited by L-701,324.
Effect of joint administration of DCS and IMI in the FST
The effects of a combined administration of DCS and IMI on total duration of immobility in mice are shown in Fig. 3a [ANOVA: F(3,35) = 5.016, p = 0.0054]. DCS at a dose of 2.5 mg/kg had no effect on the immobility time in mice. IMI given alone at the dose of 15 mg/kg was also ineffective. DCS (2.5 mg/kg) injected in combination with IMI (15 mg/kg) significantly reduced the immobility time in mice.
Effect of joint administration of d-cycloserine (DCS) and a imipramine (IMI), b fluoxetine (FX) or c reboxetine (RB) on the total duration of immobility in the forced swim test in mice. DCS and antidepressant drugs were administered i.p. 60 min before the test. The values represent means ± SEM of 9–11 mice. **p < 0.01 versus control (vehicle-treated animals) (Student–Newman–Keuls post hoc test)
Effect of joint administration of DCS and FX in the FST
The effects of a combined administration of DCS and FX on total duration of immobility in mice are shown in Fig. 3b [ANOVA: F(3,35) = 5.016, p = 0.0054]. DCS at a dose of 2.5 mg/kg had no effect on the immobility time in mice. FX given alone at a dose of 5 mg/kg was also ineffective. DCS (2.5 mg/kg) injected in combination with FX (5 mg/kg) significantly reduced the immobility time in mice.
Effect of joint administration of DCS and RB in the FST
The effects of a combined administration of DCS and RB on total duration of immobility in mice are shown in Fig. 3c [ANOVA: F(3,34) = 0.7426, p = 0.5341]. DCS at a dose of 2.5 mg/kg had no effect on the immobility time in mice. RB given alone at a dose of 2.5 mg/kg was also ineffective. DCS (2.5 mg/kg) injected in combination with RB (2.5 mg/kg) was ineffective in this test.
Effect of pretreatment with p-CPA on the antidepressant-like activity of DCS in the FST
The effects of DCS and p-CPA on the behavior in the FST are shown in Fig. 2c [ANOVA: F(3,33) = 10.47, p < 0.0001]. In control animals, DCS (5 mg/kg) significantly reduced the immobility time in comparison with saline. The administration of p-CPA (200 mg/kg i.p. once a day for 3 consecutive days) did not alter baseline behavior during the FST. However, depletion of serotonin completely blocked the decrease of immobility time elicited by DCS.
Effect of pretreatment with DSP-4 on the antidepressant-like activity of DCS in the FST
The effects of DCS and DSP-4 on the behavior in the FST are shown in Fig. 2d [ANOVA: F(3,28) = 6.11, p < 0.0025]. DCS at a dose of 5 mg/kg significantly reduced the immobility time in comparison with saline. The administration of DSP-4 (50 mg/kg i.p. once a day for 3 consecutive days) did not alter baseline behavior during the FST and did not change the reduction of immobility time elicited by DCS.
Effect of joint administration of d-serine and IMI in the FST
The effects of a combined administration of IMI and d-serine (the glycine B receptor agonist) on the total duration of immobility in mice are shown in Fig. 4a [ANOVA: F(3,33) = 12.74, p < 0.0001]. IMI at a dose of 30 mg/kg significantly reduced the immobility time in mice. d-serine alone, given i.c.v. at a dose of 100 nmol/mouse, had no effect on the immobility time. However, when combined with IMI, it abolished the IMI-induced antidepressant-like effect.
Effect of joint administration of d-serine (DS) and a imipramine (IMI), b fluoxetine (FX) or c reboxetine (RB) on the total duration of immobility in the forced swim test in mice. DS was administered intracerebroventricularly (i.c.v.) 15 min before the test and antidepressant drugs were administered i.p. 45 min before DS. The values represent means ± SEM of 9–12 mice. *p < 0.05, ***p < 0.001 versus control (vehicle-treated animals); # p < 0.05, ## p < 0.01 versus respective antidepressant drug given alone (Student–Newman–Keuls post hoc test)
Effect of joint administration of d-serine and FX in the FST
The effects of a combined administration of FX and d-serine on total duration of immobility in mice are shown in Fig. 4b [ANOVA: F(3,35) = 20.74, p < 0.0001]. FX at a dose of 10 mg/kg significantly reduced the immobility time in mice. d-serine given alone at a dose of 100 nmol/mouse i.c.v. had no effect on the immobility time; however, when combined with FX, it abolished the FX-induced antidepressant-like effect.
Effect of joint administration of d-serine and RB in the FST
The effects of a combined administration of RB and d-serine on total duration of immobility in mice are shown in Fig. 4c [ANOVA: F(3,36) = 3.777, p = 0.0187]. RB at a dose of 5 mg/kg significantly reduced the immobility time in mice. d-serine given alone at a dose of 100 nmol/mouse i.c.v. had no effect on the immobility time; however, when combined with RB, it abolished the RB-induced antidepressant-like effect.
Locomotor activity
Effect of combined treatment with L-701,324 and antidepressant drugs (imipramine—IMI, reboxetine—RB or fluoxetine—FX) on spontaneous locomotor activity in mice
Results presented in Table 1A of the effects of L-701,324, IMI and RB on a locomotor activity were analyzed by 3-way ANOVA repeated measures (between-subjects factors of antidepressant, L-701,324 and repeated factor of time). The administration of antidepressants did not affect activity: F(2,48) = 1.98, NS; however, a co-treatment with L-701,324 reduced activity: F(1,48) = 4.51, p < 0.05. There was also a significant effect of time: F(1,48) = 32.99, p < 0.0001. Since there were no significant effects of any interaction, the post hoc tests were not performed.
Results presented in Table 1B were analyzed by 2-way repeated measures ANOVA (drug as a between groups factor and time as a repeated measures factor) showed a significant effect of time: F(1,35) = 139.1, p < 0.0001. Administration of neither FX nor L-701,324 affected the activity. However, there were significant effects of interactions between FX treatment and L-701,324 treatment: F(1,35) = 5.35, p < 0.05 as well as between L-701,324 treatment and time: F(1,35) = 5.46, p < 0.05. These were mostly due to a decrease in activity of L-701,324-treated mice versus controls in the 10th min of activity measurements (p < 0.05, Newman-Keuls post hoc test).
Effect of d-cycloserine (DCS) and L-701,324 on spontaneous locomotor activity in DSP-4 and p-CPA-lesioned mice
Results from Table 2A (analyzed by 2-way ANOVA repeated measures) revealed significant effects of DSP-4 treatment: F(1,40) = 4.2, p < 0.05 and time: F(1,40) = 228.9, p < 0.0001. There was also a significant interaction between DCS treatment and time: F(1,40) = 6.33, p < 0.05, mostly due to the fact that in the controls, DCS reduced activity in the 5th min as compared with the vehicle treatment in the 10th minute, while such an effect was not observed in DSP-4-treated mice.
However, the two-way ANOVA repeated measures of the data presented in the Table 2B and C demonstrated only a significant effect of time: F(1,38) = 92, p < 0.0001 and F(1,54) = 137, p < 0.0001, respectively.
Effect of combined treatment with d-cycloserine (DCS) and antidepressant drugs (imipramine—IMI, Fluoxetine—FX, reboxetine—RB) on spontaneous locomotor activity in mice
Results presented in Table 3A analyzed by 2-way ANOVA repeated measures showed a significant effect of time: F(1,36) = 231, p < 0.0001 and a significant interaction between time, DCS and IMI treatments: F(1,36) = 8.23, p < 0.01. It appeared that at the 5th min, DCS reduced the activity independently on IMI-co-administration, but at the 10th min of the test, DCS increased it in the controls but decreased it in IMI-co-treated animals. However, the post hoc test did not reveal any changes in activity as compared with vehicle-vehicle controls.
Results presented in Table 3B and C analyzed by analyzed by 2-way ANOVA repeated measures showed that only the time factor was significant: F(1,35) = 257, p < 0.001 and F(1,36) = 331, p < 0.0001, respectively.
Effect of combined treatment with d-serine (DS) and antidepressant drugs (imipramine—IMI, fluoxetine—FX, reboxetine—RB) on spontaneous locomotor activity in mice
Results presented in Table 4A analyzed by 3-way ANOVA repeated measures showed a significant effect of time: F(1,48) = 113.6, p < 0.0001 and of DS treatment: F(1,48) = 10.38, p < 0.01. The latter effects could be attributed to a decrease in activity in DS-treated animals independently of the time and the antidepressant treatment.
Results presented in Table 4B analyzed by 2-way ANOVA repeated measures showed significant effects of time: F(1,34) = 126, p < 0.0001 and of the interaction between time and DS treatment: F(1,34) = 9.28, p < 0.01. The latter effect could be explained by a significant decrease in activity in the 10th min in DS + RB group as compared with RB treated group. However, the post hoc test did not reveal any changes in activity as compared with vehicle-treated controls.
Discussion
A growing amount of data supports the role of the excitatory glutamatergic neurotransmission in the pathophysiology of depressive disorders. In the clinical studies, disturbances of the glutamate level in depressed patients were found (Mathis et al. 1988; Altamura et al. 1993; Levine et al. 2000). The brain NMDA receptor abnormalities in human suicide victims (Nowak et al. 1995) and major depressives (Law and Deakin 2001; Karolewicz et al. 2005; Feyissa et al. 2009; Karolewicz et al. 2009) were observed. Moreover, a noncompetitive NMDA antagonist, ketamine, produced rapid and sustained antidepressant effects in depressed patients (Berman et al. 2000; Zarate et al. 2006a; Phelps et al. 2009; Price et al. 2009), as well as reports that riluzole, which inhibits glutamate release, is effective in patients with bipolar depression (Zarate et al. 2004, 2005). Recently, the antidepressant effect of NR2B subunit antagonist, traxoprodil (CP-101, 606) was demonstrated in patients unresponsive to a serotonin selective reuptake inhibitor (Kemp and McKernan 2002; Preskorn et al. 2008).
Numerous reports from preclinical studies have documented the antidepressant-like activity of structurally diverse NMDA receptor antagonists in animal behavioral paradigms. Thus, NMDA channel blockers (dizocilpine), a competitive antagonist of NMDA receptor (AP-7, CGP 37848), a polyamine site antagonist (eliprodil), divalent-cation antagonists (Mg2+, Zn2+) and a glycine site antagonist (L-701,324), and partial agonist (DCS) were all active in the FST (Skolnick et al. 1989; Trullas and Skolnick 1990; Kroczka et al. 2001; Paul and Skolnick 2003; Poleszak et al. 2004, 2005a). Additionally, the glycine partial agonist 1-aminocyclopropanecarboxylic acid (ACPC) produced a more rapid onset of action in the chronic mild stress model than it was typically observed for biogenic-amine-based agents (Papp and Moryl 1996).
The glycine binding site is the regulatory domain of the NMDA receptor complex with affinity for the endogenous ligands, glycine (or d-serine, another endogenous agonist of the glycine site) (Wood et al. 1989, 1996). Activation of this site by glycine or d-serine is absolutely required for NMDA receptor activation by l-glutamate (Kemp and Leeson 1993). It was proved that glycine receptor antagonists and partial agonists have favorable safety profile compared with competitive and noncompetitive NMDA receptor antagonists (Hawkinson et al. 1997; Danysz and Parsons 1998; Bordi et al. 1999; Beardsley et al. 2002) making them a potential candidates for new antidepressant drugs (Kemp and Leeson 1993; Danysz and Parsons 1998).
In the experimental screening procedures, both full glycine antagonist (L-701,324) and partial agonists at the glycine site (ACPC, DCS) exhibit activity comparable to that of clinically used antidepressants (Trullas and Skolnick 1990; Przegaliński et al. 1997; Vamvakides 1998). In mice the effective dose of L-701,324 in this test was 2 mg/kg (Przegaliński et al. 1998), which was confirmed in our previous study (Poleszak et al. 2007a). DCS, a partial agonist of glycine site of the NMDA receptor complex (Hood et al. 1989) was also active in the FST (an effect comparable to that observed with IMI (Vamvakides 1998). Its antidepressant-like activity was first shown in tuberculosis patients (Kendig et al. 1956; Lewis et al. 1957) and provides indirect support for the hypothesis that glycine site partial agonists may be potentially useful as antidepressants (Trullas and Skolnick 1990; Skolnick et al. 1992). As partial agonist DCS may act as agonist or antagonist of the glycine B receptor depending on the dose, that is, a lower dose acts as agonist but higher doses act as antagonist of glycine binding site of the NMDA receptor (Hood et al. 1989; Watson et al. 1990; Emmett et al. 1991). In our previous study, the effective dose of DCS in the FST was the dose 5 mg/kg, while the lower dose (2.5 mg/kg) was not effective itself, but potentiated the antidepressant effects of magnesium or zinc (Poleszak et al. 2007b; Szewczyk et al. 2010).
In the present study, we have demonstrated the effects of glycine site ligands (L-701,324 and DCS) on the action of antidepressants with different pharmacological profiles: IMI—a nonselective serotonin/noradrenaline reuptake inhibitor (Westenberg 1999), FX—a selective serotonin reuptake inhibitor (SSRI) (Rickels and Schweizer 1990) and RB—a potent, selective, and specific noradrenergic reuptake inhibitor (NARI) (Versiani et al. 1999). L-701,324 and DCS given with RB (administered in subeffective doses) did not change the behavior of animals in the forced swim test, the behavioral despair procedure commonly used to detect antidepressant agents (Borsini and Meli 1988). However, a potentiating effect was seen when both tested glycine site ligands were given jointly with IMI or FX in this test. This apparent potentiation was manifested as a reduction of the immobility time, but no increase in locomotor activity was evidenced. Similarly, such interaction was demonstrated between ionic NMDA antagonists zinc and magnesium with antidepressants in the FST (Poleszak 2007; Szewczyk et al. 2009).
In the present study, in order to confirm a possible contribution of the serotonergic system to the antidepressive effect of DCS and L-701,324, mice were pretreated with p-CPA, an inhibitor of serotonin synthesis or with DSP-4, a selective noradrenergic neurotoxin, and examined in the FST. The lesion of noradrenaline nerve terminals produced by DSP-4 did no alter the baseline activity or influence antidepressant-like action caused by L-701,324 or DCS. The depletion of serotonin by p-CPA did not alter baseline activity in the FST; however, completely antagonized the antidepressant-like action caused by L-701,324 and DCS. Thus, we proved that interaction with serotonergic, but not noradrenergic system is necessary for antidepressant-like activity of glycine/NMDA site ligands.
Our recent study has demonstrated that the activation of the NMDA receptor complex by d-serine blocks the antidepressant-like effect of an antagonist of the glycine/NMDA receptor L-701,324 and ionic antagonists, such as zinc and magnesium, in the FST (Poleszak et al. 2007a, b, 2008). Now we show that the antidepressant-like effects of IMI, FX and RB were abolished by d-serine pretreatment. Thus, these data indicate that antidepressant activity of both serotonin and noradrenaline-based antidepressant are related to the mechanism connected with a reduction of the activity of the NMDA receptor complex (particularly glycine/NMDA sites). Contrary to this notion, Boulay et al. (2008) demonstrated the antidepressant-like action of glycine transporter type 1 inhibitor SSR103800 which may indicate that activation of glycine/NMDA receptor is related to an antidepressant activity. However, the glycine/NMDA receptor dependence of the antidepressant-like action of SSR103800 in the FST was not demonstrated (e.g., antagonism by glycine/NMDA antagonists). The discrepancy between present data and that of Boulay et al. (2008) may also be due to the use of different species (mice vs. rats, gerbils). The interaction between NMDA receptor and serotonergic pathway is more obvious than NMDA receptor and noradrenergic one (Yan et al. 1997). There is emerging evidence that the interaction between excitatory amino acids and serotonin may be important for the control of many brain activities. Both NMDA and non-NMDA receptor antagonists have been found to release serotonin and to increase its turnover in some brain structures (Löscher et al. 1993).
To summarize, the present study demonstrates complex interaction between glycine/NMDA receptor ligands and conventional serotonin/noradrenaline-based antidepressants in the FST. While glycine/NMDA receptor functional antagonists enhance an antidepressant-like action of serotonin, but not noradrenaline-based antidepressants and their such activity depends on serotonin but not noradrenaline pathway, the antidepressant-like activity of both types of antidepressants rely on the dampening of the NMDA receptor complex. This study may provide some guidelines about how to optimally use combinations of the NMDA receptor antagonists and biogenic amine-based antidepressant drugs. The NMDA receptor antagonists as antidepressants may be on the near horizon, and the ability to use lower doses of, e.g., traxoprodil when combined with the appropriate agent may be an interesting approach to antidepressant therapy.
References
Altamura CA, Mauri MC, Ferrara A, Moro AR, D’Andrea G, Zamberlan F (1993) Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry 150:1731–1733
Beardsley PM, Ratti E, Balster RL, Willetts J, Trist D (2002) The selective glycine antagonist gavestinel lacks phencyclidine-like behavioral effects. Behav Pharmacol 13:583–592
Bechtholt-Gompf AJ, Smith KL, John CS, Kang HH, Carlezon WA Jr, Cohen BM, Öngür D (2011) CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology (Berl) (in press) doi:10.1007/s00213-011-2169-8
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354
Bordi F, Terron A, Reggiani A (1999) The neuroprotective glycine receptor antagonist GV150526 does not produce neuronal vacuolization or cognitive deficits in rats. Eur J Pharmacol 378:153–160
Borsini F, Meli A (1988) Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 94:147–160
Boulay D, Pichat P, Dargazanli G, Estenne-Bouhtou G, Terranova JP, Rogacki N, Stemmelin J, Coste A, Lanneau C, Desvignes C, Cohen C, Alonso R, Vige X, Biton B, Steinberg R, Sevrin M, Oury-Donat F, George P, Bergis O, Griebel G, Avenet P, Scatton B (2008) Characterization of SSR103800, a selective inhibitor of the glycine transporter-1 in models predictive of therapeutic activity in schizophrenia. Pharmacol Biochem Behav 91:47–58
Danysz W, Parsons CG (1998) Glycine and N-methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev 50:597–664
Emmett MR, Mick SJ, Cler JA, Rao TS, Iyengar S, Wood PL (1991) Actions of d-cycloserine at the N-methyl-d-aspartate-associated glycine receptor site in vivo. Neuropharmacology 30:1167–1171
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B (2009) Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 33:70–75
Hawkinson JE, Huber KR, Sahota PS, Han HH, Weber E, Whitehouse MJ (1997) The N-methyl-d-aspartate (NMDA) receptor glycine site antagonist ACEA 1021 does not produce pathological changes in rat brain. Brain Res 744:227–234
Hollister LE, Csernansky JG (1990) Clinical pharmacology of psychotherapeutic drugs. Churchill Livingstone, New York
Hood WF, Compton RP, Monahan JB (1989) d-cycloserine: a ligand for the N-methyl-d-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett 98:91–95
Karolewicz B, Stockmeier CA, Ordway GA (2005) Elevated levels of the NR2C subunit of the NMDA receptor in the locus coeruleus in depression. Neuropsychopharmacology 30:1557–1567
Karolewicz B, Szebeni K, Gilmore T, Maciąg D, Stockmeier CA, Ordway GA (2009) Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol 12:143–153
Kemp JA, Leeson PD (1993) The glycine site of the NMDA receptor–five years on. Trends Pharmacol Sci 14:20–25
Kemp JA, McKernan RM (2002) NMDA receptor pathways as drug targets. Nat Neurosci 5(Suppl):1039–1042
Kendig IV, Charen S, Lepine LT (1956) Psychological side effects induced by cycloserine in the treatment of pulmonary tuberculosis. Am Rev Tuberc 73:438–441
Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH (1982) Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr 232:299–304
Koe BK, Weissman A (1966) p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther 154:499–516
Kroczka B, Zięba A, Dudek D, Pilc A, Nowak G (2000) Zinc exhibits an antidepressant-like effect in the forced swimming test in mice. Pol J Pharmacol 52:403–406
Kroczka B, Brański P, Pałucha A, Pilc A, Nowak G (2001) Antidepressant-like properties of zinc in rodent forced swim test. Brain Res Bull 55:297–300
Law AJ, Deakin JF (2001) Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport 12:2971–2974
Layer RT, Popik P, Olds T, Skolnick P (1995) Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715). Pharmacol Biochem Behav 52:621–627
Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW (2000) Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry 47:586–593
Lewis WC, Calden G, Thurston JR, Gilson WE (1957) Psychiatric and neurological reactions to cycloserine in the treatment of tuberculosis. Dis Chest 32:172–182
Lipman JJ, Spencer PS (1980) Rapid intracerebroventricular injection assisted by an automatic syringe. J Pharmacol Methods 4:327–333
Löscher W, Annies R, Hönack D (1993) Comparison of competitive and uncompetitive NMDA receptor antagonists with regard to monoaminergic neuronal activity and behavioural effects in rats. Eur J Pharmacol 242:263–274
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352
Maj J, Rogóż Z, Skuza G, Sowińska H (1992a) Effects of MK-801 and antidepressant drugs in the forced swimming test in rats. Eur Neuropsychopharmacol 2:37–41
Maj J, Rogóż Z, Skuza G, Sowińska H (1992b) The effect of CGP 37849 and CGP 39551, competitive NMDA receptor antagonists, in the forced swimming test. Pol J Pharmacol Pharm 44:337–346
Maj J, Rogóż Z, Skuza G, Kołodziejczyk K (1994) Some central effects of kynurenic acid, 7-chlorokynurenic acid and 5, 7-dichloro-kynurenic acid, glycine site antagonists. Pol J Pharmacol 46:115–124
Mathis P, Schmitt L, Benatia M, Granier F, Ghisolfi J, Moron P (1988) Plasma amino acid disturbances and depression. Encephale 14:77–82
Matsumoto K, Ojima K, Watanabe H (1995) Noradrenergic denervation attenuates desipramine enhancement of aggressive behavior in isolated mice. Pharmacol Biochem Behav 50:481–484
Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, Invernizzi G (1998) Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology 37:124–129
Mitani H, Shirayama Y, Yamada T, Kawahara R (2006) Plasma levels of homovanillic acid, 5-hydroxyindoleacetic acid and cortisol, and serotonin turnover in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 30:531–534
Moryl E, Danysz W, Quack G (1993) Potential antidepressive properties of amantadine, memantine and bifemelane. Pharmacol Toxicol 72:394–397
Nowak G, Ordway GA, Paul IA (1995) Alterations in the N-methyl-d-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res 675:157–164
Nowak G, Siwek M, Dudek D, Zięba A, Pilc A (2003) Effect of zinc supplementation on antidepressant therapy in unipolar depression: a preliminary placebo-controlled study. Pol J Pharmacol 55:1143–1147
Nowak G, Partyka A, Pałucha A, Szewczyk B, Wierońska JM, Dybała M, Metz M, Librowski T, Froestl W, Papp M, Pilc A (2006) Antidepressant-like activity of CGP 36742 and CGP 51176, selective GABAB receptor antagonists, in rodents. Br J Pharmacol 149:581–590
Papp M, Moryl E (1996) Antidepressant-like effects of 1-aminocyclopropanecarboxylic acid and d-cycloserine in an animal model of depression. Eur J Pharmacol 316:145–151
Paul IA, Skolnick P (2003) Glutamate and depression: clinical and preclinical studies. Ann NY Acad Sci 1003:250–272
Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA Jr (2009) Family history of alcohol dependence and initial antidepressant response to an N-methyl-d-aspartate antagonist. Biol Psychiatry 65:181–184
Poleszak E (2007) Modulation of antidepressant-like activity of magnesium by serotonergic system. J Neural Transm 114:1129–1134
Poleszak E, Szewczyk B, Kędzierska E, Wlaź P, Pilc A, Nowak G (2004) Antidepressant- and anxiolytic-like activity of magnesium in mice. Pharmacol Biochem Behav 78:7–12
Poleszak E, Wlaź P, Kędzierska E, Radziwoń-Zaleska M, Pilc A, Fidecka S, Nowak G (2005a) Effects of acute and chronic treatment with magnesium in the forced swim test in rats. Pharmacol Rep 57:654–658
Poleszak E, Wlaź P, Szewczyk B, Kędzierska E, Wyska E, Librowski T, Szymura-Oleksiak J, Fidecka S, Pilc A, Nowak G (2005b) Enhancement of antidepressant-like activity by joint administration of imipramine and magnesium in the forced swim test: behavioral and pharmacokinetic studies in mice. Pharmacol Biochem Behav 81:524–529
Poleszak E, Wlaź P, Kędzierska E, Nieoczym D, Wróbel A, Fidecka S, Pilc A, Nowak G (2007a) NMDA/glutamate mechanism of antidepressant-like action of magnesium in forced swim test in mice. Pharmacol Biochem Behav 88:158–164
Poleszak E, Wlaź P, Wróbel A, Dybała M, Sowa M, Fidecka S, Pilc A, Nowak G (2007b) Activation of the NMDA/glutamate receptor complex antagonizes the NMDA antagonist-induced antidepressant-like effects in the forced swim test. Pharmacol Rep 59:595–600
Poleszak E, Szewczyk B, Wlaź A, Fidecka S, Wlaź P, Pilc A, Nowak G (2008) d-serine, a selective glycine/N-methyl-d-aspartate receptor agonist, antagonizes the antidepressant-like effects of magnesium and zinc in mice. Pharmacol Rep 60:996–1000
Popik P, Kos T, Sowa-Kućma M, Nowak G (2008) Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacology (Berl) 198:421–430
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101, 606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637
Price RB, Nock MK, Charney DS, Mathew SJ (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66:522–526
Przegaliński E, Tatarczyńska E, Dereń-Wesołek A, Chojnacka-Wójcik E (1997) Antidepressant-like effects of a partial agonist at strychnine-insensitive glycine receptors and a competitive NMDA receptor antagonist. Neuropharmacology 36:31–37
Przegaliński E, Tatarczyńska E, Chojnacka-Wójcik E (1998) Anxiolytic- and antidepressant-like effects of an antagonist at glycine B receptors. Pol J Pharmacol 50:349–354
Rickels K, Schweizer E (1990) Clinical overview of serotonin reuptake inhibitors. J Clin Psychiatry 51(Suppl B):9–12
Rosenzweig-Lipson S, Beyer CE, Hughes ZA, Khawaja X, Rajarao SJ, Malberg JE, Rahman Z, Ring RH, Schechter LE (2007) Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther 113:134–153
Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF (2004) Subtype-specific alterations of γ-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 61:705–713
Siwek M, Dudek D, Paul IA, Sowa-Kućma M, Zięba A, Popik P, Pilc A, Nowak G (2009) Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J Affect Disord 118:187–195
Skolnick P (1999) Antidepressants for the new millennium. Eur J Pharmacol 375:31–40
Skolnick P, Marvizón JC, Jackson BW, Monn JA, Rice KC, Lewin AH (1989) Blockade of N-methyl-d-aspartate induced convulsions by 1-aminocyclopropanecarboxylates. Life Sci 45:1647–1655
Skolnick P, Miller R, Young A, Boje K, Trullas R (1992) Chronic treatment with 1-aminocyclopropanecarboxylic acid desensitizes behavioral responses to compounds acting at the N-methyl-d-aspartate receptor complex. Psychopharmacology (Berl) 107:489–496
Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R (1996) Adaptation of N-methyl-d-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry 29:23–26
Skolnick P, Popik P, Trullas R (2009) Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci 30:563–569
Szewczyk B, Poleszak E, Sowa-Kućma M, Siwek M, Dudek D, Ryszewska-Pokraśniewicz B, Radziwoń-Zaleska M, Opoka W, Czekaj J, Pilc A, Nowak G (2008) Antidepressant activity of zinc and magnesium in view of the current hypotheses of antidepressant action. Pharmacol Rep 60:588–589
Szewczyk B, Poleszak E, Wlaź P, Wróbel A, Blicharska E, Cichy A, Dybała M, Siwek A, Pomierny-Chamioło L, Piotrowska A, Brański P, Pilc A, Nowak G (2009) The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry 33:323–329
Szewczyk B, Poleszak E, Sowa-Kućma M, Wróbel A, Słotwiński S, Listos J, Wlaź P, Cichy A, Siwek A, Dybała M, Gołembiowska K, Pilc A, Nowak G (2010) The involvement of NMDA and AMPA receptors in the mechanism of antidepressant-like action of zinc in the forced swim test. Amino Acids 39:205–217
Trullas R, Skolnick P (1990) Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol 185:1–10
Vamvakides A (1998) d-cycloserine is active in the adult mouse and inactive in the aged mouse, in the forced swim test. Ann Pharm Fr 56:209–212
Versiani M, Mehilane L, Gaszner P, Arnaud-Castiglioni R (1999) Reboxetine, a unique selective NRI, prevents relapse and recurrence in long-term treatment of major depressive disorder. J Clin Psychiatry 60:400–406
Watson GB, Bolanowski MA, Baganoff MP, Deppeler CL, Lanthorn TH (1990) d-cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Res 510:158–160
Westenberg HG (1999) Pharmacology of antidepressants: selectivity or multiplicity? J Clin Psychiatry 60(Suppl 17):4–8
Willetts J, Balster RL, Leander JD (1990) The behavioral pharmacology of NMDA receptor antagonists. Trends Pharmacol Sci 11:423–428
Wood PL, Emmett MR, Rao TS, Mick S, Cler J, Iyengar S (1989) In vivo modulation of the N-methyl-d-aspartate receptor complex by d-serine: potentiation of ongoing neuronal activity as evidenced by increased cerebellar cyclic GMP. J Neurochem 53:979–981
Wood PL, Hawkinson JE, Goodnough DB (1996) Formation of d-serine from l-phosphoserine in brain synaptosomes. J Neurochem 67:1485–1490
Yan QS, Reith ME, Jobe PC, Dailey JW (1997) Dizocilpine (MK-801) increases not only dopamine but also serotonin and norepinephrine transmissions in the nucleus accumbens as measured by microdialysis in freely moving rats. Brain Res 765:149–158
Zarate CA Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK (2004) An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry 161:171–174
Zarate CA Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK (2005) An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 57:430–432
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006a) A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864
Zarate CA Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK, Charney DS (2006b) A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry 163:153–155
Acknowledgments
This study was supported by Funds for Statutory Activity of Maria Curie-Skłodowska University, Lublin, Medical University of Lublin, Institute of Pharmacology, Polish Academy of Sciences, and Jagiellonian University Collegium Medicum, Kraków, Poland. The authors wish to thank Polpharma S.A. (Starogard Gdański, Poland) for a generous gift of fluoxetine.
Conflict of interest
The authors declare they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Poleszak, E., Wlaź, P., Szewczyk, B. et al. A complex interaction between glycine/NMDA receptors and serotonergic/noradrenergic antidepressants in the forced swim test in mice. J Neural Transm 118, 1535–1546 (2011). https://doi.org/10.1007/s00702-011-0630-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-011-0630-9