Abstract
Bacterial infectious diseases are severe threats to human health and increase substantial financial burdens. Nanomaterials have shown great potential in timely and accurate bacterial identification, detection, and monitoring to improve the cure rate and reduce mortality. Recently, carbon dots have been evidenced to be ideal candidates for bacterial identification and detection due to their superior physicochemical properties and biocompatibility. This review outlines the detailed recognition elements and recognition strategies with functionalized carbon dots (FCDs) for bacterial identification and detection. The advantages and limitations of different kinds of FCDs-based sensors will be critically discussed. Meanwhile, the ongoing challenges and perspectives of FCDs-based sensors for bacteria sensing are put forward.
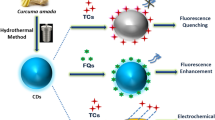
Graphical abstract













Similar content being viewed by others
References
Alafeef M, Dighe K, Pan D (2019) Label-free pathogen detection based on yttrium-doped carbon nanoparticles up to single-cell resolution. ACS Appl Mater Inter 11(46):42943–42955. https://doi.org/10.1021/acsami.9b14110
Li T, Wang ZL, Guo JH, de la Fuente-Nunez Cesar, Wang JQ, Han B, Tao H, Liu J, Wang XM (2023) Bacterial resistance to antibacterial agents: mechanisms, control strategies, and implications for global health. Sci Total Environ 860:160461. https://doi.org/10.1016/j.scitotenv.2022.160461
Ray PC, Khan SA, Singh AK, Senapati D, Fan Z (2012) Nanomaterials for targeted detection and photothermal killing of bacteria. Chem Soc Rev 41(8):3193–3209. https://doi.org/10.1039/C2CS15340H
O’Neill J (2014) Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist. https://amrreview.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf. Accessed 2 Mar 2020
Zhen XM, Li YY, Chen YX, Dong P, Liu S, Dong HJ (2018) Effect of multiple drug resistance on total medical costs among patients with intraabdominal infections in China. PLoS One 13(3):e0193977. https://doi.org/10.1371/journal.pone.0193977
Chen J, Andler SM, Goddard JM, Nugen SR, Rotello VM (2017) Integrating recognition elements with nanomaterials for bacteria sensing. Chem Soc Rev 46(5):1272–1283. https://doi.org/10.1039/C6CS00313C
Li D, Kumari B, Makabenta JM, Gupta A, Rotello V (2019) Effective detection of bacteria using metal nanoclusters. Nanoscale 11(46):22172–22181. https://doi.org/10.1039/C9NR08510F
Yu T, Xianyu YL (2021) Array-based biosensors for bacteria detection: from the perspective of recognition. Small 17(21):2006230. https://doi.org/10.1002/smlP202006230
Xu XY, Ray R, Gu YH, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737. https://doi.org/10.1021/ja040082h
Mintz KJ, Bartoli M, Rovere M, Zhou Y, Hettiarachchi SD, Paudyal S, Chen J, Domena JB, Liyanage PY, Sampson R, Khadka D, Pandey RR, Huang S, Chusuei CC, Tagliaferro A, Leblanc RM (2021) A deep investigation into the structure of carbon dots. Carbon 173:433–447. https://doi.org/10.1016/j.carbon.2020.11.017
Zhu SJ, Song YB, Zhao XH, Shao JR, Zhang JH, Yang B (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 8(2):355–381. https://doi.org/10.1007/s12274-014-0644-3
He C, Xu P, Zhang XH, Long WJ (2022) The synthetic strategies, photoluminescence mechanisms and promising applications of carbon dots: current state and future perspective. Carbon 186:91–127. https://doi.org/10.1016/j.carbon.2021.10.002
Li S, Li L, Tu HY, Zhang H, Silvester DS, Banks CE, Zou GQ, Hou HS, Ji XB (2021) The development of carbon dots: from the perspective of materials chemistry. Mater Today 51:188–207. https://doi.org/10.1016/j.mattod.2021.07.028
Arcudi F, Đorđević L, Prato M (2019) Design, synthesis, and functionalization strategies of tailored carbon nanodots. Acc Chem Res 52(8):2070–2079. https://doi.org/10.1021/acs.accounts.9b00249
Sun XC, Lei Y (2017) Fluorescent carbon dots and their sensing applications. TrAC Trend Anal Chem 89:163–180. https://doi.org/10.1016/j.trac.2017.02.001
KhairolAnuar NK, Tan HL, Lim YP, So’aib MS, Abu Bakar NF (2021) A review on multifunctional carbon-dots synthesized from biomass waste: design/fabrication, characterization and applications. Front Energy Res 9:626549. https://doi.org/10.3389/fenrg.2021.626549
Park Y, Yoo J, Lim B, Kwon W, Rhee SW (2016) Improving the functionality of carbon nanodots: doping and surface functionalization. J Mater Chem A 4(30):11582–11603. https://doi.org/10.1039/C6TA04813G
Li F, Yang DY, Xu HP (2019) Non-metal-heteroatom-doped carbon dots: synthesis and property. Chem Eur J 25(5):1165–1176. https://doi.org/10.1002/chem.201802793
Lin LP, Luo YX, Tsai P, Wang JJ, Chen X (2018) Metal ions doped carbon quantum dots: synthesis, physicochemical properties, and their applications. TrAC Trend Anal Chem 103:87–101. https://doi.org/10.1016/j.trac.2018.03.015
Anand A, Manavalan G, Mandal RP, Chang HT, Chiou YR, Huang CC (2020) Carbon dots for bacterial detection and antibacterial applications-a minireview. Curr Pharm Design 25(46):4848–4860. https://doi.org/10.2174/1381612825666191216150948
Cui FC, Ye YL, Ping JF, Sun XL (2020) Carbon dots: current advances in pathogenic bacteria monitoring and prospect applications. Biosens Bioelectron 156:112085. https://doi.org/10.1016/j.bios.2020.112085
Lin FM, Bao YW, Wu FG (2019) Carbon dots for sensing and killing microorganisms. C-J Carbon Res 5(2):33. https://doi.org/10.3390/c5020033
Li YD, Xu XK, Wu Y, Zhuang JL, Zhang XJ, Zhang HR, Lei BF, Hu CF, Liu YL (2020) A review on the effects of carbon dots in plant systems. Mater Chem Front 4(2):437–448. https://doi.org/10.1039/C9QM00614A
Kamal Z, ZareiGhobadi M, Mohseni SM, Ghourchian H (2021) High-performance porphyrin-like graphene quantum dots for immuno-sensing of Salmonella typhi. Biosens Bioelectron 188:113334. https://doi.org/10.1016/j.bios.2021.113334
Zhao Y, Li YZ, Zhang PP, Yan ZH, Zhou YG, Du YP, Qu CY, Song YJ, Zhou D, Qu SN, Yang RF (2021) Cell-based fluorescent microsphere incorporated with carbon dots as a sensitive immunosensor for the rapid detection of Escherichia coli O157 in milk. Biosens Bioelectron 179:113057. https://doi.org/10.1016/j.bios.2021.113057
Song Y, Ostermeyer GP, Du D, Lin YH (2021) Carbon nanodot-hybridized silica nanospheres assisted immunoassay for sensitive detection of Escherichia coli. Sensor Actuator B: Chem 349:130730. https://doi.org/10.1016/j.snb.2021.130730
Yang XZ, Feng LQ, Qin X (2018) Preparation of the Cf-GQDs-Escherichia coli O157: H7 bioprobe and its application in optical imaging and sensing of Escherichia coli O157: H7. Food Anal Method 11(8):2280–2286. https://doi.org/10.1007/s12161-018-1207-0
Sirdeshmukh VV, Apte HR, Kale AA (2019) Graphene quantum dots as promising probes in electrochemical immunoassay for rapid and sensitive detection of pathogenic Staphylococcus aureus. IEEE 13th International Conference on Nano/Molecular Medicine & Engineering (NANOMED). IEEE 108–114. https://doi.org/10.1109/NANOMED49242.2019.9130608
Jampasa S, Ngamrojanavanich N, Rengpipat S, Chailapakul O, Kalcher K, Chaiyo S (2021) Ultrasensitive electrochemiluminescence sensor based on nitrogen-decorated carbon dots for Listeria monocytogenes determination using a screen-printed carbon electrode. Biosens Bioelectron 188:113323. https://doi.org/10.1016/j.bios.2021.113323
Chen SF, Chen XQ, Zhang LJ, Gao JJ, Ma Q (2017) Electrochemiluminescence detection of Escherichia coli O157:H7 based on a novel polydopamine surface Imprinted polymer biosensor. ACS Appl Mater Inter 9(6):5430–5436. https://doi.org/10.1021/acsami.6b12455
Wang HY, Chi Z, Cong Y, Wang ZZ, Jiang F, Geng JY, Zhang P, Ju P, Dong QJ, Liu CG (2018) Development of a fluorescence assay for highly sensitive detection of Pseudomonas aeruginosa based on an aptamer-carbon dots/graphene oxide system. RSC Adv 8(57):32454–32460. https://doi.org/10.1039/C8RA04819C
Pan T, Shan XL, Jiang D, Qi L, Wang WC, Chen ZD (2021) Fluorometric aptasensor for determination of Escherichia coli O157:H7 by FRET effect between aminated carbon quantum dots and graphene oxide. Anal Sci 37(6):833–838. https://doi.org/10.2116/analsci.20P306
Yao S, Zhao C, Shang MY, Li J, Wang J (2021) Enzyme-free and label-free detection of Staphylococcus aureus based on target-inhibited fluorescence signal recovery. Food Chem Toxicol 150:112071. https://doi.org/10.1016/j.fct.2021.112071
Pebdeni AB, Hosseini M, Ganjali MR (2020) Fluorescent turn-on aptasensor of Staphylococcus aureus based on the FRET between green carbon quantum dot and gold nanoparticle. Food Anal Method 13(11):2070–2079. https://doi.org/10.1007/s12161-020-01821-4
Hu XT, Li YX, Xu YW, Gan ZY, Zou XB, Shi JY, Huang XW, Li ZH, Li YH (2021) Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk. Food Chem 339:127775. https://doi.org/10.1016/j.foodchem.2020.127775
Wang RJ, Xu Y, Zhang T, Jiang Y (2015) Rapid and sensitive detection of Salmonella typhimurium using aptamer-conjugated carbon dots as fluorescence probe. Anal Method 7(5):1701–1706. https://doi.org/10.1039/C4AY02880E
Guo Z, Huang XW, Li ZH, Shi JY, Zhai XD, Hu XT, Liang NN, Zou XB (2020) Rapid and highly sensitive detection of Salmonella typhimurium in lettuce by using magnetic fluorescent nanoparticles. Anal Method 12(48):5861–5868. https://doi.org/10.1039/D0AY01744B
Bahari D, Babamiri B, Salimi A, Salimizand H (2021) Ratiometric fluorescence resonance energy transfer aptasensor for highly sensitive and selective detection of Acinetobacter baumannii bacteria in urine sample using carbon dots as optical nanoprobes. Talanta 221:121619. https://doi.org/10.1016/j.talanta.2020.121619
Ranjbar S, Shahrokhian S (2018) Design and fabrication of an electrochemical aptasensor using Au nanoparticles/carbon nanoparticles/cellulose nanofibers nanocomposite for rapid and sensitive detection of Staphylococcus aureus. Bioelectrochemistry 123:70–76. https://doi.org/10.1016/j.bioelechem.2018.04.018
Jiang D, Yang CQ, Fan YD, Polly Leung HM, Inthavong K, Zhang Y, Li ZY, Yang M (2021) Ultra-sensitive photoelectrochemical aptamer biosensor for detecting E. coli O157:H7 based on nonmetallic plasmonic two-dimensional hydrated defective tungsten oxide nanosheets coupling with nitrogen-doped graphene quantum dots (dWO3•H2O@N-GQDs). Biosens Bioelectron 183:113214. https://doi.org/10.1016/j.bios.2021.113214
Lin XF, Mei YQ, He C, Luo Y, Yang M, Kuang Y, Ma XM, Zhang HF, Huang QT (2021) Electrochemical biosensing interface based on carbon dots-Fe3O4 nanomaterial for the determination of Escherichia coli O157: H7. Front Chem 9:769648. https://doi.org/10.3389/fchem.2021.769648
Cui FC, Sun JD, de Dieu Habimana J, Yang XX, Ji J, Zhang YZ, Lei HT, Li ZJ, Zheng JY, Fan MH, Sun XL (2019) Ultrasensitive fluorometric angling determination of Staphylococcus aureus in vitro and fluorescence imaging in vivo using carbon dots with full-color emission. Anal Chem 91(22):14681–14690. https://doi.org/10.1021/acs.analchem.9b03916
Bruce JA, Clapper JC (2020) Conjugation of carboxylated graphene quantum dots with Cecropin P1 for bacterial biosensing applications. ACS Omega 5(41):26583–26591. https://doi.org/10.1021/acsomega.0c03342
Shen YZ, Wu TT, Chen HH, Ye YW, Xu JJ (2022) Ratiometric fluorescence detection of pathogenic bacteria based on dual-recognition nanoprobes with controllable G-quadruplex release. Chem Commun 58(3):447–450. https://doi.org/10.1039/D1CC05966A
Wang ZZ, Wang HY, Cheng XH, Geng JY, Wang L, Dong QJ, Liu CG, Chi ZM, Chi Z (2021) Aptamer-superparamagnetic nanoparticles capture coupling siderophore-Fe3+ scavenging actuated with carbon dots to confer an “off-on” mechanism for the ultrasensitive detection of Helicobacter pylori. Biosens Bioelectron 193:113551. https://doi.org/10.1016/j.bios.2021.113551
Gao R, Zhong ZT, Gao XM, Jia L (2018) Graphene oxide quantum dots assisted construction of fluorescent aptasensor for rapid detection of Pseudomonas aeruginosa in food samples. J Agr Food Chem 66(41):10898–10905. https://doi.org/10.1021/acs.jafc.8b02164
Savas S, Altintas Z (2019) Graphene quantum dots as nanozymes for electrochemical sensing of Yersinia enterocolitica in milk and human serum. Materials 12(13):2189. https://doi.org/10.3390/ma12132189
Shi YH, Sun YH, Qu XW, Zhou L, Yue TL, Yuan YH (2021) Preparation of species-specific monoclonal antibody and development of fluorescence immunoassay based on fluorescence resonance energy transfer of carbon dots for accurate and sensitive detection of Alicyclobacillus acidoterrestris in apple juice. Food Chem 347:129069. https://doi.org/10.1016/j.foodchem.2021.129069
Yang L, Deng WF, Cheng C, Tan YM, Xie QJ, Yao SZ (2018) Fluorescent immunoassay for the detection of pathogenic bacteria at the single-cell level using carbon dots-encapsulated breakable organosilica nanocapsule as labels. ACS Appl Mater Inter 10(4):3441–3448. https://doi.org/10.1021/acsami.7b18714
Yao S, Zhao C, Liu YS, Nie HR, Xi GL, Cao XL, Li ZL, Pang B, Li J, Wang J (2020) Colorimetric immunoassay for the detection of Staphylococcus aureus by using magnetic carbon dots and sliver nanoclusters as o-phenylenediamine-oxidase mimetics. Food Anal Method 13(4):833–838. https://doi.org/10.1007/s12161-019-01683-5
Wang ZH, Yao XL, Wang R, Ji YW, Yue TL, Sun J, Li T, Wang JL, Zhang DH (2019) Label-free strip sensor based on surface positively charged nitrogen-rich carbon nanoparticles for rapid detection of Salmonella enteritidis. Biosens Bioelectron 132:360–367. https://doi.org/10.1016/j.bios.2019.02.061
Pangajam A, Theyagarajan K, Dinakaran K (2020) Highly sensitive electrochemical detection of E. coli O157:H7 using conductive carbon dot/ZnO nanorod/PANI composite electrode. Sens Bio-Sens Res 29:100317. https://doi.org/10.1016/j.sbsr.2019.100317
Ebbensgaard A, Mordhorst H, Aarestrup FM, Hansen EB (2018) The role of outer membrane proteins and lipopolysaccharides for the sensitivity of Escherichia coli to antimicrobial peptides. Front Microbiol 9:2153. https://doi.org/10.3389/fmicb.2018.02153
MartínezPeriñán E, GarcíaMendiola T, Enebral Romero E, del Caño R, Vera Hidalgo M, Vázquez Sulleiro M, Navío C, Pariente F, Pérez EM, Lorenzo E (2021) A MoS2 platform and thionine-carbon nanodots for sensitive and selective detection of pathogens. Biosens Bioelectron 189:113375. https://doi.org/10.1016/j.bios.2021.113375
Li S, Liu JL, Chen ZT, Lu YL, Low SS, Zhu LH, Cheng C, He Y, Chen QM, Su B, Liu QJ (2019) Electrogenerated chemiluminescence on smartphone with graphene quantum dots nanocomposites for Escherichia Coli detection. Sensor Actuator B: Chem 297:126811. https://doi.org/10.1016/j.snb.2019.126811
Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11(14):1620–1636. https://doi.org/10.1002/smll.201402648
Chandra S, Chowdhuri AR, Mahto TK, Samui A, Sk S (2016) One-step synthesis of amikacin modified fluorescent carbon dots for the detection of Gram-negative bacteria like Escherichia coli. RSC Adv 6(76):72471–72478. https://doi.org/10.1039/C6RA15778E
Putri FAR, Mudasir M, Morita K, Suherman S (2019) Microwave-assisted synthesis of amikacin modified N, S co-doped carbon dots for Escherichia coli detection. Chemosensors 7(4):61. https://doi.org/10.3390/chemosensors7040061
Chandra S, Mahto TK, Chowdhuri AR, Das B, Sk S (2017) One step synthesis of functionalized carbon dots for the ultrasensitive detection of Escherichia coli and iron (III). Sensor Actuator B: Chem 245:835–844. https://doi.org/10.1016/j.snb.2017.02.017
Zhong D, Zhuo Y, Feng YJ, Yang XM (2015) Employing carbon dots modified with vancomycin for assaying Gram-positive bacteria like Staphylococcus aureus. Biosens Bioelectron 74:546–553. https://doi.org/10.1016/j.bios.2015.07.015
Gao Z, Yang DZ, Wan Y, Yang YL (2020) One-step synthesis of carbon dots for selective bacterial inactivation and bacterial differentiation. Anal Bioanal Chem 412(4):871–880. https://doi.org/10.1007/s00216-019-02293-0
Wang N, Wang YT, Guo TT, Yang T, Chen ML, Wang JH (2016) Green preparation of carbon dots with papaya as carbon source for effective fluorescent sensing of iron (III) and Escherichia coli. Biosens Bioelectron 85:68–75. https://doi.org/10.1016/j.bios.2016.04.089
Lai IPJ, Harroun SG, Chen SY, Unnikrishnan B, Li YJ, Huang CC (2016) Solid-state synthesis of self-functional carbon quantum dots for detection of bacteria and tumor cells. Sensor Actuator B: Chem 228:465–470. https://doi.org/10.1016/j.snb.2016.01.062
Weng CI, Chang HT, Lin CH, Shen YW, Unnikrishnan B, Li YJ, Huang CC (2015) One-step synthesis of biofunctional carbon quantum dots for bacterial labeling. Biosens Bioelectron 68:1–6. https://doi.org/10.1016/j.bios.2014.12.028
Choi CA, Mazrad ZAI, Lee G, In I, Lee KD, Park SY (2018) Boronate-based fluorescent carbon dot for rapid and selectively bacterial sensing by luminescence off/on system. J Pharmaceut Biomed 159:1–10. https://doi.org/10.1016/j.jpba.2018.06.043
Zhang LX, Zhang ZS, Gao ZW, Xie Y, Shu S, Ke YE, Wang Y, Deng B, Yu RJ, Geng HL (2020) Facile synthesis of N, B-co-doped carbon dots with the gram-scale yield for detection of iron (III) and E. coli. Nanotechnology 31(39):395702. https://doi.org/10.1088/1361-6528/ab9b4c
Wang JL, Teng JY, Jia T, Shu Y (2018) Detection of yeast Saccharomyces cerevisiae with ionic liquid mediated carbon dots. Talanta 178:818–824. https://doi.org/10.1016/j.talanta.2017.10.029
Kumari M, Chaudhary S (2020) Modulating the physicochemical and biological properties of carbon dots synthesised from plastic waste for effective sensing of E. coli. Colloid Surface B 196:111333. https://doi.org/10.1016/j.colsurfb.2020.111333
Jo HJ, Robby AI, Kim SG, Lee G, Lee BC, Park SY (2021) Reusable biosensor-based polymer dot-coated electrode surface for wireless detection of bacterial contamination. Sensor Actuator B: Chem 346:130503. https://doi.org/10.1016/j.snb.2021.130503
Mazrad ZAI, Choi CA, Kwon YM, In I, Lee KD, Park SY (2017) Design of surface-coatable NIR-responsive fluorescent nanoparticles with PEI passivation for bacterial detection and killing. ACS Appl Mater Inter 9(38):33317–33326. https://doi.org/10.1021/acsami.7b10688
Pathak A, Venugopal P, Nair BG, Suneesh PV, SatheeshBabu TG (2020) Facile pH-sensitive optical detection of pathogenic bacteria and cell imaging using multi-emissive nitrogen-doped carbon dots. MicroChem J 159:105324. https://doi.org/10.1016/j.microc.2020.105324
Suherman S, Audio Haryanto N, Tri Wahyuni E, Ilmi M, Morita K, Oki Y (2019) Carbon dots modification for Escherichia coli detection: variation of colistin sulphate concentration. Orient J Chem 35(1):49–55. https://doi.org/10.13005/ojc/350105
Nandi S, Ritenberg M, Jelinek R (2015) Bacterial detection with amphiphilic carbon dots. Analyst 140(12):4232–4237. https://doi.org/10.1039/C5AN00471C
Zhao WB, Wang RT, Liu KK, Du MR, Wang Y, Wang YQ, Zhou R, Liang YC, Ma RN, Sui LZ, Lou Q, Hou L, Shan CX (2021) Near-infrared carbon nanodots for effective identification and inactivation of Gram-positive bacteria. Nano Res 15(3):1699–1708. https://doi.org/10.1007/s12274-021-3818-9
Yang JJ, Zhang XD, Ma YH, Gao G, Chen XK, Jia HR, Li YH, Chen Z, Wu FG (2016) Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Appl Mater Inter 8(47):32170–32181. https://doi.org/10.1021/acsami.6b10398
Yang JJ, Gao G, Zhang XD, Ma YH, Chen XK, Wu FG (2019) One-step synthesis of carbon dots with bacterial contact-enhanced fluorescence emission: fast Gram-type identification and selective Gram-positive bacterial inactivation. Carbon 146:827–839. https://doi.org/10.1016/j.carbon.2019.02.040
Fu L, Chen QM, Jia L (2022) Carbon dots and gold nanoclusters assisted construction of a ratiometric fluorescent biosensor for detection of Gram-negative bacteria. Food Chem 374:131750. https://doi.org/10.1016/j.foodchem.2021.131750
Hiremath SD, Bhosle AA, Nayse A, Biswas S, Biswas M, Bhasikuttan AC, Banerjee M, Chatterjee A (2021) A redox-coupled carbon dots-MnO2 nanosheets based sensory platform for label-free and sensitive detection of E. coli. Sensor Actuator B: Chem 339:129918. https://doi.org/10.1016/j.snb.2021.129918
Bhattacharya S, Nandi S, Jelinek R (2017) Carbon-dot–hydrogel for enzyme-mediated bacterial detection. RSC Adv 7(2):588–594. https://doi.org/10.1039/C6RA25148J
Zhao MY, Gao X, Tao ZH, Wang XK, Lin XD, Wang S, Liu YQ (2020) Sugar-metabolism-triggered pathogenic bacteria identification based on pH-sensitive fluorescent carbon dots. Sensor Actuator B: Chem 316:128063. https://doi.org/10.1016/j.snb.2020.128063
Yang QL, Farooq U, Chen W, Ullah MW, Wang SQ (2019) Fluorimetric detection of single pathogenic bacterium in milk and sewage water using pH-sensitive fluorescent carbon dots and MALDI-TOF MS. Microorganisms 8(1):53. https://doi.org/10.3390/microorganisms8010053
Das R, Singh N (2019) Exploring electrochemistry of carbon nanodots and its application in noninvasive bacterial growth monitoring. Biosens Bioelectron 144:111640. https://doi.org/10.1016/j.bios.2019.111640
Chen YX, Jiang SM, Hu YC, Gong JM (2021) Enhanced electrochemiluminescence bioassay triggered by intracellular leakage for detection of Escherichia coli. Biosens Bioelectron 194:113575. https://doi.org/10.1016/j.bios.2021.113575
Liu WJ, Gu H, Liu WK, Lv CY, Du JJ, Fan JL, Peng XJ (2022) NIR-emitting carbon dots for discriminative imaging and photo-inactivation of pathogenic bacteria. Chem Eng J 450:137384. https://doi.org/10.1016/j.cej.2022.137384
Zhao B, Cui FY, Xu Y, Zhang XF, Li ZQ (2017) Study on interaction between mannose and bacteria using glycosylated carbon quantum dots. J Instrum Anal 36(11):1318–1324. https://doi.org/10.3969/j.issn.1004-4957.2017.11.005
Ahmadian-Fard-Fini S, Ghanbari D, Amiri O, Salavati-Niasari M (2021) Green sonochemistry assisted synthesis of hollow magnetic and photoluminescent MgFe2O4-carbon dot nanocomposite as a sensor for toxic Ni(II), Cd(II) and Hg(II) ions and bacteria. RSC Adv 11(37):22805–22811. https://doi.org/10.1039/D1RA02458B
Robby AI, Kim SG, Lee UH, In I, Lee G, Park SY (2021) Wireless electrochemical and luminescent detection of bacteria based on surface-coated CsWO3-immobilized fluorescent carbon dots with photothermal ablation of bacteria. Chem Eng J 403:126351. https://doi.org/10.1016/j.cej.2020.126351
Ahmadian-Fard-Fini S, Ghanbari D, Salavati Niasari M (2019) Photoluminescence carbon dot as a sensor for detecting of Pseudomonas aeruginosa bacteria: hydrothermal synthesis of magnetic hollow NiFe2O4-carbon dots nanocomposite material. Compos Part B: Eng 161:564–577. https://doi.org/10.1016/j.compositesb.2018.12.131
Ahmadian-Fard-Fini S, Salavati Niasari M, Ghanbari D (2018) Hydrothermal green synthesis of magnetic Fe3O4-carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteria. Spectrochim Acta Part A 203:481–493. https://doi.org/10.1016/j.saa.2018.06.021
Roh SG, Robby AI, Phuong PTM, In I, Park SY (2019) Photoluminescence-tunable fluorescent carbon dots-deposited silver nanoparticle for detection and killing of bacteria. Mat Sci Eng C 97:613–623. https://doi.org/10.1016/j.msec.2018.12.070
Jia HR, Zhu YX, Chen Z, Wu FG (2017) Cholesterol-assisted bacterial cell surface engineering for photodynamic inactivation of Gram-positive and Gram-negative bacteria. ACS Appl Mater Interfaces 9:15943–15951. https://doi.org/10.1021/acsami.7b02562
Chu XH, Wu F, Sun BH, Zhang M, Song SJ, Zhang P, Wang YL, Zhang QC, Zhou NL, Shen J (2020) Genipin cross-linked carbon dots for antimicrobial, bioimaging and bacterial discrimination. Colloid Surface B 190:110930. https://doi.org/10.1016/j.colsurfb.2020.110930
Zhou CC, Xu WH, Zhang PB, Jiang MJ, Chen YC, Kwok RTK, Lee MMS, Shan GG, Qi RL, Zhou X, Lam JWY, Wang S, Tang BZ (2019) Engineering sensor arrays using aggregation-induced emission luminogens for pathogen identification. Adv Funct Mater 29(4):1805986. https://doi.org/10.1002/adfm.201805986
Yan CR, Wang CL, Hou TT, Guan P, Qiao YB, Guo LL, Teng YG, Hu XL, Wu H (2021) Lasting tracking and rapid discrimination of live Gram-positive bacteria by peptidoglycan-targeting carbon quantum dots. ACS Appl Mater Inter 13(1):1277–1287. https://doi.org/10.1021/acsami.0c19651
Bhaisare ML, Gedda G, Khan MS, Wu HF (2016) Fluorimetric detection of pathogenic bacteria using magnetic carbon dots. Anal Chim Acta 920:63–71. https://doi.org/10.1016/j.aca.2016.02.025
Yan R, Shou ZX, Chen J, Wu H, Zhao Y, Qiu L, Jiang PJ, Mou XZ, Wang JH, Li YQ (2018) On-off-on gold nanocluster-based fluorescent probe for rapid Escherichia coli differentiation, detection and bactericide screening. ACS Sustainable Chem Eng 6(4):4504–4509. https://doi.org/10.1021/acssuschemeng.8b00112
Wang XH, Shan MY, Zhang SK, Chen X, Liu WT, Chen JZ, Liu XY (2022) Stimuli-responsive antibacterial materials: molecular structures, design principles, and biomedical applications. Adv Sci 9(13):2104843. https://doi.org/10.1002/advs.202104843
Zheng LB, Qi P, Zhang D (2019) Identification of bacteria by a fluorescence sensor array based on three kinds of receptors functionalized carbon dots. Sensor Actuator B: Chem 286:206–213. https://doi.org/10.1016/j.snb.2019.01.147
Wang SJ, Zhang YQ, Zhuo P, Hu QS, Chen ZQ, Zhou L (2020) Identification of eight pathogenic microorganisms by single concentration-dependent multicolor carbon dots. J Mater Chem B 8(27):5877–5882. https://doi.org/10.1039/D0TB00834F
Shauloff N, Morag A, Yaniv K, Singh S, Malishev R, Paz-Tal O, Rokach L, Jelinek R (2021) Sniffing bacteria with a carbon-dot artificial nose. Nano-Micro Lett 13(1):112. https://doi.org/10.1007/s40820-021-00610-w
Funding
This work was financially supported by the Open Project of Priority Disciplinary Construction at Fujian Agriculture and Forestry University (722022003) and Natural Science Foundation of Fujian Province of China (No. 2023J01453).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, L., Fang, M., Liu, W. et al. Recent advances and perspectives of functionalized carbon dots in bacteria sensing. Microchim Acta 190, 363 (2023). https://doi.org/10.1007/s00604-023-05938-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05938-1