Abstract
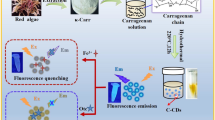
A novel carbon dot (CD) was synthesized through the facile and simple hydrothermal method from Curcuma amada, as the precursor for the first time. These CDs with an average diameter of 4.6 nm display blue fluorescence, with excitation/emission maxima at 360/445 nm and a quantum yield of 14.1%. It exhibited high stability under different conditions and was characterized using various techniques. These CDs can be employed as a dual-sensing platform to detect tetracyclines and fluoroquinolones, two antibiotic classes. Even though antibiotics are regarded as an inevitable commodity, overuse and improper management of discarded antibiotics pose a severe threat to the environment. Herein, we developed a dual-sensing, biocompatible sensor with high selectivity and sensitivity to detect antibiotics. CD was employed as a fluorescence probe and detected tetracycline and fluoroquinolone antibiotic through inner filter effect–based fluorescence quenching and hydrogen bonding–based enhancement process, respectively. The linear range was 0–16 μM and the detection limit was 33 nM for tetracycline and 2 nM for fluoroquinolone antibiotic. As an electrochemical probe, CD selectively detected tetracycline with a lower detection limit of 0.5 nM over a linear range of 0–16 μM. Using both methods, a real sample analysis of the developed sensor exhibited accurate reliability and precision.
Graphical abstract













Similar content being viewed by others
References
Chee-Sanford JC, Aminov RI, Krapac IJ, Garrigues-Jeanjean N, Mackie RI. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl Environ Microbiol. 2001;67(4):1494–502. https://doi.org/10.1128/AEM.67.4.1494-1502.2001.
Guidi LR, Santos FA, Ribeiro ACSR, Fernandes C, Silva LHM, Gloria MBA. Quinolones and tetracyclines in aquaculture fish by a simple and rapid LC-MS/MS method. Food Chem. 2018;245:1232–8. https://doi.org/10.1016/j.foodchem.2017.11.094.
Wang S, Yong W, Liu J, Zhang L, Chen Q, Dong Y. Development of an indirect competitive assay-based aptasensor for highly sensitive detection of tetracycline residue in honey. Biosens Bioelectron. 2014;57:192–8. https://doi.org/10.1016/j.bios.2014.02.032.
Tong C, Xiang G. Sensitive determination of norfloxacin by the fluorescence probe of terbium (III)- sodium dodecylbenzene sulfonate and its luminescence mechanism. J Fluoresc. 2006;16(6):831–7. https://doi.org/10.1007/s10895-006-0107-7.
Chen Z, Qian S, Chen J, Cai J, Wu S, Cai Z. Protein-templated gold nanoclusters based sensor for off–on detection of ciprofloxacin with a high selectivity. Talanta. 2012;94:240–5. https://doi.org/10.1016/j.talanta.2012.03.033.
Faridbod F, Jamali A, Ganjali MR, Hosseini M, Norouzi P. A novel cobalt-sensitive fluorescent chemosensor based on ligand capped CdS quantum dots. J Fluoresc. 2015;25(3):613–9. https://doi.org/10.1007/s10895-015-1544-y.
Jamali A, Tehrani AA, Shemirani F, Morsali A. Lanthanide metal–organic frameworks as selective microporous materials for adsorption of heavy metal ions. Dalton Trans. 2016;45(22):9193–200. https://doi.org/10.1039/c6dt00782a.
Vega D, Agüí L, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM. Voltammetry and amperometric detection of tetracyclines at multi-wall carbon nanotube modified electrodes. Anal Bioanal Chem. 2007;389(3):951–8. https://doi.org/10.1007/s00216-007-1505-7.
Gan T, Shi Z, Sun J, Liu Y. Simple and novel electrochemical sensor for the determination of tetracycline based on iron/zinc cations–exchanged montmorillonite catalyst. Talanta. 2014;121:187–93. https://doi.org/10.1016/j.talanta.2014.01.002.
Mohammad-Razdari A, Ghasemi-Varnamkhasti M, Rostami S, Izadi Z, Ensafi AA, Siadat M. Development of an electrochemical biosensor for impedimetric detection of tetracycline in milk. J Food Sci Technol. 2020;57(12):4697–706. https://doi.org/10.1007/s13197-020-04506-2.
Taghdisi SM, Danesh NM, Ramezani M, Abnous K. A novel M-shape electrochemical aptasensor for ultrasensitive detection of tetracyclines. Biosens Bioelectron. 2016;85:509–14. https://doi.org/10.1016/j.bios.2016.05.048.
Wang C-I, Wu W-C, Periasamy AP, Chang H-T. Electrochemical synthesis of photoluminescent carbon nanodots from glycine for highly sensitive detection of hemoglobin. Green Chem. 2014;16(5):2509. https://doi.org/10.1039/c3gc42325e.
Wang Y, Gao D, Zhang P, Gong P, Chen C, Gao G, Cai L. A near infrared fluorescence resonance energy transfer based aptamer biosensor for insulin detection in human plasma. Chem Commun (Camb). 2014;50(7):811–3. https://doi.org/10.1039/c3cc47649a.
Tsay JM, Michalet X. New light on quantum dot cytotoxicity. Chem Biol. 2005;12(11):1159–61. https://doi.org/10.1016/j.chembiol.2005.11.002.
Wang H, Lu Q, Hou Y, Liu Y, Zhang Y. High fluorescence S, N co-doped carbon dots as an ultra-sensitive fluorescent probe for the determination of uric acid. Talanta. 2016;155:62–9. https://doi.org/10.1016/j.talanta.2016.04.020.
Xu W, Chen J, Sun S, Tang Z, Jiang K, Song L, Wang Y, Liu C, Lin H. Fluorescent and photoacoustic bifunctional probe for the detection of ascorbic acid in biological fluids, living cells and in vivo. Nanoscale. 2018;10(37):17834–41. https://doi.org/10.1039/c8nr03435d.
Yang W, Huang T, Zhao M, Luo F, Weng W, Wei Q, Lin Z, Chen G. High peroxidase-like activity of iron and nitrogen co-doped carbon dots and its application in immunosorbent assay. Talanta. 2017;164:1–6. https://doi.org/10.1016/j.talanta.2016.10.099.
Sun S, Zhang L, Jiang K, Wu A, Lin H. Toward high-efficient red emissive carbon dots: facile preparation, unique properties, and applications as multifunctional theranostic agents. Chem Mater. 2016;28(23):8659–68. https://doi.org/10.1021/acs.chemmater.6b03695.
Baker SN, Baker GA. Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed Engl. 2010;49(38):6726–44. https://doi.org/10.1002/anie.200906623.
Zhou L, Li Z, Liu Z, Ren J, Qu X. Luminescent carbon dot-gated nanovehicles for pH-triggered intracellular controlled release and imaging. Langmuir. 2013;29(21):6396–403. https://doi.org/10.1021/la400479n.
Zhai X, Zhang P, Liu C, Bai T, Li W, Dai L, Liu W. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem Commun (Camb). 2012;48(64):7955–7. https://doi.org/10.1039/c2cc33869f.
Ju E, Liu Z, Du Y, Tao Y, Ren J, Qu X. Heterogeneous assembled nanocomplexes for ratiometric detection of highly reactive oxygen species in vitro and in vivo. ACS Nano. 2014;8(6):6014–23. https://doi.org/10.1021/nn501135m.
Yang Z, Xu M, Liu Y, He F, Gao F, Su Y, Wei H, Zhang Y. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale. 2014;6(3):1890–5. https://doi.org/10.1039/c3nr05380f.
Jaiswal A, Ghosh SS, Chattopadhyay A. One step synthesis of C-dots by microwave mediated caramelization of poly(ethylene glycol). Chem Commun (Camb). 2012;48(3):407–9. https://doi.org/10.1039/c1cc15988g.
Jiang H, Chen F, Lagally MG, Denes FS. New strategy for synthesis and functionalization of carbon nanoparticles. Langmuir. 2010;26(3):1991–5. https://doi.org/10.1021/la9022163.
Vedamalai M, Periasamy AP, Wang CW, Tseng YT, Ho LC, Shih CC, Chang HT. Carbon nanodots prepared from o-phenylenediamine for sensing of Cu2+ ions in cells. Nanoscale. 2014;6(21):13119–25. https://doi.org/10.1039/c4nr03213f.
Wang W, Li Y, Cheng L, Cao Z, Liu W. Correction: water-soluble and phosphorus-containing carbon dots with strong green fluorescence for cell labeling. J Mater Chem B. 2015;3(16):3392. https://doi.org/10.1039/c5tb90055g.
Zhou L, Lin Y, Huang Z, Ren J, Qu X. Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem Commun (Camb). 2012;48(8):1147–9. https://doi.org/10.1039/c2cc16791c.
Wang L, Bi Y, Gao J, Li Y, Ding H, Ding L. Carbon dots based turn-on fluorescent probes for the sensitive determination of glyphosate in environmental water samples. RSC Adv. 2016;6(89):85820–8. https://doi.org/10.1039/C6RA10115A.
Sharma V, Tiwari P, Mobin SM. Sustainable carbon-dots: recent advances in green carbon dots for sensing and bioimaging. J Mater Chem B. 2017;5(45):8904–24. https://doi.org/10.1039/c7tb02484c.
Malek SNA, Lee GS, Hong SL, Yaacob H, Wahab NA, Faizal Weber JF, Shah SAA. Phytochemical and cytotoxic investigations of Curcuma mangga rhizomes. Molecules. 2011;16(6):4539–48. https://doi.org/10.3390/molecules16064539.
Yu J, Yuan K, Li X, Qin R, Li L, Yang X, Yu X, Zhang X, Lu Z, Liu H. Selective detection for seven kinds of antibiotics with blue emitting carbon dots and Al3+ ions. Spectrochim Acta A Mol Biomol Spectrosc. 2019;223:117366. https://doi.org/10.1016/j.saa.2019.117366.
Wei X, Lv L, Zhang Z, Guan W. Preparation of molecularly imprinted fluorescence sensor based on carbon quantum dots via precipitation polymerization for fluorescence detection of tetracycline. J Appl Polym Sci. 2020;137(38):49126. https://doi.org/10.1002/app.49126.
Qu F, Sun Z, Liu D, Zhao X, You J. Direct and indirect fluorescent detection of tetracyclines using dually emitting carbon dots. Microchim Acta. 2016;183(9):2547–53. https://doi.org/10.1007/s00604-016-1901-9.
Korah BK, Chacko AR, Abraham T, Mathew B. Recent progress and future perspectives of carbon dots in the detection, degradation, and enhancement of drugs. Part Part Syst Charact. 2022;39(2):2100264. https://doi.org/10.1002/ppsc.202100264.
Shi W, Guo F, Han M, Yuan S, Guan W, Li H, Huang H, Liu Y, Kang Z. N, S co-doped carbon dots as a stable bio-imaging probe for detection of intracellular temperature and tetracycline. J Mater Chem B. 2017;5(18):3293–9. https://doi.org/10.1039/c7tb00810d.
Fan Y, Qiao W, Long W, Chen H, Fu H, Zhou C, et al. Detection of tetracycline antibiotics using fluorescent “turn-off” sensor based on S, N-doped carbon quantum dots. Spectrochim Acta - A Mol Biomol Spectrosc. 2022;274:121033. https://doi.org/10.1016/j.saa.2022.121033.
Jia L, Xu Z, Chen R, Chen X, Xu J. Dual-channel probe of carbon dots cooperating with lanthanide complex employed for simultaneously distinguishing and sequentially detecting tetracycline and oxytetracycline. Nanomaterials. 2021;12(1):128. https://doi.org/10.3390/nano12010128.
Xue J, Li N-N, Zhang D-M, Bi C-F, Xu C-G, Shi N-N, et al. One-step synthesis of a carbon dot-based fluorescent probe for colorimetric and ratiometric sensing of tetracycline. Anal Methods. 2020;12(42):5097–102. https://doi.org/10.1039/d0ay01699c.
Lin M, Zou HY, Yang T, Liu ZX, Liu H, Huang CZ. An inner filter effect based sensor of tetracycline hydrochloride as developed by loading photoluminescent carbon nanodots in the electrospun nanofibers. Nanoscale. 2016;8(5):2999–3007. https://doi.org/10.1039/c5nr08177g.
Li H, Zhao L, Xu Y, Zhou T, Liu H, Huang N, Ding J, Li Y, Ding L. Single-hole hollow molecularly imprinted polymer embedded carbon dot for fast detection of tetracycline in honey. Talanta. 2018;185:542–9. https://doi.org/10.1016/j.talanta.2018.04.024.
Shen Z, Zhang C, Yu X, Li J, Wang Z, Zhang Z, Liu B. Microwave-assisted synthesis of cyclen functional carbon dots to construct a ratiometric fluorescent probe for tetracycline detection. J Mater Chem C. 2018;6(36):9636–41. https://doi.org/10.1039/C8TC02982B.
Al-Hashimi B, Omer KM, Rahman HS. Inner filter effect (IFE) as a simple and selective sensing platform for detection of tetracycline using milk-based nitrogen-doped carbon nanodots as fluorescence probe. Arab J Chem. 2020;13(4):5151–9. https://doi.org/10.1016/j.arabjc.2020.02.013.
Jayaweera S, Yin K, Ng WJ. Nitrogen-doped durian shell derived carbon dots for inner filter effect mediated sensing of tetracycline and fluorescent ink. J Fluoresc. 2018;29(1):221–9. https://doi.org/10.1007/s10895-018-2331-3.
Feng Y, Zhong D, Miao H, Yang X. Carbon dots derived from rose flowers for tetracycline sensing. Talanta. 2015;140:128–33. https://doi.org/10.1016/j.talanta.2015.03.038.
Qi H, Teng M, Liu M, Liu S, Li J, Yu H, et al. Biomass-derived nitrogen-doped carbon quantum dots: highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J Colloid Interface Sci. 2019;539:332–41. https://doi.org/10.1016/j.jcis.2018.12.047.
Gao X, Qin J, Liu J, Yang Z, Zhang G, Hou J. Bioinspired carbon dots as an effective fluorescent sensing platform for tetracycline detection and bioimaging. ChemistrySelect. 2022;7(3):e202104030. https://doi.org/10.1002/slct.202104030.
Fu Y, Zhao S, Wu S, Huang L, Xu T, Xing X, Lan M, Song X. A carbon dots-based fluorescent probe for turn-on sensing of ampicillin. Dyes Pigments. 2020;172:107846. https://doi.org/10.1016/j.dyepig.2019.107846.
Xu H, Yang X, Li G, Zhao C, Liao X. Green synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples. J Agric Food Chem. 2015;63(30):6707–14. https://doi.org/10.1021/acs.jafc.5b02319.
Mehta VN, Jha S, Basu H, Singhal RK, Kailasa SK. One-step hydrothermal approach to fabricate carbon dots from apple juice for imaging of mycobacterium and fungal cells. Sens Actuators B. 2015;213:434–43. https://doi.org/10.1016/j.snb.2015.02.104.
Mehta VN, Jha S, Singhal RK, Kailasa SK. Preparation of multicolor emitting carbon dots for HeLa cell imaging. New J Chem. 2014;38(12):6152–60. https://doi.org/10.1039/C4NJ00840E.
Deka MJ, Dutta P, Sarma S, Medhi OK, Talukdar NC, Chowdhury D. Carbon dots derived from water hyacinth and their application as a sensor for pretilachlor. Heliyon. 2019;5(6):e01985. https://doi.org/10.1016/j.heliyon.2019.e01985.
Li L-S, Jiao X-Y, Zhang Y, Cheng C, Huang K, Xu L. Highly fluorescent carbon dots synthesized with binary dopants for “turn off” and “turn off-on” sensing and cell imaging. Sens Actuators B. 2018;268:84–92. https://doi.org/10.1016/j.snb.2018.03.189.
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem. 2013;52(14):4045–9. https://doi.org/10.1002/anie.201300519.
Yang M, Li H, Liu J, Kong W, Zhao S, Li C, Huang H, Liu Y, Kang Z. Convenient and sensitive detection of norfloxacin with fluorescent carbon dots. J Mater Chem B. 2014;2(45):7964–70. https://doi.org/10.1039/c4tb01385a.
Chen D, Yao D, Xie C, Liu D. Development of an aptasensor for electrochemical detection of tetracycline. Food Control. 2014;42:109–15. https://doi.org/10.1016/j.foodcont.2014.01.018.
Qian S, Qiao L, Xu W, Jiang K, Wang Y, Lin H. An inner filter effect-based near-infrared probe for the ultrasensitive detection of tetracyclines and quinolones. Talanta. 2019;194:598–603. https://doi.org/10.1016/j.talanta.2018.10.097.
Shahshahanipour M, Rezaei B, Ensafi AA, Etemadifar Z. An ancient plant for the synthesis of a novel carbon dot and its applications as an antibacterial agent and probe for sensing of an anti-cancer drug. Mater Sci Eng C Mater Biol Appl. 2019;98:826–33. https://doi.org/10.1016/j.msec.2019.01.041.
Gauthier TD, Shane EC, Guerin WF, Seitz WR, Grant CL. Fluorescence quenching method for determining equilibrium constants for polycyclic aromatic hydrocarbons binding to dissolved humic materials. Environ Sci Technol. 1986;20(11):1162–6. https://doi.org/10.1021/es00153a012.
Zhou JW, Zou XM, Song SH, Chen GH. Quantum dots applied to methodology on detection of pesticide and veterinary drug residues. J Agric Food Chem. 2018;66(6):1307–19. https://doi.org/10.1021/acs.jafc.7b05119.
Yang H, He L, Long Y, Li H, Pan S, Liu H, Hu X. Fluorescent carbon dots synthesized by microwave-assisted pyrolysis for chromium(VI) and ascorbic acid sensing and logic gate operation. Spectrochim Acta A Mol Biomol Spectrosc. 2018;205:12–20. https://doi.org/10.1016/j.saa.2018.07.015.
Yuan Y, Jiang J, Liu S, Yang J, Zhang H, Yan J, Hu X. Fluorescent carbon dots for glyphosate determination based on fluorescence resonance energy transfer and logic gate operation. Sens Actuators B. 2017;242:545–53. https://doi.org/10.1016/j.snb.2016.11.050.
Li H, Xu Y, Ding J, Zhao L, Zhou T, Ding H, Chen Y, Ding L. Microwave-assisted synthesis of highly luminescent N- and S-co-doped carbon dots as a ratiometric fluorescent probe for levofloxacin. Michrochim Acta. 2018;185(2):104. https://doi.org/10.1007/s00604-017-2619-z.
González JA, Callejón Mochón M, de la Rosa FJ. Spectrofluorimetric determination of levofloxacin in tablets, human urine and serum. Talanta. 2000;52(6):1149–56. https://doi.org/10.1016/s0039-9140(00)00484-7.
Ocaña JA, Callejón M, Barragán FJ. Terbium-sensitized luminescence determination of levofloxacin in tablets and human urine and serum. Analyst. 2000;125(10):1851–4. https://doi.org/10.1039/b004252h.
Turku I, Sainio T, Paatero E. Thermodynamics of tetracycline adsorption on silica. Environ Chem Lett. 2007;5(4):225–8. https://doi.org/10.1007/s10311-007-0106-1.
Zhou L, Li D-J, Gai L, Wang J-P, Li Y-B. Electrochemical aptasensor for the detection of tetracycline with multiwalled carbon nanotubes amplification. Sens Actuators B. 2012;162(1):201–8. https://doi.org/10.1016/j.snb.2011.12.067.
Abraham T, Gigimol MG, Priyanka RN, Punnoose MS, Korah BK, Mathew B. In-situ fabrication of Ag3PO4 based binary composite for the efficient electrochemical sensing of tetracycline. Mater Lett. 2020;279:128502. https://doi.org/10.1016/j.matlet.2020.128502.
Kushikawa RT, Silva MR, Angelo ACD, Teixeira MFS. Construction of an electrochemical sensing platform based on platinum nanoparticles supported on carbon for tetracycline determination. Sens Actuators B. 2016;228:207–13. https://doi.org/10.1016/j.snb.2016.01.009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Korah, B.K., Chacko, A.R., Mathew, S. et al. Biomass-derived carbon dots as a sensitive and selective dual detection platform for fluoroquinolones and tetracyclines. Anal Bioanal Chem 414, 4935–4951 (2022). https://doi.org/10.1007/s00216-022-04119-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04119-y