Abstract
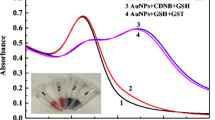
Serum levels of uric acid (UA) play an important role in the prevention of diseases. Developing a rapid and accurate way to detect UA is still a meaningful task. Hence, positively charged manganese dioxide nanosheets (MnO2NSs) with an average latter size of 100 nm and an ultra-thin thickness of below 1 nm have been prepared. They can be well dispersed in water and form stable yellow-brown solutions. The MnO2NSs can be decomposed by UA via redox reaction, leading to a decline of a characteristic absorption peak (374 nm) and a color fading of MnO2NSs solution. On this basis, an enzyme-free colorimetric sensing system for the detection of UA has been developed. The sensing system shows many advantages, including a wide linear range of 0.10–50.0 μmol/L, a limit of quantitation (LOQ) of 0.10 μmol/L, a low limit of detection (LOD) of 0.047 μmol/L (3σ/m), and rapid response without need of strict time control. Moreover, a simple and convenient visual sensor for UA detection has also been developed by adding an appropriate amount of phthalocyanine to provide a blue background color, which helps to increase visual discrimination. Finally, the strategy has been successfully applied to detect UA in human serum and urine samples.
Graphical Abstract






Similar content being viewed by others
References
Li L, Wang JL, Chen ZB (2019) Colorimetric determination of uric acid based on the suppression of oxidative etching of silver nanoparticles by chloroauric acid. Microchim Acta 187:18. https://doi.org/10.1007/s00604-019-4004-6
Wang YY, Zhang HF, Wang DH, Zhang GG NS, LinY SJQ (2020) Development of a uricase-free colorimetric biosensor for uric acid based on PPy-coated polyoxometalate-encapsulated fourfold helical metal–organic frameworks. ACS Biomater Sci Eng 6:1438–1448. https://doi.org/10.1021/acsbiomaterials.9b01922
Wu CY, Zhu LJ, Lu QJ, Li HT, Zhang YY, Yao SZ (2019) A dual-signal colorimetric and ratiometric fluorescent nanoprobe for enzymatic determination of uric acid by using silicon nanoparticles. Microchim Acta 186:754. https://doi.org/10.1007/s00604-019-3862-2
Mohammad A, Tooba H, Zahra K (2018) An enzyme-free fluorescent probe based on carbon dots MnO2 nanosheets for determination of uric acid. J Photoch Photobio A 356:603–609. https://doi.org/10.1016/j.jphotochem.2018.02.002
Yang J, Huang ZM, Hu YL, Ge J, Li JJ, Li ZH (2018) A facile fluorescence assay for rapid and sensitive detection of uric acid based on carbon dots and MnO2 nanosheets. New J Chem 42:15121–15126. https://doi.org/10.1039/C8NJ02607F
Shi BF, Su YB, Duan Y, Chen SY, Zuo WY (2019) A nanocomposite prepared from copper(II) and nitrogen-doped graphene quantum dots with peroxidase mimicking properties for chemiluminescent determination of uric acid. Mikrochim Acta 186:397. https://doi.org/10.1007/s00604-019-3491-9
Vakh C, Koronkiewicz S, Kalinowski S, Moskvin L, Bulatov A (2017) An automatic chemiluminescence method based on the multi-pumping flow system coupled with the fluidized reactor and direct-injection detector: determination of uric acid in saliva samples. Talanta 167:725–732. https://doi.org/10.1016/j.talanta.2017.02.009
Feng J, Lia Q, Cai JP, Yang T, Chen JJ, Hou XM (2019) Electrochemical detection mechanism of dopamine and uric acid on titanium nitride-reduced graphene oxide composite with and without ascorbic acid. Sens Actuators B 298:126872. https://doi.org/10.1016/j.snb.2019.126872
Guo XR, Yue HY, Song SS, Huang S, Gao X, Chen HT, Wu PF, Zhang T, Wang ZZ (2020) Simultaneous electrochemical determination of dopamine and uric acid based on MoS2 nanoflowers-graphene/ITO electrode. Microchem J 154:104527. https://doi.org/10.1016/j.microc.2019.104527
Zhao JX (2015) Simultaneous determination of plasma creatinine, uric acid, kynurenine and tryptophan by high performance liquid chromatography: method validation and in application to the assessment of renal function. Biomed Chromatogr 03:410–415. https://doi.org/10.1002/bmc.3291
Lu QJ, Deng JH, Hou YX, Wang HY, Li HT, Zhang YY (2015) One-step electrochemical synthesis of ultrathin graphitic carbon nitride nanosheets and their application to the detection of uric acid. Chem Commun 51:12251–12253. https://doi.org/10.1039/C5CC04231C
Qin X, Yuan CL, Geng GX, Shi R, Cheng XQ, Wang YL (2021) Enzyme-free colorimetric determination of uric acid based on inhibition of gold nanorods etching. Sensor Actuat B Chem 333:129638. https://doi.org/10.1016/j.snb.2021.129638
Wang Q, Peng HC, Dong YQ, Chi YW, Fu FF (2018) Colorimetric determination of glutathione by using a nanohybrid composed of manganese dioxide and carbon dots. Microchim Acta 185:291. https://doi.org/10.1007/s00604-018-2830-6
Dai YX, Wang XY, Zhu XD, Liu H, Wang PF, Wang XM, Zhang SH, Sun YL, Gao DD, Han R, Luo CN (2020) Electrochemical assays for determination of H2O2 and prostate-specific antigen based on a nanocomposite consisting of CeO2 nanoparticle-decorated MnO2 nanospheres. Microchim Acta 187:428. https://doi.org/10.1007/s00604-020-04403-7
Sun Y, Gao JL, Chen JY, Yan J, Sun JW, Jun DQ, Hu XY (2020) A near−infrared fluorescent sensor based on the architecture of low−toxic Ag2S quantum dot and MnO2 nanosheet for sensing glutathione in human serum sample. Talanta 221:121475. https://doi.org/10.1016/j.talanta.2020.121475
Sun YF, Xiong PY, Tang J, Zeng ZY, Tang DP (2020) Ultrasensitive split-type electrochemical sensing platform for sensitive determination of organophosphorus pesticides based on MnO2 nanoflower-electron mediator as a signal transduction system. Anal Bioanal Chem 412:6939–6945. https://doi.org/10.1007/s00216-020-02824-0
He LY, Wang FH, Chen Y, Liu YY (2017) Rapid and sensitive colorimetric detection of ascorbic acid in food based on the intrinsic oxidase-like activity of MnO2 nanosheets. Luminescence 7:1–8. https://doi.org/10.1002/bio.3384
Liu J, Meng L, Fei Z, Dyson PJ, Jing X, Liu X (2017) MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens Bioelectron 90:69–74. https://doi.org/10.1016/j.bios.2016.11.046
Su YB, Zhang J, Liu K, Huang Z, Ren XC, Wang CA (2017) Simple synthesis of a double-shell hollow structured MnO2@TiO2 composite as an anode material for lithium ion batteries. RSC Adv 7:46263–46270. https://doi.org/10.1039/C7RA09628C
Liu ZP, Ma RZ, Ebina Y, Takada K, Sasaki T (2007) Synthesis and delamination of layered manganese oxide nanobelts. Chem Mater 19:6504–6512. https://doi.org/10.1021/cm7019203
Kai K, Yoshida Y, Kageyama H, Saito G, Ishigaki T, Furukawa Y, Kawamata J (2008) Room-temperature synthesis of sanganese oxide monosheets. J Am Chem Soc 130:15938–15943. https://doi.org/10.1021/ja804503f
Ragupathy P, Park DH, Campet G, Vasan HN, Hwang S-J, Choy J-H, Munichandraiah N (2009) Remarkable capacity retention of nanostructured manganese oxide upon cycling as an electrode material for supercapacitor. J Phys Chem C 113:6303–6309. https://doi.org/10.1021/jp811407q
Rajabi HR, Sajadiasl F, Karimi H, Alvand ZM (2020) Green synthesis of zinc sulfide nanophotocatalysts using aqueous extract of Ficus Johannis plant for efficient photodegradation of some pollutants. J Mater Res Technol 9(6):15638–15647. https://doi.org/10.1016/j.jmrt.2020.11.017
Hatakeyama T, Okamoto NL, Ichitsubo T (2022) Thermal stability of MnO2 polymorphs. J Solid State Chem 302:122683. https://doi.org/10.1016/j.jssc.2021.122683
Gole A, Murphy CJ (2004) Seed-mediated synthesis of gold nanorods: role of the size and nature of the seed chem. Mater 16:3633–3640. https://doi.org/10.1021/cm0492336
Zhu ZM, Lin XY, Wang LN, Zhao CF, Zheng YJ, Liu AL, Lin LQ, Lin XH (2018) “Switch-On” fluorescent nanosensor based on nitrogen-doped carbon dots-MnO2 nanocomposites for probing the activity of acid phosphatase. Sens Actuators B 274:609–615. https://doi.org/10.1016/j.snb.2018.08.011
He Y, Huang W, Liang Y, Yu HL (2015) A low-cost and label-free assay for hydrazine using MnO2 nanosheets as colorimetric probes. Sens Actuators B 220:927–931. https://doi.org/10.1016/j.snb.2015.06.025
Alvand ZM, Rajabi HR, Mirzaei A, Sajadiasl F (2020) Combination of plant-mediated and sonochemical-assisted synthesis for preparation of low-toxic cadmium selenide semiconductor nanoparticles: study of the effect of extraction techniques, characterization, comparative study of biological activities. Surf Interfaces 25:101182. https://doi.org/10.1016/j.surfin.2021.101182
Funding
This study was financially supported by the National Natural Science Foundation of China (21976029) and the Natural Science Foundation of Fujian Province (2020Y0074, 2022Y0059, 2022J0113).
Author information
Authors and Affiliations
Contributions
Prof. Y. Dong and Prof. F. Fu performed the experimental design, data analysis and interpretation, and manuscript writing. R. Hu, T. Guo, C. Zeng, and X. Fu performed the experiments. Prof. Z. Lin and Dr. B. Dong co-performed data analysis and interpretation. The manuscript was written through contributions of all authors, and all authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supporting information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, R., Guo, T., Zeng, C. et al. Colorimetric and visual determination of uric acid based on decolorization of manganese dioxide nanosheet dispersions. Microchim Acta 190, 217 (2023). https://doi.org/10.1007/s00604-023-05767-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05767-2