Abstract
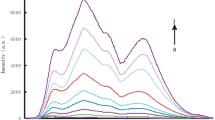
An aptamer based method is described for the determination of 8-hydroxy-2′-deoxyguanosine (8-OHdG) using resonance light scattering (RLS). Magnetic nanoparticles (MNPs) were employed as RLS probes. The probe DNA was placed on the surface of MNPs, which produces a rather low RLS signal. If, however, probe DNA hybridizes with the aptamer against 8-OHdG, a sandwich structure will be formed. This results in a significant enhancement of RLS intensity. The aptamer was used as the recognition element to capture 8-OHdG. 8-OHdG has a stronger affinity for the aptamer than probe DNA, and the conformation of the aptamer therefore switches from a double-stranded to a G-quadruplex structure. As a result, MNPs labeled with probe DNA are released, and RLS intensity decreases. The method allows 8-OHdG to be detected with a linear response in the 32 pM − 12.0 nM concentration range and an 11 pM limit of detection (at 3.29SB/m, according to the recent recommendation of IUPAC). The MNPs can be reused 5 times by applying an external magnetic field for collection. The method was successfully applied to analyze human urine samples for its content of 8-OHdG. It was also found that the levels of 8-OHdG noticeably increased with the increase of the Air Quality Index. Conceivably, the method is a viable tool to investigate the relationship between 8-OHdG levels and the effect of air pollution.

A reusable sensing strategy was constructed to detect urinary 8-OHdG based on “turn-off” resonance light scattering. The LOD was as low as 11 pM. This study showed some preliminary data for the association between oxidative stress and air pollution.



Similar content being viewed by others
References
Floyd RA, West MS, Eneff KL, Schneider JE, Wong PK, Tingey DT, Hogsett WE (1990) Conditions influencing yield and analysis of 8-hydroxy-2′-deoxyguanosine in oxidatively damaged DNA. Anal Biochem 188:155–158. https://doi.org/10.1016/0003-2697(90)90544-J
Wallace SS (2002) Biological consequences of free radical-damaged DNA bases. Free Radic Biol Med 33:1–14. https://doi.org/10.1016/S0891-5849(02)00827-4
Wu LL, Chiou CC, Chang PY, Wu JT (2004) Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta 339:1–9. https://doi.org/10.1016/j.cccn.2003.09.010
Fan RF, Wang DL, Ramage R, She JW (2012) Fast and simultaneous determination of urinary 8-hydroxy-2′-deoxyguanosine and ten monohydroxylated polycyclic aromatic hydrocarbons by liquid chromatography/tandem mass spectrometry. Chem Res Toxicol 25:491–499. https://doi.org/10.1021/tx200517h
Rossner P Jr, Mistry V, Singh R, Sram RJ, Cooke MS (2013) Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine values determined by a modified ELISA improves agreement with HPLC–MS/MS. Biochem Biophys Res Commun 440:725–730. https://doi.org/10.1016/j.bbrc.2013.09.133
Meng XY, Suo XM, Ding WJ, Li XJ, Ding YS (2014) Determination of 8-hydroxy-2′-deoxyguanosine derivatized with 4-chloro-7- nitrobenzofurazan in urine by CE-LIF. Electrophoresis 35:1873–1879. https://doi.org/10.1002/elps.201300650
Guo Z, Liu X, Liu Y, Wu G, Lu X (2016) Constructing anovel8-hydroxy-2′-deoxyguanosine electrochemical sensor and application in evaluating the oxidative damages of DNA and guanine. Biosens Bioelectron 86:671–676. https://doi.org/10.1016/j.bios.2016.07.033
Wang JC, Wang YS, Rang WQ, Xue JH, Zhou B, Liu L, Qian QM, Wang YS, Yin JC (2014) Colorimetric determination of 8-hydroxy-2′-deoxyguanosine using label-free aptamer and unmodified gold nanoparticles. Microchim Acta 181:903–910. https://doi.org/10.1007/s00604-014-1173-1
Changenet-Barret P, Gustavsson T, Improta R, Markovitsi D (2015) Ultrafast excited-state deactivation of 8-hydroxy-2′-deoxyguanosine studied by femtosecond fluorescence spectroscopy and quantum-chemical calculations. J Phys Chem A 119:6131–6139. https://doi.org/10.1021/acs.jpca.5b00688
Wang JC, Wang YS, Xue JH, Zhou B, Qian QM, Wang YS, Yin JC, Zhao H, Liu H, Liu SD (2014) An ultra sensitive label-free assay of 8-hydroxy-2′-deoxyguanosine based on the conformational switching of aptamer. Biosens Bioelectron 58:22–26. https://doi.org/10.1016/j.bios.2014.02.047
Pasternack RF, Collings PJ (1995) Resonance light scattering: a new technique for studying chromophore aggregation. Science 269:935–939. https://doi.org/10.1126/science.7638615
Liu S, Luo H, Li N, Liu Z, Zheng W (2001) Resonance Rayleigh scattering study of the interaction of heparin with some basic diphenyl naphthylmethane dyes. Anal Chem 73:3907–3914. https://doi.org/10.1021/ac001454h
Feng DQ, Liu GL, Zheng WJ, Chen TF, Li D (2013) A new light-scattering sensor forscreening G-quadruplex stabilizers based on DNA-folding-mediated assemblyof gold nanoparticles, J. Mater. Chem. B 1. https://doi.org/10.1039/C3TB20291G
Tang NL, Shan YQ, Zhang RH, Meng XL (2015) Sensitive and simple detection of trace hydrogen peroxide based on a resonance light scattering assay. Anal Methods 7:8750–8756. https://doi.org/10.1039/C5AY01414J
Navarro JRG, Werts MHV (2013) Resonant light scattering spectroscopy of gold, silver and gold-silver alloy nanoparticles and optical detection in microfluidic channels. Analyst 138:583–592. https://doi.org/10.1039/c2an36135c
Bi SY, Wang Y, Zhou HF, Zhao TT (2016) Assembly of AuNRs and eugenol for trace analysis of eugenol using resonance light scattering technique. Mater Sci Eng C 58:1001–1007. https://doi.org/10.1016/j.msec.2015.09.051
Feng D-Q, Chen M, Liu GL, Zhu WJ, Sun WS, Zhu R, Wang W (2015) A novel resonance light scattering sensing for glucose based on theconversion of gold nanoclusters into gold nanoparticles. Sensors Actuators B Chem 219:133–138. https://doi.org/10.1016/j.snb.2015.05.019
Feng D-Q, Liu GL, Wang W (2015) A novel biosensor for copper(II) ions based on turn-on resonance light scattering of ssDNA templated silver nanoclusters. J Mater Chem B 3:2083–2088. https://doi.org/10.1039/C4TB01940G
Zou X, Huang H, Gao Y, Su XG (2012) Detection of avian influenza virus based on magnetic silica nanoparticles resonance light scattering system. Analyst 137:648–653. https://doi.org/10.1039/c1an16041a
Yue QL, Shen TF, Wang JT, Wang L, Xu SL, Li HB, Liu JF (2013) A reusable biosensor for detecting mercury(II) at the subpicomolar level based on "turn-on" resonance light scattering. Chem Commun 49:1750–1752. https://doi.org/10.1016/j.bios.2017.02.024
Hou YN, Liu JF, Hong M, Li X, Ma YH, Yue QL, Li C-Z (2017) A reusable aptasensor of thrombin based on DNA machine employing resonance light scattering technique. Biosens Bioelectron 92:259–265. https://doi.org/10.1016/j.bios.2017.02.024
Bell ML (2012) Assessment of the health impacts of particulate matter characteristics. Res Rep Health Eff Inst 27:5–38 http://www.healtheffects.org/system/files/Bell-161.pdf
Sauvain J-J, Setyan A, Wild P, Tacchini P, Lagger G, Storti F, Deslarzes S, Guillemin M, Rossi MJ (2011) Riediker M, biomarkers of oxidative stress and its association with the urinary reducing capacity in bus maintenance workers. J Occup Med Toxicol 6:18. https://doi.org/10.1186/1745-6673-6-18
Guo C, Li XF, Wang R, Yu JK, Ye MF, Mao LN, Zhang SZ, Zheng S (2016) Association between oxidative DNA damage and risk of colorectal cancer: sensitive determination of urinary 8-hydroxy-2′-deoxyguanosine by UPLC-MS/MS analysis. Sci Rep 6:32581. https://doi.org/10.1038/srep32581
Khalil WKB, Girgis E, Emam AN, Mohamed MB, Rao KV (2011) Genotoxicity evaluation of nanomaterials: DNA damage, micronuclei, and 8-hydroxy-2-deoxyguanosine induced by magnetic doped CdSe quantum dots in male mice. Chem Res Toxicol 24:640–650. https://doi.org/10.1021/tx2000015
Satoh M, Fujimoto S, Horike H, Ozeki M, Nagasu H, Tomita N, Sasaki T, Kashihara N (2011) Mitochondrial damage-induced impairment of angiogenesis in the aging rat kidney, lab. Invest 91:190–202. https://doi.org/10.1038/labinvest.2010.175
Tong XC, Smith LM (1992) Solid-phase method for the purification of DNA sequencing reactions. Anal Chem 64:2672–2677. https://doi.org/10.1021/ac00046a004
Liu YJ, Wei M, Zhang LQ, Zhang YJ, Wei W, Yin LH, Pu YP, Liu SQ (2016) Chiroplasmonic assemblies of gold nanoparticles for ultrasensitive detection of 8-hydroxy-2′-deoxyguanosine in human serum sample. Anal Chem 88:6509–6514. https://doi.org/10.1021/acs.analchem.6b01258
Shang T, Wang P, Liu X, Jiang X, Hu Z, Lu X (2018) Facile synthesis of porous single-walled carbon nanotube for sensitive detection of 8-hydroxy-2′-deoxyguanosine. J Electroanal Chem 808:28–34. https://doi.org/10.1016/j.jelechem.2017.11.064
Hao J, Wu K, Wan C, Tang Y (2018) Reduced graphene oxide-ZnO nanocomposite based electrochemical sensor for sensitive and selective monitoring of 8-hydroxy-2′-deoxyguanosine. Talanta 185:50–556. https://doi.org/10.1016/j.talanta.2018.04.028
Liu H, Wang Y-S, Tang X, Yang H-X, Chen S-H, Zhao H, Liu S-D, Zhu Y-F, Wang X-F, Huang Y-Q (2016) A novel fluorescence aptasensor for 8-hydroxy-2′-deoxyguanosinebased on the conformational switching of K+-stabilized G-quadruplex. J Pharm Biomed Anal 118:177–182. https://doi.org/10.1016/j.jpba.2015.10.035
Gutiérrez A, Osegueda S, Gutiérrez-Granados S, Alatorre A, García MG (2008) Amperometric detection and quantification of 8-hydroxy-2′-deoxyguanosine (8-OHdG) using dendrimermodified electrodes. Electroanalysis 21:2294–2300. https://doi.org/10.1002/elan.200804324
Wu ZS, Guo MM, Shen GL, Yu RQ (2007) G-rich oligonucleotide-functionalized gold nanoparticle aggregation. Anal Bioanal Chem 387:2623–2626. https://doi.org/10.1007/s00216-007-1126-1
Kim JY, Mukherjee S, Ngo LC (2004) Christiani DC, urinary 8-hydroxy-2′-deoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to fine particulates. Environ Health Perspect 112:666–671. https://doi.org/10.1289/ehp.6827
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (91543206), the Natural Science Foundation (ZR2014BQ017, ZR2015BM024, and 2013SJGZ07) and the Tai-Shan Scholar Research Fund of Shandong Province and research foundation of Liaocheng University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 1.21 mb)
Rights and permissions
About this article
Cite this article
Tao, L., Yue, Q., Hou, Y. et al. Resonance light scattering aptasensor for urinary 8-hydroxy-2′-deoxyguanosine based on magnetic nanoparticles: a preliminary study of oxidative stress association with air pollution. Microchim Acta 185, 419 (2018). https://doi.org/10.1007/s00604-018-2937-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2937-9