Abstract
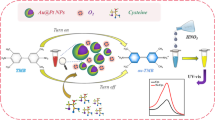
The authors report that the peroxidase-like activity of Au@Pt core-shell nanohybrids (Au@PtNHs) is selectively inhibited by cysteine. This finding has led to a highly sensitive colorimetric assay for cysteine that is based on the nanohybrid-catalyzed oxidation of TMB by H2O2 to form a blue product. The method has a detection limit of 5.0 nM and a linear range from 10 nM to 20 μM. The assay is highly selective over other amino acids. It was successfully applied to the determination of cysteine in an injection containing a mixture of amino acids.

The peroxidase-like activity of Au@Pt core-shell nanohybrids (Au@PtNHs) is selectively inhibited by cysteine, enabling the determination of cysteine.







Similar content being viewed by others
References
Li R, Zhang H, Ling J, Huang C, Wang J (2016) Plasmonic platforms for colorimetric sensing of cysteine. Appl Spectrosc Rev 51:129–147. doi:10.1021/ac010541m
Lunar ML, Rubio S, Pérez-Bendito D, Carreto ML, McLeod CW (1997) Hexadecylpyridinium chloride micelles for the simultaneous kinetic determination of cysteine and cystine by their induction of the iodine-azide reaction. Anal Chim Acta 337:341–349. doi:10.1016/S0003-2670(96)00430-8
Salimi A, Pourbeyram S (2003) Renewable sol–gel carbon ceramic electrodes modified with a Ru-complex for the amperometric detection of l-cysteine and glutathione. Talanta 60:205–214. doi:10.1016/S0039-9140(03)00125-5
Kuśmierek K, Głowacki R, Bald E (2006) Analysis of urine for cysteine, cysteinylglycine, and homocysteine by high-performance liquid chromatography. Anal Bioanal Chem 385:855–860. doi:10.1007/s00216-006-0454-x
Tcherkas YV, Denisenko AD (2001) Simultaneous determination of several amino acids, including homocysteine, cysteine and glutamic acid, in human plasma by isocratic reversed-phase high-performance liquid chromatography with fluorimetric detection. J Chromatogr A 913:309–313. doi:10.1016/S0021-9673(00)01201-2
Amjadi M, Abolghasemi-Fakhri Z, Hallaj T (2015) Carbon dots-silver nanoparticles fluorescence resonance energy transfer system as a novel turn-on fluorescent probe for selective determination of cysteine. J Photochem Photobiol A Chem 309:8–14. doi:10.1016/j.jphotochem.2015.04.016
Chen Z, Lu D, Cai Z, Dong C, Shuang S (2014) Bovine serum albumin-confined silver nanoclusters as fluorometric probe for detection of biothiols. Luminescence 29:722–727. doi:10.1002/bio.2613
Xu X, Qiao J, Li N, Qi L, Zhang S (2015) Fluorescent probe for turn-on sensing of l-cysteine by ensemble of AuNCs and polymer protected AuNPs. Anal Chim Acta 879:97–103. doi:10.1016/j.aca.2015.03.036
Chen S, Gao H, Shen W, Lu C, Yuan Q (2014) Colorimetric detection of cysteine using noncrosslinking aggregation of fluorosurfactant-capped silver nanoparticles. Sensors Actuators B Chem 190:673–678. doi:10.1016/j.snb.2013.09.036
Gao H, Shen W, Lu C, Liang H, Yuan Q (2013) Surface plasmon resonance additivity of gold nanoparticles for colorimetric identification of cysteine and homocysteine in biological fluids. Talanta 115:1–5. doi:10.1016/j.talanta.2013.03.073
Park JI, Nguyen TD, de Queiros SG, Bahng JH, Srivastava S, Zhao G, Sun K, Zhang P, Glotzer SC, Kotov NA (2014) Terminal supraparticle assemblies from similarly charged protein molecules and nanoparticles. Nat Commun 5:3593. doi:10.1038/ncomms4593
Hou XY, Chen S, Tang J, Long YF (2014) Visual determination of trace cysteine based on promoted corrosion of triangular silver nanoplates by sodium thiosulfate. Spectrochim Acta A Mol Biomol Spectrosc 125:285–289. doi:10.1016/j.saa.2014.01.098
Lin X-Q, Deng H-H, Wu G-W, Peng H-P, Liu A-L, Lin X-H, Xia X-H, Chen W (2015) Platinum nanoparticles/graphene-oxide hybrid with excellent peroxidase-like activity and its application for cysteine detection. Analyst 140:5251–5256. doi:10.1039/C5AN00809C
Sun Y, Wang J, Li W, Zhang J, Zhang Y, Fu Y (2015) DNA-stabilized bimetallic nanozyme and its application on colorimetric assay of biothiols. Biosens Bioelectron 74:1038–1046. doi:10.1016/j.bios.2015.08.001
Wei X, Qi L, Tan J, Liu R, Wang F (2010) A colorimetric sensor for determination of cysteine by carboxymethyl cellulose-functionalized gold nanoparticles. Anal Chim Acta 671:80–84. doi:10.1016/j.aca.2010.05.006
Hsiao Y-P, Su W-Y, Cheng J-R, Cheng S-H (2011) Electrochemical determination of cysteine based on conducting polymers/gold nanoparticles hybrid nanocomposites. Electrochim Acta 56:6887–6895. doi:10.1016/j.electacta.2011.06.031
Taei M, Hasanpour F, Salavati H, Banitaba SH, Kazemi F (2016) Simultaneous determination of cysteine, uric acid and tyrosine using Au-nanoparticles/poly(E)-4-(p-tolyldiazenyl)benzene-1,2,3-triol film modified glassy carbon electrode. Mater Sci Eng C 59:120–128. doi:10.1016/j.msec.2015.10.004
Wang L, Tricard S, Yue P, Zhao J, Fang J, Shen W (2016) Polypyrrole and graphene quantum dots @ Prussian blue hybrid film on graphite felt electrodes: application for amperometric determination of l-cysteine. Biosens Bioelectron 77:1112–1118. doi:10.1016/j.bios.2015.10.088
Zhang L, Ning L, Zhang Z, Li S, Yan H, Pang H, Ma H (2015) Fabrication and electrochemical determination of l-cysteine of a composite film based on V-substituted polyoxometalates and Au@2Ag core–shell nanoparticles. Sensors Actuators B Chem 221:28–36. doi:10.1016/j.snb.2015.06.070
Yu X, Wang Q, Liu X, Luo X (2012) A sensitive chemiluminescence method for the determination of cysteine based on silver nanoclusters. Microchim Acta 179:323–328. doi:10.1007/s00604-012-0893-3
Xie J, Zhang X, Wang H, Zheng H, Huang Y, Xie J (2012) Analytical and environmental applications of nanoparticles as enzyme mimetics. TrAC Trends Anal Chem 39:114–129. doi:10.1016/j.trac.2012.03.021
Lien CW, Chen YC, Chang HT, Huang CC (2013) Logical regulation of the enzyme-like activity of gold nanoparticles by using heavy metal ions. Nanoscale 5:8227–8234. doi:10.1039/c3nr01836a
Long YJ, Li YF, Liu Y, Zheng JJ, Tang J, Huang CZ (2011) Visual observation of the mercury-stimulated peroxidase mimetic activity of gold nanoparticles. Chem Commun 47:11939–11941. doi:10.1039/C1CC14294A
Lien CW, Tseng YT, Huang CC, Chang HT (2014) Logic control of enzyme-like gold nanoparticles for selective detection of lead and mercury ions. Anal Chem 86:2065–2072. doi:10.1021/ac4036789
Zhao D, Chen C, Lu L, Yang F, Yang X (2015) A label-free colorimetric sensor for sulfate based on the inhibition of peroxidase-like activity of cysteamine-modified gold nanoparticles. Sensors Actuators B Chem 215:437–444. doi:10.1016/j.snb.2015.04.010
Gao Z, Tang D, Tang D, Niessner R, Knopp D (2015) Target-induced nanocatalyst deactivation facilitated by core@shell nanostructures for signal-amplified headspace-colorimetric assay of dissolved hydrogen sulfide. Anal Chem 87:10153–10160. doi:10.1021/acs.analchem.5b03008
Wang N, Sun J, Chen L, Fan H, Ai S (2015) A Cu2(OH)3Cl-CeO2 nanocomposite with peroxidase-like activity, and its application to the determination of hydrogen peroxide, glucose and cholesterol. Microchim Acta 182:1733–1738. doi:10.1007/s00604-015-1506-8
Xiang Z, Wang Y, Ju P, Zhang D (2016) Optical determination of hydrogen peroxide by exploiting the peroxidase-like activity of AgVO3 nanobelts. Microchim Acta 183:457–463. doi:10.1007/s00604-015-1670-x
Čunderlová V, Hlaváček A, Horňáková V, Peterek M, Němeček D, Hampl A, Eyer L, Skládal P (2016) Catalytic nanocrystalline coordination polymers as an efficient peroxidase mimic for labeling and optical immunoassays. Microchim Acta 183:651–658. doi:10.1007/s00604-015-1697-z
Zhang Y, Lu F, Yan Z, Wu D, Ma H, Du B, Wei Q (2015) Electrochemiluminescence immunosensing strategy based on the use of Au@Ag nanorods as a peroxidase mimic and NH4CoPO4 as a supercapacitive supporter: application to the determination of carcinoembryonic antigen. Microchim Acta 182:1421–1429. doi:10.1007/s00604-015-1473-0
Liang G, Liu X (2015) G-quadruplex based impedimetric 2-hydroxyfluorene biosensor using hemin as a peroxidase enzyme mimic. Microchim Acta 182:2233–2240. doi:10.1007/s00604-015-1565-x
Ni P, Dai H, Wang Y, Sun Y, Shi Y, Hu J, Li Z (2014) Visual detection of melamine based on the peroxidase-like activity enhancement of bare gold nanoparticles. Biosens Bioelectron 60:286–291. doi:10.1016/j.bios.2014.04.029
Gao Z, Xu M, Lu M, Chen G, Tang D (2015) Urchin-like (gold core)@(platinum shell) nanohybrids: a highly efficient peroxidase-mimetic system for in situ amplified colorimetric immunoassay. Biosens Bioelectron 70:194–201. doi:10.1016/j.bios.2015.03.039
Yang Z, Chai Y, Yuan R, Zhuo Y, Li Y, Han J, Liao N (2014) Hollow platinum decorated Fe3O4 nanoparticles as peroxidase mimetic couple with glucose oxidase for pseudobienzyme electrochemical immunosensor. Sensors Actuators B Chem 193:461–466. doi:10.1016/j.snb.2013.11.010
Peng C-F, Pan N, Xie Z-J, Wu L-L (2016) Highly sensitive and selective colorimetric detection of Hg2+ based on the separation of Hg2+ and formation of catalytic DNA–gold nanoparticles. Anal Methods 8:1021–1025. doi:10.1039/c5ay02843d
Chen H, Wang F, Li K, Woo KC, Wang J, Li Q, Sun L-D, Zhang X, Lin H-Q, Yan C-H (2012) Plasmonic percolation: Plasmon-manifested dielectric-to-metal transition. ACS Nano 6:7162–7171. doi:10.1021/nn302220y
Wang F, Shen YR (2006) General properties of local Plasmons in metal nanostructures. Phys Rev Lett 97:206806
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583. doi:10.1038/nnano.2007.260
Ray C, Dutta S, Sarkar S, Sahoo R, Roy A, Pal T (2014) Intrinsic peroxidase-like activity of mesoporous nickel oxide for selective cysteine sensing. J Mater Chem B 2:6097. doi:10.1039/c4tb00968a
Kumara VV, Anthony SP (2014) AuNPs based selective colorimetric sensor for cysteine at wide pH range: investigation of capping molecule structure on the colorimetric sensing and catalytic properties. RSC Adv 4:18467–18472. doi:10.1039/C4RA00345D
Bernardi F, Traverse A, Olivi L, Alves MCM, Morais J (2011) Correlating sulfur reactivity of PtxPd1–x nanoparticles with a bimetallic interaction effect. J Phys Chem C 115:12243–12249. doi:10.1021/jp200182a
Fei S, Chen J, Yao S, Deng G, He D, Kuang Y (2005) Electrochemical behavior of l-cysteine and its detection at carbon nanotube electrode modified with platinum. Anal Biochem 339:29–35. doi:10.1016/j.ab.2005.01.002
Lu C-H, Wang Y-W, Ye S-L, Chen G-N, Yang H-H (2012) Ultrasensitive detection of Cu2+ with the naked eye and application in immunoassays. NPG Asia Mater 4:e10
Wang F, Liu X, Lu C-H, Willner I (2013) Cysteine-mediated aggregation of Au nanoparticles: the development of a H2O2 sensor and oxidase-based biosensors. ACS Nano 7:7278–7286. doi:10.1021/nn402810x
Xiao Q, Shang F, Xu X, Li Q, Lu C, Lin J-M (2011) Specific detection of cysteine and homocysteine in biological fluids by tuning the pH values of fluorosurfactant-stabilized gold colloidal solution. Biosens Bioelectron 30:211–215. doi:10.1016/j.bios.2011.09.013
Oliveira E, Núñez C, Santos HM, Fernández-Lodeiro J, Fernández-Lodeiro A, Capelo JL, Lodeiro C (2015) Revisiting the use of gold and silver functionalised nanoparticles as colorimetric and fluorometric chemosensors for metal ions. Sensors Actuators B Chem 212:297–328. doi:10.1016/j.snb.2015.02.026
Yu C-J, Chen T-H, Jiang J-Y, Tseng W-L (2014) Lysozyme-directed synthesis of platinum nanoclusters as a mimic oxidase. Nanoscale 6:9618–9624. doi:10.1039/C3NR06896J
He W, Liu Y, Yuan J, Yin J-J, Wu X, Hu X, Zhang K, Liu J, Chen C, Ji Y, Guo Y (2011) Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials 32:1139–1147. doi:10.1016/j.biomaterials.2010.09.040
Acknowledgments
The work was supported by the National Natural Science Foundation of China (31371767), the National S&T support program of China (2015BAD17B02) and the Natural Science Foundation of Jiangsu Province (BK20141108).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 506 kb)
Rights and permissions
About this article
Cite this article
Pan, N., Li-Ying, W., Wu, LL. et al. Colorimetric determination of cysteine by exploiting its inhibitory action on the peroxidase-like activity of Au@Pt core-shell nanohybrids. Microchim Acta 184, 65–72 (2017). https://doi.org/10.1007/s00604-016-1981-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1981-6