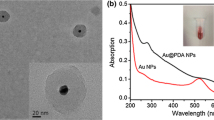
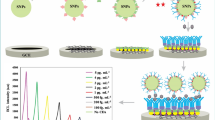
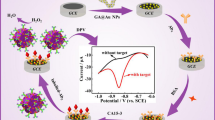
Abstract
The luminol-H2O2 system is widely applied in electrochemiluminescence (ECL) assays but has limited stability. We are presenting an alternative immunosensing strategy by making use of Au@Ag nanorods that mimic the enzyme peroxidase. It also makes use of the supercapacitive supporter NH4CoPO4 as a supporter substrate that facilitates ion movement due to its many nanogaps between the assembled nanoplates. It also plays a vital role for stabilizing the ECL signal of luminol. The immunosensor was constructed by first placing a chitosan film containing NH4CoPO4, Au@Ag and luminol on a glassy carbon electrode (GCE), and the immobilizing anti-CEA on its surface. ECL is generated via electrochemical reaction of luminol on the surface of the Au@Ag-luminol film in the presence of H2O2. The assay was evaluated with respect to effects of pH value, time and temperature of incubation, specificity, reproducibility, and stability in a lab setting. A linear relationship between ECL intensity and CEA concentration is found for the 0.1 pg · mL−1 to 380 ng · mL−1 range, and the lower detection limit is as low as 30 fg · mL−1. In our perception, this immunoassay has a large scope in that numerous other immunoassays will become feasible by using other antibodies and, possibly, aptamers.

We describe an electro-chemiluminescence based immunosensing strategy for CEA based on the use of Au@Ag nanorods as a peroxidase mimic and NH4CoPO4 as a supercapacitive supporter.





Similar content being viewed by others
References
Cao W, Ferrance JP, Demas J, Landers JP (2006) Quenching of the electrochemiluminescence of tris (2, 2′-bipyridine) ruthenium (II) by ferrocene and its potential application to quantitative DNA detection. J Am Chem Soc 128:7572–7578
Hu L, Xu G (2010) Applications and trends in electrochemiluminescence. Chem Soc Rev 39:3275–3304
Miao W (2008) Electrogenerated chemiluminescence and its biorelated applications. Chem Rev 108:2506–2553
Jie G, Liu P, Wang L, Zhang S (2010) Electrochemiluminescence immunosensor based on nanocomposite film of CdS quantum dots-carbon nanotubes combined with gold nanoparticles-chitosan. Electrochem Commun 12:22–26
Taleat Z, Khoshroo A, Mazloum-Ardakani M (2014) Screen-printed electrodes for biosensing: a review (2008–2013). Microchim Acta 181:865–891
Wu M, Yuan D, Xu J, Chen H (2013) Sensitive electrochemiluminescence biosensor based on Au-ITO hybrid bipolar electrode amplification system for cell surface protein detection. Anal Chem 85:11960–11965
Nan Chen G, Zhang L, Er Lin R, Cong Yang Z, Ping Duan J, Qing Chen H, Brynn Hibbert D (2000) The electrogenerated chemiluminescent behavior of hemin and its catalytic activity for the electrogenerated chemiluminescence of lucigenin. Talanta 50:1275–1281
Jiang X, Chai Y, Wang H, Yuan R (2014) Electrochemiluminescence of luminol enhanced by the synergetic catalysis of hemin and silver nanoparticles for sensitive protein detection. Biosens Bioelectron 54:20–26
Joung H, Oh YK, Kim M (2014) An automatic enzyme immunoassay based on a chemiluminescent lateral flow immunosensor. Biosens Bioelectron 53:330–335
Ge L, Wang P, Ge S, Li N, Yu J, Yan M, Huang J (2013) Photoelectrochemical lab-on-paper device based on an integrated paper supercapacitor and internal light source. Anal Chem 85:3961–3970
Xiong B, Xu R, Zhou R, He Y, Yeung ES (2014) Preventing UV induced cell damage by scavenging reactive oxygen species with enzyme-mimic Au–Pt nanocomposites. Talanta 120:262–267
Asati A, Santra S, Kaittanis C, Nath S, Perez JM (2009) Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem Int Ed 48:2308–2312
Shi W, Zhang X, He S, Huang Y (2011) CoFe2O4 magnetic nanoparticles as a peroxidase mimic mediated chemiluminescence for hydrogen peroxide and glucose. Chem Commun 47:10785–10787
Tang Z, Wu H, Zhang Y, Li Z, Lin Y (2011) Enzyme-mimic activity of ferric nano-core residing in ferritin and its biosensing applications. Anal Chem 83:8611–8616
Gui R, Wang Y, Sun J (2014) Protein-stabilized fluorescent nanocrystals consisting of a gold core and a silver shell for detecting the total amount of cysteine and homocysteine. Microchim Acta 181:1231–1238
Cheng Y, Lei J, Chen Y, Ju H (2014) Highly selective detection of microRNA based on distance-dependent electrochemiluminescence resonance energy transfer between CdTe nanocrystals and Au nanoclusters. Biosens Bioelectron 51:431–436
Hou S, Hu X, Wen T, Liu W, Wu X (2013) Core–shell noble metal nanostructures templated by gold nanorods. Adv Mater 25:3857–3862
Won Y, Huh K, Stanciu LA (2011) Au nanospheres and nanorods for enzyme-free electrochemical biosensor applications. Biosens Bioelectron 26:4514–4519
Breedon M, Miura N (2013) Augmenting H2 sensing performance of YSZ-based electrochemical gas sensors via the application of Au mesh and YSZ coating. Sensors Actuators B 182:40–44
Chen X, Wu G, Cai Z, Oyama M, Chen X (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Jayabal S, Ramaraj R (2013) Synthesis of core/shell Au/Ag nanorods embedded in functionalized silicate sol–gel matrix and their applications in electrochemical sensors. Electrochim Acta 88:51–58
Li D, Li J, Jia X, Xia Y, Zhang X, Wang E (2013) A novel Au–Ag–Pt three-electrode microchip sensing platform for chromium (VI) determination. Anal Chim Acta 804:98–103
Wang L, Wang F, Shang L, Zhu C, Ren W, Dong S (2010) AuAg bimetallic nanoparticles film fabricated based on H2O2-mediated silver reduction and its application. Talanta 82:113–117
Wang C, Chen W, Chang H (2012) Enzyme mimics of Au/Ag nanoparticles for fluorescent detection of acetylcholine. Anal Chem 84:9706–9712
Chen L, Zhang Z, Zhang P, Zhang X, Fu A (2011) An ultra-sensitive chemiluminescence immunosensor of carcinoembryonic antigen using HRP-functionalized mesoporous silica nanoparticles as labels. Sensors Actuators B 155:557–561
Dai H, Lin Y, Xu G, Gong L, Yang C, Ma X, Chen G (2012) Cathodic electrochemiluminescence of luminol using polyaniline/ordered mesoporous carbon (CMK-3) hybrid modified electrode for signal amplification. Electrochim Acta 78:508–514
Pang H, Yan Z, Wang W, Chen J, Zhang J, Zheng H (2012) Facile fabrication of NH4CoPO4 center dot H2O nano/microstructures and their primarily application as electrochemical supercapacitor. Nanoscale 4:5946–5953
Zhou J, Tang J, Chen G, Tang D (2014) Layer-by-layer multienzyme assembly for highly sensitive electrochemical immunoassay based on tyramine signal amplification strategy. Biosens Bioelectron 54:323–328
Nikoobakht B, El-Sayed MA (2003) Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater 15:1957–1962
Sau TK, Murphy CJ (2004) Seeded high yield synthesis of short Au nanorods in aqueous solution. Langmuir 20:6414–6420
Liu M, Guyot-Sionnest P (2004) Synthesis and optical characterization of Au/Ag core/shell nanorods. J Phys Chem B 108:5882–5888
Guo X, Zhang Q, Sun Y, Zhao Q, Yang J (2012) Lateral etching of core–shell Au@ metal nanorods to metal-tipped Au nanorods with improved catalytic activity. ACS Nano 6:1165–1175
Dai Y, Cai Y, Zhao Y, Wu D, Liu B, Li R, Yang M, Wei Q, Du B, Li H (2011) Sensitive sandwich electrochemical immunosensor for alpha fetoprotein based on prussian blue modified hydroxyapatite. Biosens Bioelectron 28:112–116
Yu S, Wei Q, Du B, Wu D, Li H, Yan L, Ma H, Zhang Y (2013) Label-free immunosensor for the detection of kanamycin using Ag@Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens Bioelectron 48:224–229
Yang X, Wang Y, Liu Y, Jiang X (2013) A sensitive hydrogen peroxide and glucose biosensor based on gold/silver core–shell nanorods. Electrochim Acta 108:39–44
Ren R, Leng C, Zhang S (2010) Detection of DNA and indirect detection of tumor cells based on circular strand-replacement DNA polymerization on electrode. Chem Commun 46:5758–5760
Cui H, Xu Y, Zhang Z (2004) Multichannel electrochemiluminescence of luminol in neutral and alkaline aqueous solutions on a gold nanoparticle self-assembled electrode. Anal Chem 76:4002–4010
Li F, Cui H (2013) A label-free electrochemiluminescence aptasensor for thrombin based on novel assembly strategy of oligonucleotide and luminol functionalized gold nanoparticles. Biosens Bioelectron 39:261–267
Lin Z, Zhang G, Yang W, Qiu B, Chen G (2012) CEA fluorescence biosensor based on the FRET between polymer dots and Au nanoparticles. Chem Commun 48:9918–9920
Liu N, Feng F, Liu Z, Ma Z (2014) Porous platinum nanoparticles and PdPt nanocages for use in an ultrasensitive immunoelectrode for the simultaneous determination of the tumor markers CEA and AFP. Microchim Acta. doi:10.1007/s00604-014-1435-y
Feng D, Lu X, Dong X, Ling Y, Zhang Y (2013) Label-free electrochemical immunosensor for the carcinoembryonic antigen using a glassy carbon electrode modified with electrodeposited Prussian Blue, a graphene and carbon nanotube assembly and an antibody immobilized on gold nanoparticles. Microchim Acta 180:767–774
Ge L, Yan J, Song X, Yan M, Ge S, Yu J (2012) Three-dimensional paper-based electrochemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials 33:1024–1031
Acknowledgments
This study was supported by the Natural Science Foundation of China (No.21375047), the Natural Science Foundation of Shandong Province (No.ZR2013BL003) and the Natural Science Foundation of UJN (No. XKY1305). QW thanks the Special Foundation for Taishan Scholar Professorship of Shandong Province and UJN (No. TS20130937). All of the authors express their deep thanks.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 807 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Lu, F., Yan, Z. et al. Electrochemiluminescence immunosensing strategy based on the use of Au@Ag nanorods as a peroxidase mimic and NH4CoPO4 as a supercapacitive supporter: Application to the determination of carcinoembryonic antigen. Microchim Acta 182, 1421–1429 (2015). https://doi.org/10.1007/s00604-015-1473-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1473-0