Abstract
Background
Lumbar spinal stenosis is a common disease in the aging population. Decompression surgery represents the treatment standard, however, a risk of segmental destabilization depending on the approach and extent of decompression is discussed. So far, biomechanical studies on techniques were mainly conducted on non-degenerated specimens. This biomechanical in vitro study aimed to investigate the increase in segmental range of motion (ROM) depending on the extent of decompression in degenerated segments.
Methods
Ten fresh frozen lumbar specimens were embedded in polymethyl methacrylate (PMMA) and loaded in a spine tester with pure moments of ± 7.5 Nm. The specimens were tested in their intact state for lateral bending (LB), flexion/extension (FE) and axial rotation (AR). Subsequently, four different decompression techniques were performed: unilateral interlaminar decompression (DC1), unilateral with "over the top" decompression (DC2), bilateral interlaminar decompression (DC3) and laminectomy (DC4). The ROM of the index segment was reported as percent (%) of the native state.
Results
Specimens were measured in their intact state prior to decompression. The mean ROM was defined as 100% (FE:6.3 ± 2.3°; LB:5.4 ± 2.8°; AR:3.0 ± 1.6°). Interventions showed a continuous ROM increase: FE (DC1: + 4% ± 4.3; DC2: + 4% ± 4.5; DC3: + 8% ± 8.3;DC4: + 20% ± 15.9), LB(DC1: + 4% ± 6.0; DC2: + 5% ± 7.3; DC3: + 8% ± 8.3; DC4: + 11% ± 9.9), AR (DC1: + 7% ± 6.0; DC2: + 9% ± 7.9; DC3: + 15% ± 11.5; DC4: + 19% ± 10.5). Significant increases in ROM for all motion directions (p < 0.05) were only obtained after complete laminectomy (DC4).
Conclusion
Unilateral and/or bilateral decompressive surgery resulted in a statistically insignificant ROM increase, whereas complete laminectomy showed statistically significant ROM increase. If this ROM increase also has an impact on the clinical outcome and how to identify segments at risk for secondary lumbar instability should be evaluated in further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Lumbar spinal stenosis (LSS) is a common disease of the aging population and therefore implies a growing socioeconomic importance. [1, 2] Typical symptoms include low back pain, radiating pain and neurogenic claudication with significant impact on mobility and quality of life. LSS often is a result of multifactorial degenerative changes. Those degenerations of the intervertebral discs, facet joints and surrounding soft tissue lead to narrowing of the spinal and/ or the intervertebral foramina. Treatment options include conservative management, as well as the surgical approach, primarily aiming to decompress neural structures. Previous research clearly demonstrated the superiority of surgical over conservative treatment. [3,4,5] Regarding the surgical management, a decompression of the LSS is known to be the standard procedure. [6] The interlaminar approach is the most commonly used technique in case of a unilateral central or lateral recess stenosis. This approach allows a laminotomy to resect the flava ligaments by preserving as much as possible of the lateral facet joint. In case of a bilateral central or lateral recess stenosis, a unilateral interlaminar approach with “over the top” decompression or a bilateral interlaminar approach might be mandatory. [7] In special cases, laminectomies with additional bilateral facetectomies of the affected segments may also be an option.
From a surgical point of view, the resection margin is increasing from a unilateral to a bilateral interlaminar decompression and reaches the greatest extend for a laminectomy. Extensive decompression of neural structures carries some well reported disadvantages, such as extensive muscle trauma and the risk for secondary lumbar instability (SLI), contributing a poor outcome in up to 50% of patients. [7, 8] So far, biomechanical studies on decompression techniques were mainly conducted on non-degenerated specimens. [9,10,11,12].
In the past, lumbar laminectomy was described as the gold standard. Currently, however, unilateral or bilateral decompression and the ultimately established unilateral decompression with undercutting from the ipsilateral approach-related side to the contralateral side are considered as the gold standard due to the preservation of the dorsal midline structures. [13] This biomechanical in vitro study aimed to investigate the increase in segmental range of motion (ROM) depending on the extent of a decompression in degenerated segments and not the effect of degeneration on the segmental ROM, which was investigated in former studies [14,15,16]. The hypothesis of the study was that a unilateral decompression and ipsilateral plus over the top decompression does not cause a significant increase in ROM in degenerated segments, while a laminectomy results in a significant increase in ROM of degenerated segments. Additionally, we wanted to observe, if in some specimens, a decompression causes an abnormal ROM increase.
Material and methods
Specimens
For this study from a pool of 24 fresh frozen human lumbar spines, twelve specimens with at least one degenerated segment indicated for decompression surgery were selected by an experienced spinal surgeon (PK). During testing, two specimens developed fractures (one spinous process fracture and one sacrum fracture) and had to be excluded. The remaining ten specimens for data evaluation had a mean age of 73 (± 7.7 years and a male-to-female ratio of 4:1) (Table 1). Each specimen was composed of four vertebral bodies and three intervertebral discs. A preoperative quantitative Computed Tomography (qCT) scan (General Electrics, Lightspeed VCT 16) was used to evaluate and quantify the progress of degeneration according to Weishaupt et al. [17]. (Table 2) The segment to be decompressed was chosen according to its grade of degeneration. In case of multilevel degeneration, the level with the highest grade of degeneration was chosen as index level to be decompressed and if possible, specimen length was adapted to allow for a cranial and caudal adjacent segment (Table 1). Specimens were kept frozen and vacuum sealed in plastic bags. For preparation, specimens were thawed overnight at 6 ℃ and all soft tissue was removed, whereas ligaments, joint capsules and other supporting tissue were preserved. The upper half of the cranial vertebra and the lower half of the caudal vertebra were embedded in polymethyl methacrylate (PMMA) (Technovit 3040, Heraeus Kulzer GmbH, Wehrheim, Germany). Flanges to mount the specimens in the spine simulator were affixed at the cured PMMA cranially and caudally. At the ventral side of the vertebrae, a motion analysis system (Winbiomechanics, Zebris, Isny, Germany) was fixed to measure the segmental ROM in a six degree of freedom spine tester.
Surgical technique
Four different extents of a decompression with increasing bone removal as well as ligamentous structures were performed. After the fourth decompression, the decompressed segment was stabilized with a posterior pedicle screw system (Verticale, Silony Medical, Germany; 6.2 × 45 mm or 6.25 × 50 mm screws and 5,5 mm titanium rods).The four decompressions encompassed the following structures and were carried out with standard surgical instruments in the hands of trained neurosurgeons (SL, SH).
Mild decompression (DC1)
The interlaminar window was unilaterally enlarged by drilling the cranial and caudal lamina in a crescent shape. The yellow ligament was bluntly dissected at its cranial transition zone was removed in a craniocaudal technique. The lateral bony decompression including the facet joint was kept as small as possible to minimize the risk for iatrogenic instability according to the normal decompression procedure in a “real-life scenario”. The decompression was accomplished as the ipsilateral traversing nerve root was fully visible and no recessal compression was identified. (Fig. 1; DC1).
Medium decompression (DC2)
Starting from the aforementioned unilateral approach, the operating angle was adjusted, and the spinous process was undercut with a high speed drill. An “over the top” decompression according to the proposed technique of Spetzger et al. [18] was performed by drilling the contralateral lamina until the contralateral transition zone of the yellow ligament could be identified. The contralateral ligament was left intact, so that a subflaval recessal decompression with preservation of the contralateral facet joint was accomplished. (Fig. 1; DC2).
Large decompression (DC3)
The contralateral interlaminar window was enlarged by drilling the remaining laminae in a crescent shape. The yellow ligamentum was bluntly dissected and removed in the same fashion as on contralateral side. The lateral bony structures were spared as much as possible, until the traversing nerve root was fully identified and no compression of the dural sac or the traversing nerve root was present. The interspinous ligament was preserved during the hole procedure. (Fig. 1; DC3).
Full decompression (DC4)
The spinous process was removed with a Luer bone rongeur, and the remaining parts of the adjacent cranial and caudal laminae as well as remaining soft tissue were removed with a Kerrison punch, so that a one-level laminectomy with an increased craniocaudal undercutting was performed. (Fig. 1; DC4).
Biomechanical testing
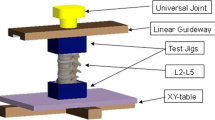
The order of experimental steps can be seen in Table 3. Range of motion (ROM)/flexibility tests were carried out in a six degrees of freedom spine tester (Fig. 2) in flexion/extension (FE), lateral bending (LB) and axial rotation (AR) with pure bending moments of ± 7.5 Nm according to the recommendation for testing of spinal implants. [18] Due to the experimental setup with pure moment loading, we did not apply an additional shear loading and therefore did not measure translations. The spine tester is equipped with a six-component load cell with feedback control and is connected to a stepper motor to control the loading of the specimens with pure moments. The intersegmental motion was measured using an ultrasound-based 3D motion analysis system (Winbiomechanics, Zebris, Isny, Germany) affixed to the ventral side of the vertebral bodies of the decompressed and adjacent segment. All flexibility tests were carried out at room temperature and the specimens were kept moist for the duration of the testing.
Exemplary picture of the spine tester with 6° of freedom; three in translation (green) and three in rotation (yellow). With a stepper motor and cable cords, the bending moment (red) can be applied to the specimens. A 6-component load cell measures the deformation during motion, and the sensors of the 3D motion analysis system are mounted on the ventral side of the PMMA blocks
Statistics and data evaluation
ROM was evaluated from each tested state (intact, DC 1–4, instrumented) from the hysteresis curves. Values are expressed by mean ± standard deviation (SD). The Kolmogorov–Smirnov test was used for testing normal distribution. The unpaired Student's t test was performed to analyze differences in characteristics and variables. Furthermore, a paired t test was performed to analyze for differences between the native state and sequential decompressions. A Bonferroni correction was made to correct for multiple testing. A p value < 0.05 was considered as statistically significant. All statistical evaluations were performed with SPSS Version 21.0 (IBM Corp. Released 2012. IBM SPSS Statistics for Mac OS X, Version 21.0, NY: IBM Corp.). Figures and tables were designed using Microsoft Excel (Version 15.36 for Mac OS X, Microsoft Corporation 2017, Redmond, USA).
Results
Specimens
The radiographic grading of the individual degeneration level revealed grade 1 in 20% (n = 2) and grade 2 degeneration in 50% of used specimens (n = 5), whereas 30% (n = 3) showed grade 3 degeneration (Table 1).
Biomechanical testing
ROM of the decompressed segment in LB
The means and standard deviations (mean ± SD) for ROM in LB (° degree) for the intact state, DC1, DC2, DC3, DC4 and posterior instrumentation (PI) were 5.4 ± 2.8°, 5.6 ± 2.9°, 5.7 ± 2.9°, 5.7 ± 2.9°, 5.9 ± 2.9° and 1.7 ± 0.9°, respectively. The increase of means and SD for normalized ROM in LB compared to the intact state (100%) was + 4 ± 6.0%, + 5 ± 7.3%, + 8 ± 8.3%, + 11 ± 9.9% and − 61 ± 23.1% for DC1, DC2, DC3, DC4 and PI, respectively (Fig. 3).
ROM of the decompressed segment in FE
The means and standard deviations (mean ± SD) for ROM in FE (°) for the native state, DC1, DC2, DC3, DC4 and PI were 6.3 ± 2.2°, 6.6 ± 2.3°, 6.6 ± 2.4°, 6.9 ± 2.5°, 7.4 ± 2.9° and 1.6 ± 0.7°, respectively. The mean normalized ROM and SD increase in FE (%) in respect to the native state was + 4 ± 4.3%, + 4 ± 4.5%, + 8 ± 8.3%, + 20 ± 15.9% and − 71 ± 12.8% for DC1, DC2, DC3, DC4 and PI, respectively (Fig. 4).
ROM of the decompressed segment in AR
The means and standard deviations (mean ± SD) for ROM in AR (°) for the native state, DC1, DC2, DC3, DC4 and PI were 3.0 ± 1.6°, 3.1 ± 1.7°, 3.2 ± 1.7°, 3.4 ± 1.8°, 3.5 ± 1.8° and 1.2 ± 0.6°, respectively. The mean normalized ROM and SD increase in AR (%) in respect to the native state was + 7 ± 6.0%, + 9 ± 7.9%, + 15 ± 11.5%, + 19 ± 10.5% and − 54 ± 21.1% for DC1, DC2, DC3, DC4 and PI, respectively (Fig. 5).
Compared to the intact state, a clear increase in the normalized ROM was measured after DC4 for all motion directions (Table 4). This was especially true for two specimens (S05 and S06) showing a mean increase of ROM of 34% after DC3 and 36% after DC4 in AR, while the mean increase of ROM in AR after DC4 of the remaining specimens accounted for 14% (S01:15%, S02:17%, S03:17%, S04:11%, S07:25%, S08:7%, S09:18%, S10:8%).
Discussion
We hereby report the results of an in vitro biomechanical investigation, assessing the ROM increase with progressive states of lumbar decompression. The authors' hypothesis prior to this study proved to be true, showing no significant clinical difference between decompressions DC1-3. However, resection and loss of dorsal tethering (DC4) showed a clinically significant increase of ROM. In this biomechanical in vitro study, solely degenerated lumbar spine segments were selected to investigate the effect of decompression surgery as equivalent as possible. Test results showed a slow, but progressive increase of ROM according to the extent of decompression for lumbar spinal stenosis.
LSS constitutes a treatment requiring disease, especially in the elderly population. [20] Regarding the surgical management, there are still controversial opinions, and a decompression of the LSS was known to be the standard procedure. [6] In order to avoid the commonly reported secondary lumbar instability, additional fusion was recommended, and numbers of complex spinal procedures were rising. [21,22,23,24] To overcome the disadvantages of extensive laminectomy procedures, less invasive microsurgical decompression techniques have been developed. [7] However, reoperation and secondary fusion rates are still high, and especially perioperative surgical risk factors for detecting instability need to be identified to guarantee the administration of the most promising treatment strategy.
The results of our biomechanical in vitro investigation, conducted on degenerated lumbar spines, were able to demonstrate that the unilateral decompression only showed minimal increases in LB (+ 4%), FE (+ 4%) and AR (+ 7%) compared to the intact state and therefore does not seem to have significant clinical impact on the development of instability of the lumbar spine (p > 0.05). Interestingly, the same accounts for a unilateral approach with a contralateral “over the top decompression”, as changes in all dimensions were very small as well. Mean increases of the ROM in all dimensions were still less than 10% (p > 0.05). Those results coincide with previous studies, showing no significant increase of instability following unilateral approaches with contralateral “over the top” decompression. [25] With further amount of decompression and the likelihood of promoting instability in case of a bilateral approach with bilateral interlaminar decompression, the ROM further increased, showing statistically significant trends (p < 0.05). Nevertheless, when compared to the intact condition, the ROM increased less than 10% in LB and FE about 15% in AR, which should not have any clinical impact, as absolute values failed to exceed an increase + 0.5°. Previous studies reported similar results when biomechanically assessing unilateral versus bilateral laminotomies [26].
Regarding the selection of specimens, a model representing the population suffering from LSS was chosen. Mean age was 73 years and male-to-female ratio was 4:1. [27] Index levels were distributed relatively balanced between L3 and S1 as described in the results section. Still, an influence of age, gender and index level on degeneration as well as potential instability cannot be ignored, as for example, female gender and age < 70 years are known risk factors for the development of SLI. [28,29,30] Also, due to anatomical reasons and facet joint alignment, different index levels in the lower lumbar spine might show different risk for an increase of ROM and therefore for the development of SLI. Furthermore, other patient related factors, i.e., the influence of body weight, could not be considered in this experimental performance. Despite these factors, our study aimed to analyze the overall risk of ROM increment due to decompressive surgery and identification of specifically more vulnerable individuals.
Nevertheless, the fact, that our model does not consider enhanced muscle trauma due to the in vitro testing setup, cannot be disregarded. It is widely known, that paraspinal muscles, and therefore also their impairment, show a significant impact on the development of SLI. [31] When carrying the decompression to the maximum extent, meaning a full laminectomy, ROM measurements showed statistically significant increases compared to the intact specimen. In LB measurements increased about 10%, which may be clinically negligible, but leading to approximately 20% in FE and AR (p < 0.05). These findings agree with previous study results, showing the highest risk for the development of lumbar instability after a complete laminectomy [32, 33].
SLI represents a widely known problem, following decompressive surgery for LSS. In the last decade, a vast array of clinical, radiological and technical factors, potentially influencing stability, have been evaluated. [31, 34, 35] Still, to date, no clear consensus in determination and definition of a possible instability as well as possible risk factors for development of SLI has been achieved. In our measurement series, two specimens showed a marked increase in normalized ROM in axial rotation, which might be a risk factor for the development of SLI. Reasons for that are numerous but may include individual destabilization of decompressed segments independent from gender, level or degeneration grade due to other anatomical or biomechanical parameters assuming after these analyses. Nevertheless, these are a very important results for further studies, as we could attest the individual risk of destabilization for specific patients. Intraoperative measurement and detection of patients with a reduced resistance in axial rotation after decompression surgery might assist to identify patients who could possibly benefit from additional stabilization after decompression. Therefore, there might be a potential for a device to intraoperatively measure the flexibility in axial rotation before and after decompression to identify patients with the need for an additional instrumentation.
The ROM of the adjacent motion segments was also measured as standard procedure for testing. However, it was not reported, as it was not significantly affected by the amount of decompression. This is due to the setup of the testing with pure moment loading recommended for testing of spinal specimens [19]. By definition of pure moment loading, each motion segment is loaded with the same bending moment. If a segment is not manipulated, the ROM of adjacent segments will remain the same. If multisegmental ligamentous structures are resected, the ROM of adjacent segments may show small changes. Therefore, loading spinal specimens with pure moment loading does not give any information on ROM changes of adjacent segments. Pure moment testing does not fully reflect the physiological loading of the spine with external loads and muscle forces in patients. However, it is a well standardized load protocol to investigate the effect a surgical treatment/intervention on the ROM of a treated functional spine unit. Limitations include the above-mentioned issues as well as the solely experimental setting lacking physiological influence such as paraspinal muscles, varying bodyweight and gender. Therefore, prior to translating these results directly in to clinical practice, it should be considered that the results of this experimental and biomechanical study were derived from standardized pure moment loading without additional muscle forces.
To the best of our knowledge, this is the first biomechanical in vitro study using preselected degenerated lumbar spines with radiographic indications for surgical decompression to assess the ROM changes after sequential decompression. Due to advances in surgical techniques in the last years, involving minimally invasive approaches and microsurgery, intraoperative destabilization can be kept limited [36, 37].
Conclusion
Unilateral as well as bilateral decompressive surgery only shows a mild increase in ROM and therefore likely presents a safe strategy to treat LSS. Nevertheless, after laminectomy, the ROM increase was most pronounced and statistically significant. If this statistically significant, increase in ROM has also an impact on the clinical outcome and how to identify segments at risk for SLI should be evaluated in further studies.
References
Abbas J, Hamoud K, May H, Peled N, Sarig R, Stein D et al (2013) Socioeconomic and physical characteristics of individuals with degenerative lumbar spinal stenosis. Spine 38(9):E554–E561
Schizas C, Schmit A, Schizas A, Becce F, Kulik G, Pierzchała K (2014) Secular changes of spinal canal dimensions in Western Switzerland: a narrowing epidemic? Spine 39(17):1339–1344
Atlas SJ, Keller RB, Robson D, Deyo RA, Singer DE (2000) Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the Maine Lumbar Spine study. Spine 25(5):556–562
Jarrett MS, Orlando JF, Grimmer-Somers K (2012) The effectiveness of land based exercise compared to decompressive surgery in the management of lumbar spinal-canal stenosis: a systematic review. BMC Musculoskelet Disord 13:30
Weinstein JN, Tosteson TD, Lurie JD, Tosteson ANA, Blood E, Hanscom B et al (2008) (1976) Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 358(8):794–810
Rosenberg NJ (1976) Degenerative spondylolisthesis: surgical treatment. Clin Orthop Relat Res 117:112–120
Thomé C, Zevgaridis D, Leheta O, Bäzner H, Pöckler-Schöniger C, Wöhrle J et al (2005) Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine 3(2):129–141
Postacchini F, Cinotti G, Perugia D, Gumina S (1993) The surgical treatment of central lumbar stenosis. Multiple laminotomy compared with total laminectomy. J Bone Joint Surg 75(3):386–392
Quint U, Wilke HJ, Löer F, Claes LE (1998) Funktionelle Folgen operativer Dekompressionen am lumbalen Bewegungssegment–eine biomechanische Studie in vitro [Functional sequelae of surgical decompression of the lumbar spine–a biomechanical study in vitro]. Z Orthop Ihre Grenzgeb 136(4):350–357
Delank KS, Gercek E, Kuhn S, Hartmann F, Hely H, Röllinghoff M et al (2010) How does spinal canal decompression and dorsal stabilization affect segmental mobility? A biomechanical study. Arch Orthop Trauma Surg 130(2):285–292
Hartmann F, Janssen C, Böhm S, Hely H, Rommens PM, Gercek E (2012) Biomechanical effect of graded minimal-invasive decompression procedures on lumbar spinal stability. Arch Orthop Trauma Surg 132(9):1233–1239
Costa F, Ottardi C, Volkheimer D, Ortolina A, Bassani T, Wilke HJ, Galbusera F (2018) Bone-preserving decompression procedures have a minor effect on the flexibility of the lumbar spine. J Korean Neurosurg Soc 61(6):680–688
Zhang C, Chen L, Li J, Huang D, Zhang W, Lin J (2021) Should posterior midline structures be preserved in decompression surgery for lumbar spinal stenosis? A systematic review and meta-analysis. Clin Spine Surg. https://doi.org/10.1097/BSD.0000000000001268
Kettler A, Rohlmann F, Ring C, Mack C, Wilke HJ (2011) Do early stages of lumbar intervertebral disc degeneration really cause instability? Evaluation of an in vitro database. Eur Spine J 20(4):578–584 (Epub 2010 Dec 2)
Galbusera F, Niemeyer F, Tao Y, Cina A, Sconfienza LM, Kienle A, Wilke HJ (2021) ISSLS prize in bioengineering science 2021: in vivo sagittal motion of the lumbar spine in low back pain patients-a radiological big data study. Eur Spine J 30(5):1108–1116 (Epub 2021 Jan 21)
Mimura M, Panjabi MM, Oxland TR, Crisco JJ, Yamamoto I, Vasavada A (1994) Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine 19(12):1371–1380
Weishaupt D, Zanetti M, Boos N, Hodler J (1999) MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol 28(4):215–219
Spetzger U, Bertalanffy H, Reinges MH, Gilsbach JM (1997) Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis Part II: clinical experiences. Acta Neurochir 139(5):397–403
Wilke HJ, Wenger K, Claes L (1998) Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur spine J 7(2):148–154
Kitab S, Habboub G, Abdulkareem SB, Alimidhatti MB, Benzel E (2019) Redefining lumbar spinal stenosis as a developmental syndrome: does age matter? J Neurosurg Spine 31(3):357–365
Herkowitz HN, Kurz LT (1991) Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 73(6):802–808
Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C (1993) The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord 6(6):461–472
Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG (2010) Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 303(13):1259–1265
Kepler CK, Vaccaro AR, Hilibrand AS, Anderson DG, Rihn JA, Albert TJ et al (2014) National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine 39(19):1584–1589
Smith ZA, Vastardis GA, Carandang G, Havey RM, Hannon S, Dahdaleh N et al (2014) Biomechanical effects of a unilateral approach to minimally invasive lumbar decompression. PLoS ONE 9(3):e92611
Ho Y-H, Tu Y-K, Hsiao C-K, Chang C-H (2015) Outcomes after minimally invasive lumbar decompression: a biomechanical comparison of unilateral and bilateral laminotomies. BMC Musculoskelet Disord 16:208
Ishimoto Y, Yoshimura N, Muraki S, Yamada H, Nagata K, Hashizume H et al (2012) Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: the wakayama spine study. Osteoarthritis Cartilage 20(10):1103–1108 (Epub 2012 Jul 10)
Urakawa H, Jones T, Samuel A, Vaishnav AS, Othman Y, Virk S et al (2020) The necessity and risk factors of subsequent fusion after decompression alone for lumbar spinal stenosis with lumbar spondylolisthesis: 5 years follow-up in two different large populations. Spine J 20(10):1566–1572
Hopp E, Tsou PM (1988) Postdecompression lumbar instability. Clin Orthop Relat Res 227:143–151
Johnsson KE, Redlund-Johnell I, Udén A, Willner S (1989) Preoperative and postoperative instability in lumbar spinal stenosis. Spine 14(6):591–593
Kalichman L, Suri P, Guermazi A, Li L, Hunter DJ (2009) Facet orientation and tropism: associations with facet joint osteoarthritis and degeneratives. Spine 34(16):E579–E585
Bisschop A, van Engelen SJPM, Kingma I, Holewijn RM, Stadhouder A, van der Veen AJ et al (2014) Single level lumbar laminectomy alters segmental biomechanical behavior without affecting adjacent segments. Clin Biomech 29(8):912
Lee KK, Teo EC (2004) Effects of laminectomy and facetectomy on the stability of the lumbar motion segment. Med Eng Phys 26(3):183–192
Lattig F, Fekete TF, Grob D, Kleinstück FS, Jeszenszky D, Mannion AF (2012) Lumbar facet joint effusion in MRI: a sign of instability in degenerative spondylolisthesis? Eur spine J 21(2):276–281
Simmonds AM, Rampersaud YR, Dvorak MF, Dea N, Melnyk AD, Fisher CG (2015) Defining the inherent stability of degenerative spondylolisthesis: a systematic review. J Neurosurg Spine 23(2):178–189
Denis DR, Hirt D, Shah S, Lu DC, Holly LT (2016) Minimally invasive surgery for lumbar synovial cysts with coexisting degenerative spondylolisthesis. Int J spine Surg 10:37
Hasan S, Härtl R, Hofstetter CP (2019) The benefit zone of full-endoscopic spine surgery. J spine Surg 5(Suppl 1):S41-56
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lener, S., Schmölz, W., Abramovic, A. et al. The effect of various options for decompression of degenerated lumbar spine motion segments on the range of motion: a biomechanical in vitro study. Eur Spine J 32, 1358–1366 (2023). https://doi.org/10.1007/s00586-023-07587-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07587-7