Abstract
Purpose
The aim of this study was to elucidate segmental range of motion (ROM) before and after common decompression and fusion procedures on the lumbar spine.
Methods
ROM of fourteen fresh-frozen human cadaver lumbar segments (L1/2: 4, L3/4: 5, L5/S1: 5) was evaluated in six loading directions: flexion/extension (FE), lateral bending (LB), lateral shear (LS), anterior shear (AS), axial rotation (AR), and axial compression/distraction (AC). ROM was tested with and without posterior instrumentation under the following conditions: 1) native 2) after unilateral laminotomy, 3) after midline decompression, and 4) after nucleotomy.
Results
Median native ROM was FE 6.8°, LB 5.6°, and AR 1.7°, AS 1.8 mm, LS 1.4 mm, AC 0.3 mm. Unilateral laminotomy significantly increased ROM by 6% (FE), 3% (LB), 12% (AR), 11% (AS), and 8% (LS). Midline decompression significantly increased these numbers to 15%, 5%, 21%, 20%, and 19%, respectively. Nucleotomy further increased ROM in all directions, most substantially in AC of 153%. Pedicle screw fixation led to ROM decreases of 82% in FE, 72% in LB, 42% in AR, 31% in AS, and 17% in LS. In instrumented segments, decompression only irrelevantly affected ROM.
Conclusions
The amount of posterior decompression significantly impacts ROM of the lumbar spine. The here performed biomechanical study allows creation of a simplified rule of thumb: Increases in segmental ROM of approximately 10%, 20%, and 50% can be expected after unilateral laminotomy, midline decompression, and nucleotomy, respectively. Instrumentation decreases ROM by approximately 80% in bending moments and accompanied decompression procedures only minorly destabilize the instrumentation construct.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degenerative disease of the lumbar spine, which typically presents as low back, gluteal, and leg pain, is an ever-increasing burden for the health care system worldwide [1, 2]. The most common conditions leading to surgery are spinal stenosis and spondylolisthesis, and the numbers of surgical procedures performed for these conditions have increased steadily over the past decades [2,3,4]. The two mainstays of surgery are decompression of neural structures alone and decompression with spinal instrumentation and fusion.[5, 6].
Discussions about which patient should undergo decompression at all and to what extent and in whom a fusion should be attempted are as old as the treatment options themselves [7]. In the early era of spine surgery, nucleotomy, or removal of parts of the intervertebral disks, was considered to lead to fusion through the degenerated disk over time, but many studies have reported more reliable results with posterior spinal instrumentation and interbody fusion [7, 8]. Whereas fusion surgery inherits the disadvantages of implant-related infections, painful pseudarthrosis, screw loosening, and adjacent segment disease, decompression alone may provoke spinal instability and lead to rapid advancement of degenerative processes, inadequate spinal balance, and undesirable clinical outcomes. The modern literature continues to report conflicting results regarding whether patients with spinal canal stenosis and degenerative spondylolisthesis should undergo decompression alone or decompression and instrumentation, and the topic still provokes heated debates [9,10,11,12,13,14,15,16].
A recent meta-analysis by Lang et al. [17] found similar revision rates after decompression alone and decompression with instrumentation with rates of around 20% within 5 years in both groups, but the indications for revision differed. While the reasons for revision after decompression surgery alone were recurrent stenosis, further degeneration, spinal instability, and other forms of same segment diseases in 52–100% of the cases [11, 18]; the most common reason for revision after decompression and fusion surgery was adjacent segment disease with proportion ranging from 42 to 100% [11, 19]. Iatrogenic spinal instability after spinal surgery has been described as driver for further degeneration in both same segment disease after decompression and adjacent segment disease after instrumentation [10, 20]. Although spinal instability is most commonly reported as excessive gain of ROM beyond 8°–15° for angular motion and 3–4.5 mm for translation, we believe that already smaller amounts of ROM increase by decompression surgery may play a role in further segment degeneration [20]. This assumption is for example backed by the study of Kim et al. [21], who found higher reoperation rates for more extensive decompression techniques, with rates of 19%, 14%, and 12% within 5 years after laminectomy, open discectomy, and endoscopic discectomy, respectively.
While important efforts were made to study clinical outcomes after decompression and instrumentation techniques, the biomechanical understanding of spinal motion after these procedures is still fairly limited. In our opinion, understanding iatrogenic alterations in biomechanical behavior caused by surgery are likely to be as important as detailed anatomic knowledge and other patient individual factors to achieve successful clinical outcomes. The purpose of this study was to comprehensively elucidate segmental range of motion before and after ascending extents of decompression procedures and posterior instrumentation on the lumbar spine [10, 20]. Because the most commonly performed decompression techniques for disk herniation and spinal stenosis in our tertiary spine center are unilateral laminotomy, midline decompression and nucleotomy, we aimed to biomechanically investigate these surgical interventions with and without pedicle screw instrumentation. We hypothesized that, segmental motion increases depending on the amount of structural harm of the segment by decompression, and that posterior instrumentation decreases segmental motion.
Methods
Specimen preparation
Fourteen fresh-frozen lumbar segments (L1/2: 4, L3/4: 5, L5/S1: 5) from 11 human cadavers were procured from Science Care (Phoenix, AZ, USA). The median age of the cadavers was 59 years (range 50–68 years); eight were male, and three were female. The median BMI was 27.9 (range 16.1–34.5). Computed tomography (CT) and magnetic resonance imaging (MRI) were performed to exclude specimens with fractures, tumors, and CT-graphic signs of poor bone quality measured with Hounsfield Units on clinical CT protocol [22] (Table 1). Furthermore, the degeneration of the disk was graded from 0° to 4° on MRI using the Pfirrmann classification on T2-weighted sagittal images [23]. Degeneration of the facet joint was assessed on CT and MRI and scored from 0° to 3° using the Weishaupt classification [24]. No assessment regarding spinal canal stenosis or nerve root compression was possible, because the neural structures of the spine cadavers were empty of spinal fluid and fully collapsed. The cadavers were stored at − 20 °C until thawing overnight in plastic bags. The specimens were cleaned of connective tissue and paraspinal musculature, leaving the intervertebral ligaments, disks, and facet joint capsule intact. Titanium alloy polyaxial pedicle screws (Medacta International, Castel San Pietro, Switzerland) were placed into all vertebrae with a 3D-printed, patient-specific guide navigation technique with optimized screw diameter (6.0–7.0 mm) and length (45–55 mm).
Experimental setup
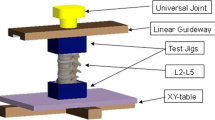
A previously established biomechanical test protocol was used for this study, and the specimen was mounted with 3D-printed clamps [25, 26]. Force-controlled displacements were measured after the application of a predefined load to the cranial vertebra with the caudal vertebra fixed to a semi-constrained test apparatus (Zwick/Roell AllroundLine 10 kN, Germany) (Fig. 1). Bending moments were applied around the vertical axis (z-axis), while coupled translational motion in the horizontal plane was unconstrained with the use of an x–y-table. This approach eliminated axial forces in the horizontal plane, whereby vertical compressive and bending moments around the x and y-axis were not prevented. Similarly, the shear and compressive forces were employed along the vertical axis with the x–y-table preventing the buildup of translational forces in the horizontal plane, while rotational motion around all 3 axes were constrained. Flexion–extension (FE), lateral bending (LB), and axial rotation (AR) were tested with a velocity of 1°/sec, attaining a torque of ± 7.5 Nm. Anterior shear (AS) and lateral shear (LS) were measured at 0.5 mm/sec with 150 N applied to each direction and axial compression/decompression (AC) at 0.1 mm/sec until + 400N compression and − 150 N distraction were reached. After completion of five preconditioning cycles, the range of motion (ROM) of the sixth cycle was recorded. Infrared-emitting markers were positioned on each vertebra to enable stereophotogrammetrical measurements of ROM with 10 Hz and 0.09 mm accuracy (Fusion Track 500, Atracsys, Puidoux, Switzerland). Additional markers were set on the 3D-printed mounting clamps to ensure adequate fit of the vertebrae in the clamps.
Experimental Setup. a vertical position for testing of axial rotation and axial compression. b Spine segment installed in the horizontal, prone position to measure lateral bending and anterior shear. c Spine segment installed in the horizontal, lateral position to measure flexion/extension and lateral shear
Test protocol
All procedures were performed by two experienced and specialty-trained spine surgeons (one orthopedic and one neurosurgeon). With the aforementioned setup, the ROM of each specimen was measured in the following sequence (Fig. 2):
-
A)
Intact spinal segment without decompression and without vertical rods attached to the pedicle screws
-
B)
Intact segment after instrumentation (= attachment of the titanium rods on each side)
-
C)
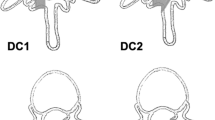
Non-instrumented, left unilateral laminotomy, and resection of the ligamentum flavum
-
D)
Instrumented, unilateral laminotomy
-
E)
Non-instrumented, midline decompression (bilateral laminotomy and removal of interspinous ligaments)
-
F)
Instrumented, midline decompression
-
G)
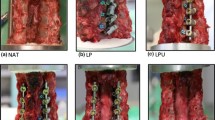
Non-instrumented, nucleotomy (description in Fig. 3)
-
H)
Instrumentation and complete removal of the nucleus pulposus and insertion of two posterior lumbar interbody fusion (PLIF) cages (MectaLIF Posterior, Medacta International, Switzerland). The segments were distracted with − 100 N prior to PLIF insertion and compressed with + 200 N before tightening the vertical rods.
Test Protocol. a intact, non-instrumented, b intact, instrumented, c unilateral laminotomy, non-instrumented, d unilateral laminotomy, instrumented, e midline decompression, non-instrumented, f midline decompression, instrumented, g nucleotomy, non-instrumented, h complete nucleotomy and posterior lumbar interbody fusion (PLIF)
Statistical analysis
Statistical analysis was performed using MATLAB (MATLAB 2019a, MathWorks, Massachusetts, USA). Medians, 25th and 75th percentiles of absolute and relative ROM are reported. Wilcoxon signed rank tests were used. A Bonferroni correction of p values was applied due to multiple comparisons. Statistical significance was defined as α < 0.05.
Results
All specimen revealed age-related signs of degeneration with median degree of degeneration of the intervertebral disk of 2° (range 2–4°) according to Pfirrmann [23] and facet joint 2° (range 0–3°) according to Weishaupt [24] (Table 2). Median ROM of the native segments was FE 6.8° (4.7–9.1°), LB 5.6° (4.4–6.6°), AR 1.7° (1.1–3.3°), AS 1.8 mm (1.2–2.5 mm), LS 1.4 mm (0.7–1.8 mm), and AC 0.3 mm (0.2–0.5 mm) (Table 2). The segments L5/S1 tended toward higher ROM in FE, LB, and AS than the s L1/2 and L3/4, but the relative ROM changes with decompression and instrumentation were similar. All investigated decompression steps led to an increase in ROM and the instrumentation steps did reliably decrease motion (Table 2; Figs. 4 and 5).
Change of range of motion (ROM) relative to the intact spinal segment. Non-instrumented (= light gray) and instrumented (= dark gray) decompression states relative to non-instrumented and instrumented, intact states, respectively. Values above 0 represent relative ROM increase and below 0 decrease. NI non-instrumented. I instrumented. Ul laminotomy unilateral laminotomy. Midline deco midline decompression. The boxplots contain the median, the 25 and 75 percentiles and the whiskers represent the min. and max. values
Unilateral laminotomy versus traditional midline decompression
In the non-instrumented segments, unilateral laminotomy led to small but significant ROM increases in all loading directions, except of AC. Increases in ROM after unilateral laminotomy were in FE 6% (5–10%), LB 3% (1–5%), AR 12% (4–22%), AS 11% (6–18%), and LS 8% (3–14%), all p < 0.001. The ROM increases were even more evident after midline decompression, with an increase of 15% (11–18%) in FE, 5% (2–8%) in LB, 21% (10–33%) in AR, 20% (16–31%) in AS, and 19% (8–27%) in LS. ROM increases were significantly higher after midline decompression than after unilateral laminotomy in all loading directions.
Nucleotomy
Nucleotomy led to substantial increases in ROM, which were highly significant (p < 0.0001) in all loading directions when compared to the intact segment (= state A) but also in comparison with the decompressed segments (= states C and E). The largest ROM increases were found in AC of 153% (79–337%) compared to intact. FE was increased by 46% (40–65%), LB by 15% (12–38%), AR by 52% (24–135%), AS by 45% (30–72%), and LS by 52% (26–75%).
Decompression and instrumentation
Instrumentation through connecting the inserted pedicle screws led to a significant decrease in ROM in all loading directions, except AC (Table 1). The largest ROM reductions were found in FE, with median relative reductions of 82% (81–85%; p < 0.001), followed by LB 72% (68–78%; p < 0.001), AR 42% (30–53%), AS 31% (26–48%), and LS 17% (9–35%) in the intact segment. Higher relative ROM reductions were observed with more extensive decompression. AC was not affected by instrumentation in any surgical decompression state, except for a significant ROM decrease of 75% (53–59%) with instrumentation and PLIF compared to after nucleotomy without instrumentation (p < 0.001). In the instrumented segment, both unilateral laminotomy and midline decompression significantly increased ROM in LS by 8% (3–14%; p < 0.001) and 19% (8–27%; p < 0.001) but did not significantly affect ROM in any other loading direction (Fig. 5). In instrumented segments, relative ROM increases were significantly larger after midline decompression than after unilateral laminotomy in both LS (19% vs. 8%; p = 0.012) and AS (20% vs. 11%; p = 0.050). In the instrumented segments, a tendency to increased ROM after midline decompression was also observed in FE and LB by 11% ((− 4)–18%) and 9% (0–11%), respectively, which did not reach statistical significance. No other loading directions were affected by laminotomy or midline decompression of the instrumented segment. PLIF did not lead to any significant change in ROM in any loading direction compared to instrumentation without interbody fusion. PLIF did, however, show the greatest relative ROM reductions when compared to decompression and nucleotomy, with the largest relative ROM reductions in FE of 87% (84–89%), followed by LB 78% (70–81%) and AC 75% (53–86%), all p < 0.001.
Discussion
Although biomechanical understanding of the human body is of primary importance in spine and orthopedic surgery in general, awareness and knowledge of the alteration of segmental spinal motion after a decompression or fusion procedure are fairly low. The present study aims to fill this gap by providing detailed analysis of segmental ROM after common decompression and instrumentation procedures on the lumbar spine. The main findings are that even unilateral laminotomy leads to a significant increase in segmental ROM, which is further increased after midline decompression and nucleotomy. Pedicle screw instrumentation significantly reduced segmental ROM in each case, since overall posterior decompression procedures only negligibly destabilized the fusion construct.
ROM of spinal segments differ between different levels and also depend on degree of degeneration [27]. In this study, we found a trend toward higher ROM in FE, LB, and AS in the segments L5/S1 than the segments L1/2 and L3/4, but the relative ROM changes with decompression and instrumentation were similar. Although wide variation in absolute ROM between the specimen was observed, the relative ROM changes with decompression and instrumentation were in close ranges. For example, absolute ROM in FE of the native segments ranged between 2.9° and 12.6° (= 434% of 2.9°), while relative FE decreases ranged between 76 and 88% after instrumentation and relative FE increases ranged between 8 and 23% after midline decompression (Table 1). In contrast, in absolute numbers, wide variations in ROM were observed, with FE decreases ranging between−2.3° and − 10.5° and FE increases ranging between 0.3° and 2.0° after instrumentation and midline decompression, respectively. Therefore, the higher the native ROM, the higher the absolute change in ROM with decompression and instrumentation. However, the amount of relative change in ROM after decompression and instrumentation seems to be more constant and can be more reliably estimated. Neither a connection between relative change in ROM to native ROM, nor to level of segment and nor to degree of degeneration could be observed in this study.
In cases of degenerative spinal stenosis and spondylolisthesis, it is controversial whether spinal decompression alone is an appropriate surgical treatment or whether fusion should be pursued. Also, it is often unclear whether decompression for adjacent segment disease on top of an instrumented spine is sufficient, or not. Opponents of decompression alone argue that this approach could provoke further segmental instability and further accelerate the degenerative cascade. While studies in the 1990s already suggested that patients who underwent posterior instrumentation in addition to laminectomy had better outcomes and less progression of listhesis than patients who underwent laminectomy alone [16, 17], recently published studies have failed to depict a consistent clinical benefit of additional instrumentation [12,13,14, 28]. Therefore, a clear consensus remains pending, possibly due to the lack of awareness and knowledge of the actual segmental native ROM and its alteration by surgical intervention.
Unilateral laminotomy with or without over-the-top decompression and conventional midline decompression with bilateral laminotomy and removal of the interspinous ligaments are standard procedures for the removal of herniated disks and decompression of the spinal canal and entrapped nerve roots. Former biomechanical studies found a significantly larger ROM increase the more extensive the decompression [29, 30]. Smith et al. [29] investigated ROM on six human lumbar cadaver specimen and found a significant increase in FE, LB, and AR after decompression and the ROM increases were larger, the more extensive the decompression. ROM in their study from native to > unilateral laminotomy > standard midline decompression > wide midline decompression was as follows: FE 9.2° > 9.6° > 10.7° > 12.3°, LB 8.0° > 8.4° > 8.6° > 10.4°, and AR from 3.7° > 4.0° > 4.5° > 6.3°. Grunert et al. [30] investigated ROM after minimally invasive unilateral laminotomy with over-the-top decompression and laminectomy at the adjacent level (L3/4) cranial to the instrumented spine (L4/5). Minimally invasive decompression significantly increased FE at L3-L4 by 13% and AR by 23%. Laminectomy further increased ROM by an additional 12% in FE and by 17% in AR. The results of both studies are in line with the here presented data. Although these former studies already provided valuable insights on distinct decompression techniques, no study had comprehensively investigated segmental ROM after standard decompression techniques in context with instrumentation and with nucleotomy, to date.
In our study, unilateral laminotomy increased segmental ROM by 3% (LB) to 12% (AR), whereas midline decompression showed an increase of 5% (LB) to 21% (AR). Minimally invasive and endoscopic decompression techniques have been further shown to affect segmental stability to a lesser degree in biomechanical investigations [30, 31]. Nucleotomy, routinely performed in recurrent cases or in patients at high risk for further disk herniation, aims to reduce intradiscal pressure [32]. However, fenestration of the annulus and partial resection of the nuclear substance resulted in a substantial segmental ROM increase in all loading directions, with the most prominent changes in AC of 153%. Therefore, nucleotomy could increase the risk of accelerated segmental degeneration and should be considered only as a salvage option for recurrent disk herniation in patients who are not candidates for fusion surgery.
Instrumentation with pedicle screws and connecting rods remains the gold standard in spinal fusion surgery today and is increasingly applied together with the insertion of interbody fusion cages [33]. In this study, the standard bilateral PLIF technique was compared to nucleotomy. While instrumentation of the segment significantly decreased ROM in almost all loading cases, both decompression steps significantly increased ROM in LS by 8% and 19%, respectively. However, in light of the small absolute ROM increase in LS of 0.07 mm (0.03–0.11 mm) and 0.13 mm (0.05–0.18 mm), respectively, the significance of this increase is rather academic and may be negligible from a biomechanical point of view, meaning that both unilateral decompression and midline decompression can be considered safe in instrumented segments.
The use of interbody fusion has increased rapidly in recent years [33]. Biomechanically, interbody fusion is intended to support the anterior and middle columns, resist compression forces, and reduce stress on the pedicle screw-bone and screw-rod interfaces [34, 35]. Furthermore, better clinical outcomes, higher fusion rates, and less early screw loosening due to intersomatic fusion have already been shown [33, 36]. Contrary to our expectations, bilateral PLIF insertion did not lead to noticeable ROM reductions and did not increase construct stiffness. The literature provides conflicting results regarding biomechanical effect of interbody fusion. Whereas Godzik et al. [37] found a trend to motion decrease with the use of interbody fusion compared to posterior instrumentation alone, Ntilikina et al. [38] reported a non-significant trend toward motion increase in bending and rotational directions. Furthermore, Harris et al. [39] reported that transforaminal interbody fusion led to substantial destabilization, and the additional posterior instrumentation did only reapproximate the motion to the native spine in their study. Therefore, it appears that the stabilizing effects of the intervertebral disk cannot be fully restored using a cage; in FE und AR motions, the cage implantation allowed even more motion, implying that the cage partially acts as a hypomochlion in the performance of certain movements. This potential drawback must be weighed against the advantages of anterior column support, which also aims to maintain disk height in the early stages until the bony fusion has sufficiently advanced by the inserted bone material.
Clinical significance
It is our opinion that, to capture the complete picture of a patient in spine surgery practice, understanding the biomechanical alterations after a surgical procedure are as pivotal as knowing the patient’s expectations, comorbidities, and individual anatomic factors. The results reported in this study can and should be kept in mind when counseling patients for lumbar decompression with and without instrumentation, whether in primary surgery or in revision surgery, e.g., for adjacent segment disease. Although not entirely generalizable to each individual patient, the results of this study can be roughly summarized to the following five simplified rules of thumb:
-
1)
Unilateral laminotomy increases lumbar segmental motion by 10%
-
2)
Midline decompression increases segmental motion by 20%
-
3)
Nucleotomy increases segmental motion by 50%
-
4)
Pedicle screw instrumentation reduces lumbar segmental motion by 80% of the native ROM
-
5)
Decompression only negligibly increases residual motion in instrumented spinal segments
Limitations
This biomechanical in vitro study inherits some limitations. The application of isolated load in FE, LB, AR, LS, AS, and AC enables precise comparisons between different decompression and instrumentation conditions, but represents a very rough simplification of the complex motion and force pattern of a human spine in vivo [20, 40]. Also, cadaver spine tests generally ignore the stabilizing effect of the active trunk musculature. In our opinion, the semi-constrained test protocol enables adequate investigation of the study question and most adequately balances the trade-off between exact replication of the physiological kinematics and the reduction in complexity [27]. A completely unconstrained test setup, as it has been proposed in the literature [41], allows for coupled motion in all dimensions and thus, prevents the buildup of loading in these motion planes. While this configuration might most adequately resemble in vivo kinematics, it might result in more complex and potentially less replicable motion between the investigated surgical intervention steps; since due to the low stiffness of spinal segments in their neutral zone, only minimal load is required for relatively large motion. In contrast, a completely constrained testing configuration forces the specimen to move in the same pattern with each repetition and the effect of the surgical intervention would be most reliably measured in this exact motion. However, the complete restriction of all coupled motions might result in unphysiological loading and motion patterns (e.g., when the axis of rotation of the testing apparatus is not aligned with the physiological center of rotation of the spinal segment). The authors regard the semi-constrained setup used in this study to represent an adequate compromise between the two aspects, as center of rotations is not forced on the specimens, while (unnecessary) evasive motion is prevented.
Although all included spinal segments did reveal some degree of degeneration of the intervertebral disk and/or facet joint, spinal stenosis or nerve root compression could not be assessed, because of collapsed neural structures. Therefore, the cadavers tested do only limitedly represent candidates for decompression and fusion surgery and iatrogenic alterations of segmental motion after these procedures can only roughly be estimated. For example, in a patient with disk herniation, a weakened anulus fibrosus and disk could already have altered the segmental mobility and decompression, and nucleotomy might result in different amounts of ROM increases than in intact spinal segments. However, age-related signs of degeneration were found in all specimen, and their age range of 50–68 years does match with the patient population undergoing spinal decompression and fusion surgery, which is reportedly 57 ± 14 years old according to Martin et al. [2]. Also, the ROM reported herein most likely represents the initial postoperative state, which would alter in the further course due to scar tissue formation and continuation of the degenerative process. CT was performed with clinical CT protocols without phantom, and no DEXA measurements were available. Therefore, the here reported Hounsfield Units only roughly describe bone quality. Correlation of native ROM, level of segment, and degree of degeneration with relative ROM were not the object of this study, and the relatively small sample size did not provide adequate power for statistical analysis of this matter. Therefore, further investigation of change of segmental motion after spinal decompression and instrumentation in dependence of degree of degeneration is warranted. Finally, because ROM was tested in six loading directions separately, and the weighting of each loading direction to the entire segmental mobility is unknown, the above-mentioned five rules of thumb are rather rough simplifications of the data than the results of exact mathematic calculations.
Conclusion
This study provides detailed insights into segmental ROM in different loading directions after decompression and instrumentation of the lumbar spine. The amount of posterior decompression significantly impacts ROM, with unilateral laminotomy, midline decompression and nucleotomy all significantly increasing ROM in ascending order. Pedicle screw instrumentation reliably decreases segmental ROM, and accompanied decompression procedures only minorly destabilize the instrumentation construct. Based on the large amount of ROM change observed in this study, surgeons should be aware of the iatrogenic alterations of spinal biomechanics after decompression and instrumentation. The reported study provides a basis for surgical strategy making in the clinical routine and for future research.
References
Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG (2010) Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 303:1259–1265. https://doi.org/10.1001/jama.2010.338
Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS (2019) Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the united states, 2004 to 2015. Spine 44:369–376. https://doi.org/10.1097/brs.0000000000002822
Rajaee SS, Bae HW, Kanim LE, Delamarter RB (2012) Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine 37:67–76. https://doi.org/10.1097/BRS.0b013e31820cccfb
Deyo RA, Nachemson A, Mirza SK (2004) Spinal-fusion surgery—the case for restraint. N Engl J Med 350:722–726. https://doi.org/10.1056/NEJMsb031771
Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H (2008) Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med 358:794–810. https://doi.org/10.1056/NEJMoa0707136
Carlson BB, Albert TJ (2019) Lumbar disc herniation: what has the spine patient outcomes research trial taught us? Int Orthop 43:853–859. https://doi.org/10.1007/s00264-019-04309-x
Cloward RB (1953) The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg 10:154–168. https://doi.org/10.3171/jns.1953.10.2.0154
Reisener MJ, Pumberger M, Shue J, Girardi FP, Hughes AP (2020) Trends in lumbar spinal fusion-a literature review. J Spine Surg 6:752–761. https://doi.org/10.21037/jss-20-492
Liang H-F, Liu S-H, Chen Z-X, Fei Q-M (2017) Decompression plus fusion versus decompression alone for degenerative lumbar spondylolisthesis: a systematic review and meta-analysis. Eur Spine J 26:3084–3095. https://doi.org/10.1007/s00586-017-5200-x
Ahmad S, Hamad A, Bhalla A, Turner S, Balain B, Jaffray D (2017) The outcome of decompression alone for lumbar spinal stenosis with degenerative spondylolisthesis. Eur Spine J 26:414–419. https://doi.org/10.1007/s00586-016-4637-7
Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN, Coumans JV, Harrington JF, Amin-Hanjani S, Schwartz JS, Sonntag VK, Barker FG 2nd, Benzel EC (2016) Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med 374:1424–1434. https://doi.org/10.1056/NEJMoa1508788
Försth P, Ólafsson G, Carlsson T, Frost A, Borgström F, Fritzell P, Öhagen P, Michaëlsson K, Sandén B (2016) A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med 374:1413–1423. https://doi.org/10.1056/NEJMoa1513721
Austevoll IM, Hermansen E, Fagerland MW, Storheim K, Brox JI, Solberg T, Rekeland F, Franssen E, Weber C, Brisby H, Grundnes O, Algaard KRH, Böker T, Banitalebi H, Indrekvam K, Hellum C (2021) Decompression with or without fusion in degenerative lumbar spondylolisthesis. N Engl J Med 385:526–538. https://doi.org/10.1056/NEJMoa2100990
Ulrich NH, Burgstaller JM, Valeri F, Pichierri G, Betz M, Fekete TF, Wertli MM, Porchet F, Steurer J, Farshad M (2022) Incidence of revision surgery after decompression with vs without fusion among patients with degenerative lumbar spinal stenosis. JAMA Netw Open 5:e2223803. https://doi.org/10.1001/jamanetworkopen.2022.23803
Gadjradj PS, Basilious M, Goldberg JL, Sommer F, Navarro-Ramirez R, Mykolajtchuk C, Ng AZ, Medary B, Hussain I, Härtl R (2023) Decompression alone versus decompression with fusion in patients with lumbar spinal stenosis with degenerative spondylolisthesis: a systematic review and meta-analysis. Eur Spine J. https://doi.org/10.1007/s00586-022-07507-1
Caelers IJMH, Mannion AF, Haschtmann D, Rijkers K, van Hemert WLW, de Bie RA, van Santbrink H (2022) Factors associated with an increased risk of developing postoperative symptomatic lumbar spondylolisthesis after decompression surgery: an explorative two-centre international cohort study. Eur Spine J. https://doi.org/10.1007/s00586-022-07403-8
Lang Z, Li J-S, Yang F, Yu Y, Khan K, Jenis LG, Cha TD, Kang JD, Li G (2019) Reoperation of decompression alone or decompression plus fusion surgeries for degenerative lumbar diseases: a systematic review. Eur Spine J 28:1371–1385. https://doi.org/10.1007/s00586-018-5681-2
Aizawa T, Ozawa H, Kusakabe T, Tanaka Y, Sekiguchi A, Hashimoto K, Kanno H, Morozumi N, Ishii Y, Sato T, Takahashi E, Kokubun S, Itoi E (2015) Reoperation rates after fenestration for lumbar spinal canal stenosis: a 20-year period survival function method analysis. Eur Spine J 24:381–387. https://doi.org/10.1007/s00586-014-3479-4
Irmola TM, Häkkinen A, Järvenpää S, Marttinen I, Vihtonen K, Neva M (2018) Reoperation rates following instrumented lumbar spine fusion. Spine 43:295–301. https://doi.org/10.1097/brs.0000000000002291
Malakoutian M, Volkheimer D, Street J, Dvorak MF, Wilke H-J, Oxland TR (2015) Do in vivo kinematic studies provide insight into adjacent segment degeneration? A qualitative systematic literature review. Eur Spine J 24:1865–1881. https://doi.org/10.1007/s00586-015-3992-0
Kim CH, Chung CK, Park CS, Choi B, Kim MJ, Park BJ (2013) Reoperation rate after surgery for lumbar herniated intervertebral disc disease: nationwide cohort study. Spine 38:581–590. https://doi.org/10.1097/BRS.0b013e318274f9a7
Zou D, Li W, Deng C, Du G, Xu N (2019) The use of CT Hounsfield unit values to identify the undiagnosed spinal osteoporosis in patients with lumbar degenerative diseases. Eur Spine J 28:1758–1766. https://doi.org/10.1007/s00586-018-5776-9
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26:1873–1878. https://doi.org/10.1097/00007632-200109010-00011
Weishaupt D, Zanetti M, Boos N, Hodler J (1999) MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol 28:215–219. https://doi.org/10.1007/s002560050503
Widmer J, Cornaz F, Scheibler G, Spirig JM, Snedeker JG, Farshad M (2020) Biomechanical contribution of spinal structures to stability of the lumbar spine-novel biomechanical insights. Spine J 20:1705–1716. https://doi.org/10.1016/j.spinee.2020.05.541
Cornaz F, Burkhard M, Fasser MR, Spirig JM, Snedeker JG, Farshad M, Widmer J (2021) 3D printed clamps for fixation of spinal segments in biomechanical testing. J Biomech 125:110577. https://doi.org/10.1016/j.jbiomech.2021.110577
Widmer J, Fornaciari P, Senteler M, Roth T, Snedeker JG, Farshad M (2019) Kinematics of the spine under healthy and degenerative conditions: a systematic review. Ann Biomed Eng 47:1491–1522. https://doi.org/10.1007/s10439-019-02252-x
Ulrich NH, Burgstaller JM, Pichierri G, Wertli MM, Farshad M, Porchet F, Steurer J, Held U (2017) Decompression surgery alone versus decompression plus fusion in symptomatic lumbar spinal stenosis: a Swiss prospective multicenter cohort study with 3 years of follow-up. Spine 42:E1077-e1086. https://doi.org/10.1097/brs.0000000000002068
Smith ZA, Vastardis GA, Carandang G, Havey RM, Hannon S, Dahdaleh N, Voronov LI, Fessler RG, Patwardhan AG (2014) Biomechanical effects of a unilateral approach to minimally invasive lumbar decompression. PLoS ONE 9:e92611. https://doi.org/10.1371/journal.pone.0092611
Grunert P, Reyes PM, Newcomb AG, Towne SB, Kelly BP, Theodore N, Härtl R (2016) Biomechanical evaluation of lumbar decompression adjacent to instrumented segments. Neurosurgery 79:895–904. https://doi.org/10.1227/neu.0000000000001419
Farshad M, Hagel V, Spirig JM, Fasser MR, Burkhard MD, Widmer J, Calek AK (2022) Biomechanics of transforaminal endoscopic approaches. Spine. https://doi.org/10.1097/brs.0000000000004471
Hijikata S (1989) Percutaneous nucleotomy. A new concept technique and 12 years’ experience. Clin Orthop Relat Res 238:9–23
Makanji H, Schoenfeld AJ, Bhalla A, Bono CM (2018) Critical analysis of trends in lumbar fusion for degenerative disorders revisited: influence of technique on fusion rate and clinical outcomes. Eur Spine J 27:1868–1876. https://doi.org/10.1007/s00586-018-5544-x
Lund T, Oxland TR, Jost B, Cripton P, Grassmann S, Etter C, Nolte LP (1998) Interbody cage stabilisation in the lumbar spine: biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br 80:351–359. https://doi.org/10.1302/0301-620x.80b2.7693
Xu M, Yang J, Lieberman I, Haddas R (2019) Stress distribution in vertebral bone and pedicle screw and screw-bone load transfers among various fixation methods for lumbar spine surgical alignment: a finite element study. Med Eng Phys 63:26–32. https://doi.org/10.1016/j.medengphy.2018.10.003
Kim DH, Hwang RW, Lee GH, Joshi R, Baker KC, Arnold P, Sasso R, Park D, Fischgrund J (2020) Comparing rates of early pedicle screw loosening in posterolateral lumbar fusion with and without transforaminal lumbar interbody fusion. Spine J 20:1438–1445. https://doi.org/10.1016/j.spinee.2020.04.021
Godzik J, Kalb S, Reis MT, Reyes PM, Singh V, Newcomb A, Chang SW, Kelly BP, Crawford NR (2018) Biomechanical evaluation of interbody fixation with secondary augmentation: lateral lumbar interbody fusion versus posterior lumbar interbody fusion. J Spine Surg 4:180–186. https://doi.org/10.21037/jss.2018.05.07
Ntilikina Y, Charles YP, Persohn S, Skalli W (2020) Influence of double rods and interbody cages on quasistatic range of motion of the spine after lumbopelvic instrumentation. Eur Spine J 29:2980–2989. https://doi.org/10.1007/s00586-020-06594-2
Harris BM, Hilibrand AS, Savas PE, Pellegrino A, Vaccaro AR, Siegler S, Albert TJ (2004) Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine 29:E65-70. https://doi.org/10.1097/01.brs.0000113034.74567.86
Volkheimer D, Malakoutian M, Oxland TR, Wilke H-J (2015) Limitations of current in vitro test protocols for investigation of instrumented adjacent segment biomechanics: critical analysis of the literature. Eur Spine J 24:1882–1892. https://doi.org/10.1007/s00586-015-4040-9
Wilke HJ, Wenger K, Claes L (1998) Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J 7:148–154. https://doi.org/10.1007/s005860050045
Acknowledgements
The authors gratefully thank Mauro Suter for his technical support during this study. The authors further thank Medacta International (Castel San Pietro, Switzerland) for providing the implants used for this study. Imaging was performed with support of the Swiss Center for Musculoskeletal Imaging, SCMI, Balgrist Campus AG, Zurich.
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors state that they have no potential conflict of interest in relation to this study’s content. MF is consultant for Medacta, but unrelated to the content of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burkhard, M.D., Calek, AK., Fasser, MR. et al. Biomechanics after spinal decompression and posterior instrumentation. Eur Spine J 32, 1876–1886 (2023). https://doi.org/10.1007/s00586-023-07694-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07694-5