Abstract
Purpose
This article aims at presenting a scale that, through the analysis of MRI images, clearly charts the various degenerative stages of the cervical spine and establishes its biological age. We have created this scale by summing together various scores linked to a selection of parameters according to which MRI images are analyzed.
Method
We examined 423 cervical spine MRI scans, belonging to patients who had been admitted to the Medical Imaging Service of the Military Hospital of Rome between January 2010 and July 2011. We selected 6 parameters for the analysis of the MRI scans of the cervical spine: (1) the degeneration of the intervertebral discs, (2) the degeneration of the yellow ligaments, (3) the degeneration of the vertebral bodies, (4) the possible presence of spondylolistheses, (5) the presence or absence of foraminal stenosis, and (6) the diameter of the spinal canal. We assigned to each parameter a score system based on a graduated scale. The cervical spine physiological age can be determined by summing up the scores obtained for each parameter.
Results
We submitted the data obtained from the study to a statistical enquiry. The results of the enquiry confirmed the suitability of the parameters selected for the evaluation of the aging process of the cervical spine.
Conclusions
The effectiveness of the various treatments for cervical spine degenerative disorders is influenced by the overall anatomical conditions of the cervical spine. Up until now there has been no objective criterion for the evaluation of these anatomical conditions. We believe that this scale will be a useful tool to homogenize retrospective studies and to correctly set up prospective studies on the degenerative conditions of the cervical spine and relative treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many scientific papers [1–4] have shown that degenerative cervical spine disorders are closely linked to aging. Lifestyle, hereditary factors, posture, sports, and work-related activities can, however, influence the course of degenerative disorders [5–7]; moreover, in a number of cases, the cervical spine biological age does not match the person’s chronological age. In short, aging of the spine appears to be a complex and inhomogeneous process.
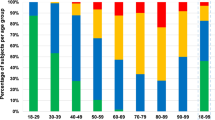
In our daily clinical practice, it is not unusual to find individuals whose cervical spine scans show a much different result than what would normally be expected taking into account the subjects’ chronological age (Fig. 1). In the literature, so far, there are no tools to measure the degree of degeneration of the cervical spine. A scale such as the one presented in this article might prove essential to standardize studies on degenerative pathologies and relative treatments. So far such standardization has not been possible. There is a distinct lack of homogeneity in treatment guidelines, so much so that selection of appropriate treatment is often wholly lead by the preference of the physician; moreover, population samples in clinical studies have been formed mainly according to chronological age [8–11]. As previously mentioned, our study shows that chronological age alone is not a comprehensive and satisfactory parameter when it comes to researching degenerative disorders of the cervical spine.
The decision on whether a patient should be treated surgically or otherwise, and, in the case of surgery, on which type of intervention should be carried out, is taken on the basis of many parameters, such as medical history, the general and neurological conditions of the patient, the presence of osteoporosis and/or osteopenia, as well as the presence or absence of clear signs of myeloradicular compression caused by degenerative pathology of the spine. Given such premise, it is, however, necessary to recognize that the general condition of the cervical spine is an element that influences the effectiveness of treatments and since such condition can greatly vary from person to person even within the same age group, it is not accurate nor helpful to carry out studies that compare tout court groups of patients homogeneous only because sharing the same age range.
This article aims at presenting a scale for the analysis of MRI images that, by clearly charting the various degenerative stages of the cervical spine, can establish with precision the overall state of degeneration of any given cervical spine, or as we prefer to call it, the spine’s biological age. The evaluation system created complies with the following requirements: objectivity, comparability, and replicability.
The cervical spine biological age is determined by summing together various scores linked to a selection of parameters according to which MRI images are analyzed.
Materials and methods
For this article, we have examined the MRI scans of the cervical spine belonging to all the patients who were admitted to the Medical Imaging Service of the Military Hospital of Rome between January 2010 and July 2011, for a total of 508 scans. The exclusion criteria applied to this sample were:
-
Patients aged under 20,
-
MRI scans performed due to recent trauma to the spine,
-
MRI scans performed due to neoplastic growths,
-
MRI scans performed after surgery to the cervical tract, and
-
MRI scans performed due to inflammatory/infectious diseases of the cervical tract.
Following these criteria, our sample was narrowed down to 423 scans.
The MRI scans were performed using a 2010 Release 2.1.5.5 Philips Achieva with gradients between 33 mT/m and 1 slew rate of 150 T/ms; T1 SE sagittal sequences with 400 ms repetition time (TR), 7.4 ms echo time (TE), 90° flip angle with a thickness of 3 mm and 3′. 43″ scanning time as well as T2 FFE sagittal sequences with 3500 ms TR, 120 ms TE, 90° flip angle with a 3 mm thickness and 3′. 44″ scanning time; axial sequences on T2 FFE 3D, 50 ms TR, 12 ms TE, 7° flip angle, 0.5 mm thickness, 3′ scanning time.
For our study, all images were re-elaborated with Osirix software.
The scans were reviewed by two independent teams. Each team included a neuroradiologist with over 15 years of experience, a senior neurosurgeon with over 15 years of experience in the field of cervical spine and a junior neurosurgeon with less than 15 years of experience.
On the grounds of literature and of our experience, we selected six parameters by which to analyze the sample MRI scans. We assigned to each parameter a score system based on a graduated scale. The cervical spine biological age could then be determined by summing up the scores obtained for each parameter.
The following six parameters were:
-
1.
The degeneration of intervertebral discs,
-
2.
The degeneration of yellow ligaments,
-
3.
The degeneration of vertebral bodies,
-
4.
The possible presence of spondylolistheses,
-
5.
The presence or absence of foraminal stenosis, and
-
6.
The diameter of the spinal canal.
All these factors were evaluated through the use of graduated ordinal scales with incremental scores, whereby each score denoted the state of one of the selected elements as it appeared on the MRI image. Each of these factors was analyzed per single subaxial cervical spine level (C2-D1) as extensively shown by Table 1.
Results
Statistical analysis
Initially, the results obtained by the two examining teams underwent the Pearson’s test to assess inter-operator dependency: the correlation coefficient equal to 0.891** showed that this scale is not dependent on the operator’s subjective view.
We then submitted the data obtained from the study to a statistical enquiry with SPSS v. 18 software.
We first carried out a descriptive statistics analysis; the results of which are displayed in Table 2.
The following variables were added to the six parameters selected:
-
Scale total (sum of the individual scores per parameter),
-
Chronological age of the subject of the MRI scan,
-
The difference between these last two variables.
As it is easily deduced from the table, the average value and standard deviation (SD) of the two variables scale total and chronological age is very similar, indicating a significant super imposability of the two diagrams. The difference of the averages between these two variables is below one point (N = 423, m = −0.929), while the SD of the difference is once again similar to the SD of the two variables, thus indicating similarity between the dispersion indexes. The Compare Means Test confirmed this observation.
We then carried out on the sample two types of inferential statistics study: Pearson’s product-moment correlation coefficient (Table 3) and Factor analysis (Table 4).
The Pearson’s product-moment correlation coefficient between the variables, ‘chronological age’ and ‘scale total’, was found to be statistically high (r = 0.726, p < 0.01); as was also the case for all the other scale parameters used as variables, since they too presented a significant positive correlation with the chronological age of the sample subjects (p < 0.01).
We then submitted the sample to a Factor analysis (Table 4): a single statistical factor (Fig. 2) was able to determine, in our sample, 56.26 % of variance in the scores obtained using the scale. We hypothesized this factor to be aging.
Discussion
To create our scale we used parameters suggested by the relevant literature on the subject. We examined age in correlation with the following anatomical structures of the cervical spine:
-
1.
Vertebral bodies. In 1988, Modic et al. [12] published the renowned work on MRI scans showing the degeneration of vertebral bodies’ bone marrow and of the adjacent endplates. From then on numerous studies were carried out on the subject. We have simplified the analysis of the degeneration of vertebral bodies using a scale with only three base measuring units or degrees:
-
Score of 1.
Absence of non-homogeneity of signal on T1 and T2-weighed images of the vertebral body.
-
Score of 2.
Presence of non-homogeneity.
-
Score of 3.
Presence of any kind of degeneration classified according to the Modic scale.
-
2.
Intervertebral discs. The progressive disc degeneration caused by aging can easily be verified by MRI scan examination. In 2001, Pfirrmann proposed a measuring system for lumbar disc degeneration [13]. For the cervical spine we adopted a similar system with five base measuring units or degrees:
-
Score of 1.
Disc that is hyper or isointense to the cerebrospinal fluid (CSF) on T2-weighted MR images.
-
Score of 2.
Hypointense disc.
-
Score of 3.
Black disc.
-
Score of 4.
Protruded or extruded disc from any side.
-
Score of 5.
Absence of disc space/presence of osteophytic bridges between vertebrae.
-
3.
Intervertebral ligaments. The degeneration of the ligaments is due to changes in the collagen fibers and in calcium content. Numerous articles [14–17] highlight how, with aging, the cervical spine ligaments present a marked tendency toward calcification, in particular toward OPLL (ossification of the posterior longitudinal ligament). We have selected the degeneration of the posterior ligamentous complex (yellow ligament/interspinous ligament), while discounting the remaining ligamentous compartment as it was already included in other parameters (disc, intervertebral foramina, presence of spondylolisthesis, and canal). For this parameter, we established three base measuring units or degrees of progressive degeneration: healthy (score of 1), calcified (score of 2), and projecting into the canal (score of 3).
-
4.
Intervertebral foramina. We can evaluate the degenerative process of the zygapophysial joints and the facet joints by examining the deterioration of connecting foramina [18]. To achieve this, we used the axial sequences for the vertebral bodies studied and the T2-weighed sagittal sequences. On the levels that were not clear, we used 2D reconstruction with Osirix software, thus obtaining the images of the foramina on an orthogonal plane compared to the axis of the foramen in consideration [19]. For each foramen, we established the following base measuring units or degrees: score of 0 if healthy, score of 1 if it presented any form of deterioration [20].
-
5.
Spinal Canal. The degenerative processes of the spine caused by aging provoke a progressive narrowing of the spinal canal with myelopathic signal manifestations in MRI scans [21, 22]. For this reason, we included a parameter to evaluate the AP diameter at the worst level. We adopted the following scale system:
-
Score of 1.
Normal diameter.
-
Score of 2.
Reduction up to 25 % compared to a normal adjacent space.
-
Score of 3.
Reduction between 25 and 50 %.
-
Score of 4.
Reduction between 50 and 75 %.
-
Score of 5.
Reduction above 75 %.
-
Score of 6.
Presence of myelopathic signal on T2 at single level.
-
Score of 7.
Presence of myelopathic signal over more levels.
-
Score of 8.
Presence of spinal cord atrophy.
-
Score of 1.
The last three degrees do not refer to the diameter of the spinal canal, but to pathologies of the spinal cord that occur in very serious anatomical conditions; in these instances, the walls of the spinal canal no longer represent the element that contains and protects the spinal cord, but they actually become the cause for pathologies of the nervous tissue.
-
6.
Alignment or misalignment between two vertebrae. Degenerative spondylolistheses, which has long been known in the lumbar region, has been studied at cervical level only since 1986 [23]. Its presence increases with aging and it has been found to be high in people over 50 [24]. This is why we chose to include this parameter in our scale by simply acknowledging its absence (score of 0) or presence (score of 1) for each vertebral unit under consideration.
We have not included osteoporosis among the parameters under observation, even though it is an element that needs to always be kept in mind for the selection of treatment for the spine, because osteoporosis represents a very clear pathology of the bone, which is not derived from the degenerative process [25–27].
The results of the statistical analysis show that to evaluate the cervical spine aging process, the choice of the aforementioned parameters has been correct. Since the degeneration caused by aging is not in itself a pathology but an unavoidable physiological occurrence for everyone without exception, whether symptoms are present or not [28], we did not consider it necessary to gather data from a “healthy” sample. Any spine expert is aware that the radiological appearance of the spine does not always correlate with the clinical picture; thus, a patient with spine degeneration may not show any symptoms and, therefore, not require treatment.
The effectiveness of the various medical, physiatrical, and surgical treatments for cervical spine degenerative disorders is influenced by the overall anatomical conditions of the cervical spine. Up until now there has been no objective criterion for the evaluation of these conditions. Moreover, as already stated, the aging processes of the spine are not always homogeneous per age band. These factors contribute to the extreme difficulty in achieving any sort of objective comparison among therapeutic strategies. We believe that this scale will be useful to homogenize retrospective studies and to correctly set up prospective studies on the degenerative disorders of the cervical spine and the relative treatments; it is effective and simple tool for the objective classification and staging of degenerative processes and for the measurement of the cervical spine’s biological age; our team has been using it for over a year and found it extremely helpful to determine the appropriate therapy for each patient. In fact, recently, we have begun a prospective study on the choice, in relation to patients’ age, of either the artificial disc or the cage as prosthesis during anterior surgery of myeloradiculopathy caused by disk herniation or by cervical spondylosis. This study involves two groups of patients. The choice of prosthesis for the first group will rely solely on the subjects’ chronological age, which is currently common practice; while for the other group, the choice will be based on the spine’s biological age, calculated according to our scale. Early data shows that all the patients who were given a disc prosthesis having scored 50 or below on our scale, irrespective of their actual age, even after two years have had no signs of prosthesis’ fusion and the consequent lessening of mobility; whereas the only two patients who were given an artificial disk because younger than 50 years old, but whose score was above 50, both showed an early prosthesis fusion process.
In conclusion, our work means to contribute, through a statistical model, to the standardization and simplification of the complex phenomenon that is cervical spine aging, and thus it offers a tool for the greater homogenization of studies concerning the treatments of pathologies linked to spinal degeneration. The sample we chose to build the scale from is statistically sufficient [29, 30]; however, the topic we chose is so varied, vast, and complex that it certainly deserves a larger sample as well as a different approach to the research. In conclusion, we consider ours a pilot study that may lead to a larger multicenter study.
References
Benoist M (2003) Natural history of the aging spine. Eur Spine J 12(Suppl 2):S86–S89
Papadakis M, Sapkas G, Papadopoulos EC, Katonis P (2011) Pathophysiology and biomechanics of the aging spine. Open Orthop J 5:335–342
Prescher A (1998) Anatomy and pathology of the aging spine. Eur J Radiol 27(3):181–195
Wilmink JT (2011) The normal aging spine and degenerative spinal disease. Neuroradiology 53(Suppl 1):S181–S183
Blum M, Harris SS, Must A, Phillips SM, Rand WM, Dawson-Hughes B (2002) Household tobacco smoke exposure is negatively associated with premenopausal bone mass. Osteoporos Int 13(8):663–668
Gopal D, Ho AL, Shah A, Chi JH (2012) Molecular basis of intervertebral disc degeneration. Adv Exp Med Biol 760:114–133
Hartvigsen J, Christensen K (2007) Active lifestyle protects against incident low back pain in seniors: a population-based 2-year prospective study of 1387 Danish twins aged 70–100 years. Spine (Phila Pa 1976) 32(1):76–81
Naderi S, Özgen S, Pamir M, Özek M, Erzen C (1998) Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery 43(1):43–49
Wang MY, Shah S, Green BA (2004) Clinical outcomes following cervical laminoplasty for 204 patients with cervical spondylotic myelopathy. Surg Neurol 62:487–493
Hukuda S, Mochizuky T, Ogata M, Shichigawa K, Shimomura Y (1985) Operation for cervical spondylotic myelopathy. A comparision of the results of anterior and posterior procedures. J Bone Joint Surg 67-B(4):609–615
Sekhon LHS (2003) Cervical arthroplasty in the management of spondylotic myelopathy. J Spinal Disord Techn 16(4):307–313
Modic M, Steinberg P, Ross J, Masaryk T, Carter J (1988) Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 166:193–199
Pfirrmann C, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 26:1873–1878
Barros EM, Rodrigues CJ, Rodrigues NR, Oliveira RP, Barros TE, Rodrigues AJ Jr (2002) Aging of the elastic and collagen fibers in the human cervical interspinous ligaments. Spine J 2(1):57–62
Keorochana G, Taghavi CE, Tzeng ST, Morishita Y, Yoo JH, Lee KB, Liao JC, Wang JC (2010) Magnetic resonance imaging grading of interspinous ligament degeneration of the lumbar spine and its relation to aging, spinal degeneration, and segmental motion. J Neurosurg Spine 13(4):494–499
Smith CF, Pugh DG, Polley HF (1955) Physiologic vertebral ligamentous calcification: an aging process. Am J Roentgenol Radium Ther Nucl Med 74(6):1049–1058
Yamada M, Tohno Y, Tohno S, Moriwake Y, Azuma C, Utsumi M, Minami T, Takano Y, Takakura Y (2004) Age-related changes of elements and relationships among elements in human tendons and ligaments. Biol Trace Elem Res 98(2):129–142
Humphreys SC, Hodges SD, Patwardhan, Eck JC, Covington LA, Sartori M (1998) The natural history of the cervical foramen in symptomatic and asymptomatic individuals aged 20–60 years as measured by magnetic resonance imaging. A descriptive approach. Spine (Phila Pa 1976) 23(20):2180–2184
Shim J, Park C, Lee J, Choi J, Lee D, Kim D (2009) A comparison of angled sagittal MRI and conventional MRI in the diagnosis of herniated disc and stenosis in the cervical foramen. Eur Spine J 18:1109–1116
Matsumoto M, Fujimura Y, Suzuki N, Nishi Y, Nakamura M, Yabe Y, Shiga H (1998) MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br 80:19–24
Goto S, Umehara J, Aizawa T, Kokubun S (2010) Comparison of cervical spinal canal diameter between younger and elder generations of Japanese. J Orthop Sci 15(1):97–103
Ishikawa M, Matsumoto M, Fujimura Y, Chiba K, Toyama Y (2003) Changes of cervical spinal cord and cervical spinal canal with age in asymptomatic subjects. Spinal Cord 41(3):159–163
Lee C, Woodring J, Rogers L, Kim K (1986) The radiographic distinction of degenerative slippage (spondylolisthesis and retrolisthesis) from traumatic slippage of the cervical spine. Skeletal Radiol 15:439–443
Park MS, Moon SH, Lee HM, Kim SW, Kim TH, Lee SY, Riew KD (2013) The effect of age on cervical sagittal alignment: normative data on 100 asymptomatic subjects. Spine (Phila Pa 1976) 38(8):E458–E463
Dequeker J, Aerssens J, FP L (2003) Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res 15:426–439
Miyakoshi N, Itoi E, Murai H, Wakabayashi I, Ito H, Minato T (2003) Inverse relation between osteoporosis and spondylosis in postmenopausal women as evaluated by bone mineral density and semiquantitative scoring of spinal degeneration. Spine (Phila Pa 1976) 28:492–495
Rizzoli R, Bruyere O, Cannata-Andia J, Devogelaer J, Lyritis G, Ringe J, Vellas B, Reginster J (2009) Management of osteoporosis in the elderly. Curr Med Res Opin 25:2373–2387
Okada E, Matsumoto M, Ichihara D, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Hashimoto T, Ogawa J, Watanabe M, Takahata T (2009) Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine (Phila Pa 1976) 34(7):706–712
Guadagnoli E, Velicer WF (1988) Relation of Sample Size to the Stability of Component Patterns. Psychol Bull 103(2):265–275
Velicer WF, Fava JL (1998) Effects of Variable and Subject Sampling on Factor Pattern Recovery. Psychol Methods 3(2):231–251
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Wierzbicki, V., Pesce, A., Marrocco, L. et al. How old is your cervical spine? Cervical spine biological age: a new evaluation scale. Eur Spine J 24, 2763–2770 (2015). https://doi.org/10.1007/s00586-014-3673-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3673-4