Abstract
Root-colonizing fungi, such as mycorrhizal fungi and dark septate endophyte fungi, are often found on pioneer plant species during early primary succession. However, little is known about which fungal species are responsible for the establishment of pioneer plants when these symbionts colonize simultaneously. We investigated the root-colonizing fungal communities of Pinus thunbergii that established prior to lichens, bryophytes, and short-lived herbaceous plants in a primary successional volcanic mudflow site on Kuchinoerabu Island, Japan. We collected a total of 54 current-year and 1- to 2-year-old seedlings. The colonization of root fungi was evaluated by direct observation of key structures (e.g., mantle, arbuscule, microsclerotia, and hyphae) and molecular analysis. Of the 34 current-year seedlings collected, only 12 individuals were colonized by ectomycorrhizal (ECM) fungi. By contrast, all 1- to 2-year-old seedlings were colonized by ECM fungi. Seedlings colonized by pine-specific ECM fungi, specifically Rhizopogon roseolus and Suillus granulatus, showed higher nitrogen and phosphorus contents in their needles compared to non-ECM seedlings. Arbuscular mycorrhizal fungi and dark septate endophyte fungi were found in only two and three individuals, respectively. The high density of mycophagous deer on Kuchinoerabu-jima may contribute to the favored dispersal of ECM fungi over other root-colonizing fungi. In conclusion, the seedling establishment of P. thunbergii at the volcanic mudflow may be largely supported by ECM fungi, with negligible effects of arbuscular mycorrhizal fungi and dark septate endophytes.
Similar content being viewed by others
Introduction
Terrestrial plants interact with various fungi through their roots (van der Putten et al. 2013; Wardle et al. 2002). Among these interactions, mycorrhizal fungi positively affect host plants by enhancing water and nutrient absorption as well as providing protection from antagonists in exchange for photosynthetic products (Smith and Read 2008). Ectomycorrhizal (ECM) and arbuscular mycorrhizal (AM) fungi are prominent mycorrhizal groups associated with dominant plant species in global ecosystems (Brundrett 2017). Furthermore, dark septate endophyte (DSE) fungi, which form characteristic structures such as melanized mycelia and microsclerotia instead of mycorrhizal structures (Andrade-Linares and Franken 2013; Rodriguez et al. 2009), have been reported from approximately 600 plant species across various ecosystems (Jumpponen 2001; Jumpponen and Trappe 1998). The coexistence of mycorrhizal and DSE fungi in both seedlings and mature trees within forest ecosystems has also been reported in high-throughput sequencing-based studies (Toju et al. 2013; Yamamoto et al. 2014). Therefore, mycorrhizal and DSE fungi are ubiquitous in plant roots, from seedlings to mature trees, in most natural ecosystems.
As mycorrhizal fungi play a crucial role in nutrient absorption by host plants, the composition of their infection source can be a limiting factor for host plant establishment, particularly in primary successional sites where the infection source can be scarce (Allen 1987; Allen et al. 1992). In the initial stage of primary succession, mycorrhizal propagules such as spores and sclerotia must be transported from the surrounding areas. While the dispersal ability of propagules varies among mycorrhizal fungal types and species, they are dispersed by both biotic (e.g., mammals, birds, and invertebrates) and abiotic (e.g., wind and water) vectors (Horton 2017; Paz et al. 2021). Previous studies have reported that mycorrhizal fungal diversity and composition vary with distance from surrounding vegetation (Ashkannejhad and Horton 2006; Cázares et al. 2005; Wu et al. 2007). In addition, mycorrhizal fungi in primary successional sites are often dominated by a few species that can maintain the infectivity of propagules for long periods in harsh environments (Ashkannejhad and Horton 2006; Dickie et al. 2013). The plant species involved in pioneer invasion also influence the composition of mycorrhizal fungi, particularly ECM fungi (Ashkannejhad and Horton 2006; Ishikawa and Nara 2023; Nara et al. 2003). Therefore, the composition of mycorrhizal fungi in primary successional sites would be affected by various factors and vary among sites.
Plants are usually classified as forming a single type of mycorrhiza. However, some plants can form mycorrhizae with both AM and ECM fungi, either simultaneously within the same root system or at different life stages (Molina et al. 1992). Teste et al. (2020) hypothesized that early colonization by AM fungi may confer advantages to seedlings if the carbon cost associated with AM fungi is lower than that of ECM fungi. Studies have reported that typical ECM host tree species can be colonized by AM fungi that form typical structures such as arbuscules and coils in the roots of the seedlings (Dickie et al. 2001; Horton et al. 1998). This dual mycorrhizal status has been observed in situations with limited infection sources of ECM fungi, such as grasslands and initially in secondary succession. Thus, in primary successional sites with limited sources of mycorrhizal fungi, AM fungi may aid the establishment of plant species that are usually associated with ECM fungi. However, most studies conducted in primary successional environments have focused exclusively on a single type of mycorrhizal fungi (Ashkannejhad and Horton 2006; Nara 2006), and none has considered multiple mycorrhizal types.
Although DSE fungi are ubiquitous among various plant species, their ecological role remains unclear as the results of previous inoculation tests vary from positive to negative (Mayerhofer et al. 2013; Newsham 2011). However, DSE fungi are considered to have neutral or positive effects in strongly stressed environments including early primary succession, particularly when mycorrhizal infection sources are limited (Cázares et al. 2005; Fukuchi et al. 2011; Tejesvi et al. 2010). Moreover, DSE fungi promote AM fungal colonization and enhance phosphorus absorption (Della Monica et al. 2015; Gooden et al. 2020; Wagg et al. 2008). DSE fungi can also interact with ECM fungi, although few previous studies have simultaneously assessed colonization rates of DSE and ECM fungi. Horton et al. (1998) observed that DSE fungi rapidly colonized Pinus muricata seedlings before mycorrhizal fungi after a forest fire but did not mention the effect on seedling growth. Thus, the interactions between DSE and mycorrhizal fungi and their effects on seedling establishment (e.g., increased biomass and nutrient absorption) during primary succession remain largely unknown.
Studies of primary succession in Japan have been conducted at various volcanic sites (Iida et al. 2021; Kamijo et al. 2002; Ohsawa 1984; Teramoto et al. 2017; Tsuyuzaki et al. 2005). Pioneer herbaceous plant species are often dominated by Polygonaceae, Cyperaceae, and Poaceae throughout the region. By contrast, the pioneer tree species vary among sites and change with altitude and latitude. For example, Salix and Alnus species are commonly observed in volcanic sites located in northern or high elevational areas, while Pinus thunbergii is recognized as a pioneer species after eruptions in southern Japan (Teramoto et al. 2017). Pinus thunbergii is also distributed in coastal forests in Japan, Korea, and China, where their ECM fungal communities have been reported in several studies (Matsuda et al. 2009; Obase et al. 2009). However, no previous studies have examined root-colonizing fungi including ECM fungi associated with pioneer P. thunbergii after volcanic eruptions.
In late May 2015, a large eruption occurred on Kuchinoerabu Island, located in southern Japan. Pinus thunbergii is the most dominant pioneer plant on bare areas formed by lahars (i.e., volcanic mudflows caused by rainwater) that occurred after the eruption, establishing in advance of lichens, bryophytes, and short-lived herbaceous plants. These pine seedlings are sporadically and individually established in this volcanic site, providing a unique opportunity to investigate the relationship between seedling establishment and root-colonizing fungi.
In this study, we investigated the effects of root-colonizing fungi, including AM, ECM, and DSE fungi, on the nutrient status of P. thunbergii seedlings in a volcanic mudflow area on Kuchinoerabu-jima. Both molecular identification and direct microscopic observation approaches were used because direct observation of fungal structures is necessary to accurately quantify fungal infection in plants (Teste et al. 2020).
Materials and methods
Study site and field sampling
This study was conducted on Kuchinoerabu Island (30°28′ N, 130°12′ E), located ~12 km west of Yakushima Island, Kagoshima Prefecture, southern Japan (Fig. 1). Kuchinoerabu-jima is located in a warm temperate zone with an annual mean temperature of 19.6 °C and annual mean precipitation of 4651.7 mm, according to records from the nearest Yakushima observatory station (from 1991 to 2020; Japan Meteorological Agency: http://www.jma.go.jp). Mount Shindake (626 m above sea level), located on the eastern part of the island, is an active volcano that repeatedly erupts at about 20-year intervals (Tameguri et al. 2016). The last eruption that occurred in 2015 was relatively large, forcing all residents on the island to be evacuated (Tameguri et al. 2016). A pyroclastic flow from this eruption spread west-northwest from the central crater and reached Mukaehama beach. This pyroclastic flow consisted mainly of hot gas, which killed almost all trees; the deposition of ashes was not extensive (Geshi and Itoh 2018). Subsequently, a lahar occurred due to heavy rains after the eruption that deposited ash-mud substrates within a valley toward Mukaehama beach in 2015 (Geshi and Itoh 2018). Lahar deposition with a depth of more than 2 m in the valley formed a bare area ~100 m wide for ~500 m from Mukaehama beach. A pyroclastic flow killed the vegetation on the northern slope of this bare area, but the secondary evergreen forest on the southern slope surrounding the valley remained. Several mature P. thunbergii trees that formed pinecones also survived in the center of the bare area, located in topographically safe patches.
Pioneer plants in this site include P. thunbergii, Toxicodendron succedaneum, Miscanthus sinensis, and Histiopteris incisa. These were often observed in the shadows of large rocks where the surface runoff water during heavy rains does not erode the soil substrates. Lichens and bryophytes were scarcely established in this bare area. The secondary evergreen forest on the southern slope of the bare area was mainly composed of Castanopsis sieboldii, Schefflera heptaphylla, Ardisia sieboldii, and Maesa perlarius var. formosana, and P. thunbergii was also distributed on the forest edge facing the bare area. In addition, the coastal forest along Mukaehama beach was mainly composed of P. thunbergii.
In Nov. 2021, we sampled 54 current- to second-year pine seedlings (< 10 cm in height). The sampling points were at least 1 m apart, and their geographical positions were recorded by GPS (Garmin 62 S; Garmin International, Olathe, KS, USA). The collected samples were placed separately in plastic bags and stored at 4 °C until use.
Location of Kuchinoerabu-jima and the study site. a Location of Kuchinoerabu-jima (black arrow). b Location of the study site (red shading) and crater (white star) on the island. c Distribution of Pinus thunbergii seedlings at the study site; white circle: current-year non- ECM seedling; red circle: current-year ECM seedlings; red diamond: 1- to 2-year-old ECM seedling; black diamond: mature P. thunbergii tree. Shaded areas represent remnant forests
Measurement of fungal colonization and needle nitrogen and phosphorus
Each seedling was separated into shoot and root parts. Root samples were carefully cleaned of soil and debris with tap water. ECM root tips were classified into morphotypes using a dissecting microscope based on their surface color, shape, texture, and emanating hyphae (Agerer 1991). We collected up to three ECM root tips upon availability for each morphotype per seedling, placed them into separate 2.0 mL tubes containing 50 µL cetyltrimethylammonium bromide (CTAB) buffer, and stored them at −30 °C until use.
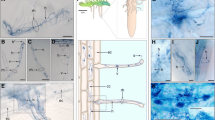
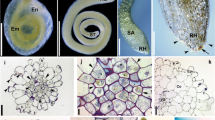
After ECM sampling, root samples were divided into halves based on fresh weight and used to measure AM and DSE fungal colonization. Half of the roots were cut into 1–2 cm fragments and autoclaved twice in 10% KCl at 121 °C for 20 min with replacement of the KCl sol. The autoclaved roots were rinsed with tap water and bleached in alkaline hydrogen peroxide (0.5% v/v NH4OH and H2O2; Teste and Laliberté 2018) at 60 °C for 45 min. After rinsing the breached roots with tap water, they were immersed in 2% HCl for 30 min. Finally, the acidified roots were stained using a 0.05% Trypan blue solution at 90 °C for 15 min. AM and DSE fungal colonization were identified by the presence/absence of AM structures (e.g., hyphae, arbuscule, and vesicle) and endophyte structures (e.g., hyphae and microsclerotia) using a light microscope (Eclipse E600; Nikon, Tokyo, Japan).
The other half of the roots were oven-dried at 70 °C for 48 h with the shoot sample and weighed. Dried root samples were stored at −30 °C until molecular extraction. Dried needle samples were digested with sulfuric acid and hydrogen peroxide (Lindner and Harley 1942). Nitrogen and phosphorus concentrations were determined by the indophenol blue method and the molybdenum blue method, respectively (Rodriguez et al. 1994; Scheiner 1976). The nitrogen and phosphorus contents of the needles were calculated from the nitrogen or phosphorus concentration per sample and the dry weight of the needles, excluding the stem.
Molecular analyses and fungal identification
We extracted DNA from each ECM root tip using the CTAB method (Nara et al. 2003). DNA was also extracted from 20 mg of fine roots collected from each of the dried root samples using a DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. The internal transcribed spacer (ITS) region of rDNA from the ECM root tip was amplified by polymerase chain reaction (PCR) using the primers ITSOF-T (forward) and LB-W (reverse) (Tedersoo et al. 2008). For unamplified ECM samples, another reverse primer (ITS4) was used (Tedersoo et al. 2008; White et al. 1990).
For the dried root samples from which DSE fungal structures were confirmed, primers ITSOF-T and ITS4 were used to amplify DSE fungi. Similarly, for dried root samples with confirmed AM structures, the small subunit (SSU) rDNA of AM fungi was amplified using the primers AML1 and AML2 (Lee et al. 2008). PCR was performed using an Emerald Amp PCR Master Mix Kit (Takara Bio, Shiga, Japan) under the following conditions: 30 cycles of 98 °C for 10 s, 56 °C for 30 s, and 72 °C for 60 s. To detect DSE and AM fungi in roots where no fungal structures were observed under the microscope, seven dried root samples were randomly selected from microscopically uncolonized seedlings and subjected to the same PCR amplification for DSE and AM. DNA amplification was confirmed by 2% agarose gel electrophoresis.
Amplified products of ECM and AM fungi were purified using ExoSAP-IT (Applied Biosystems, Foster City, CA, USA) and subjected to direct sequencing on a 3730xl DNA Analyzer (Applied Biosystems). Sequencing reactions were performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) with primers ITS1 or ITS4 (ITS regions), or AML1 or AML2 (SSU regions). Because direct sequencing was unsuccessful for DSE products, they were cloned using the Mighty TA-cloning Kit (Takara Bio) following the manufacturer’s protocol. For each DSE product, 12 positive colonies were selected and subjected to PCR with primers ITSOF-T and ITS4 under the same conditions as the initial amplification. Products of colony PCR were purified and sequenced in the same way as ECM and AM fungi.
The sequences of the ITS or SSU regions were assembled after manual trimming and correction using Sequence Scanner Software 2 (ver. 2.0; Applied Biosystems) and ATGC ver. 7 (Genetyx, Tokyo, Japan). High-quality sequences ≥ 250 bp were clustered into operational taxonomic units (OTUs) based on ≥ 97% identity threshold in the VSEARCH program (Rognes et al. 2016). OTU clustering with ≥ 98.5% identity threshold was also performed, because the most frequently used 97% threshold could be too conservative for species-level classification (Nilsson et al. 2019). The taxonomic identities of the clustered consensus sequences, as well as the unclustered sequences with ≥ 350 bp, were determined by BLAST searches against the International Nucleotide Sequence Database Collaboration (INSDC) and UNITE databases (Kõljalg et al. 2005). For DSE fungal identification, OTUs with DSE fungi in the UNITE Species Hypotheses database (at the 1.5% distance threshold; ver. 9 release 2022.10.17) closest to the query sequence were considered DSE fungi, while non-DSE fungi were excluded in this study. The identified ITS and SSU sequences have been deposited in the DNA Data Bank of Japan under accession numbers LC786761–LC786774.
Data analyses
The sample size-based rarefaction and extrapolation curve as well as the Chao2 richness estimator for ECM fungi were calculated for both current-year and 1- to 2-year-old seedlings using Estimate S ver. 9.1 (Colwell et al. 2012) with 1000 randomizations. All analyses described below were performed with R ver. 4.2.2 (R Core Team 2022). The frequency of ECM fungal species between current-year and 1- to 2-year-old seedlings was compared using Fisher’s exact test. The effects of seedling age and ECM, AM, and DSE fungal colonization on pine seedling dry weight, shoot/root ratio, and needle nitrogen or phosphorus contents were analyzed using a generalized linear model (GLM) with gamma error distributions and a log link function. The full model (response variables ~ Age + ECM + AM + DSE) was simplified to select the best model based on Akaike’s information criterion using the “StepAIC” function from the MASS package (Venables and Ripley 2002). In addition, seedling growth conditions and nutrient contents were compared among the major ECM fungal lineages (i.e., Amphinema, suilloid [Rhizopogon and Suillus], and uncolonized) by Tukey’s post hoc test with the “glht” function in the multcomp package for current-year and 1- to 2-year-old seedlings, respectively (Hothorn et al. 2008), while pine seedlings that were colonized by multiple EM fungal OTUs were excluded from this analysis because of the difficulty in isolating the effect of each EM fungus. We also analyzed the correlation between distance from remnant vegetation and colonization by root-colonizing fungi or seedling age using a GLM with binomial error distribution and logit link function.
Results
We collected 34 current-year seedlings and 20 1- to 2-year-old seedlings (Table 1). Among the current-year seedlings, 12 individuals were colonized by ECM fungi, while the remaining 22 were not. By contrast, ECM fungi colonized all 1- to 2-year-old seedlings. The ECM colonization tended to be located in close proximity to the remaining vegetation, specifically, within a 35 m distance, although no significant correlation was observed between ECM and non-ECM seedling distribution by distance (P = 0.48). Similarly, no significant correlation was found between seedling age and proximity to the remaining vegetation (P = 0.94).
After morphotyping, we collected 37 and 62 ECM root tips from current-year and 1- to 2-year-old seedlings, respectively. Subsequent sequencing of the 99 ECM root tips yielded 85 high-quality sequences (≥ 250 bp), all of which belonged to ECM fungi and were grouped into nine OTUs at both the 97% and 98.5% identity thresholds (Table 1). Suillus granulatus was the most abundant species, detected in eight individuals, followed by Rhizopogon roseolus and Amphinema sp.1, each found in seven individuals (Table 1).
A total of four ECM fungal species were identified from current-year seedlings, whereas nine were identified from 1- to 2-year-old seedlings. Rarefaction and extrapolation curves for observed ECM fungal species in this study reached a plateau for current-year seedlings but not for the others (Fig. 2). The Chao2 richness estimators were 4 and 18, respectively. Fisher’s exact tests indicated that the frequency of ECM fungal species significantly differed between the groups (Table 1). Amphinema sp.1 was most frequently detected in current-year seedlings, followed by R. roseolus. By contrast, S. granulatus was the most frequently detected species in 1- to 2-year-old seedlings, followed by R. roseolus and Amphinema sp. 2.
Sample-based rarefaction curves for ECM fungi on current-year (filled red) and 1- to 2-year-old (open black) seedlings of Pinus thunbergii in a primary successional volcanic mudflow site 6 years after the 2015 eruption on Kuchinoerabu-jima. Dotted lines indicate extrapolations. Shaded areas represent 95% confidence intervals
AM fungal structures were observed in 2 of 54 pine seedlings, one from a current-year seedling and the other from a 1- to 2-year-old seedling (Fig. 3). Both seedlings were simultaneously colonized by ECM fungi in other root tips. All AM fungal sequences obtained from these seedlings were clustered into a single OTU, Rhizophagus sp., at both the 97% and 98.5% identity thresholds. No valid AM sequences were obtained from the seven randomly selected root samples with no observable fungal colonization.
DSE fungal colonization was confirmed in three individuals, one from a current-year seedling and two from the other seedlings, with distinctive microsclerotium and septate hyphae observed under the microscope. While the current-year DSE seedling was not accompanied by any ECM fungus, the two other seedlings were simultaneously colonized by ECM fungi. After sequencing, a total of four DSE fungi were identified from microscopically confirmed roots at both the 97% and 98.5% identity thresholds. One to three DSE fungal OTUs were identified for each colonized seedling. Although some sequences of non-DSE fungal species, including yeasts and common soil fungi, were obtained from the randomly selected root samples lacking fungal structures, they were excluded from further analyses.
Seedling age significantly influenced seedling dry weight and needle nitrogen and phosphorus but not the shoot/root ratio (Table 2). In GLM analyses, ECM colonization significantly increased shoot/root ratios and needle nitrogen and phosphorus contents. The effect of DSE fungal colonization on needle nitrogen and phosphorus contents was marginally significant but the contribution was less than that explained by ECM colonization. In pairwise comparisons, both Amphinema species and suilloid species (S. granulatus and R. roseolus) increased shoot/root ratios and needle nitrogen and phosphorus contents more than non-ECM fungi in current-year seedlings (Fig. 4). In 1- to 2-year-old seedlings, growth and nutrient status did not significantly differ between Amphinema and suilloid species (Fig. S1).
Only explanatory parameters of selected models based on Akaike’s information criterion are shown.
Effects of ectomycorrhizal colonization on the growth and nutrient status of current-year Pinus thunbergii seedlings in a primary successional volcanic mudflow site 6 years after the 2015 eruption on Kuchinoerabu-jima (Tukey’s post hoc tests: *P < 0.05, **P < 0.01, ***P < 0.001). a Seedling dry weight; b shoot/root ratio; c needle nitrogen content; d needle phosphorus content; none: no ECM colonization. The Amphinema and suilloid lineages include two Amphinema species and Rhizopogon roseolus/Suillus granulatus, respectively
Discussion
Nearly 65% of current-year seedlings collected at the volcanic mudflow site on Kuchinoerabu-jima were not colonized by mycorrhizal or DSE fungi. This indicates that soil propagule banks of root-colonizing fungi have yet to develop ubiquitously in this primary successional site. While 6 years had passed since the last eruption, this duration may not be sufficient to develop soil propagule banks. Unstable volcanic mudflow substrates that easily move by rainwater would make propagule accumulation in the soil more difficult. In contrast to the current-year seedlings, all of the 1- to 2-year-old seedlings were colonized by ECM fungi. This suggests that, of the current-year seedlings, only those colonized by ECM fungi would survive the winter, whereas most non-ECM seedlings would die out due to nutrient deficiency. Another possibility is that enough ECM fungal spores are dispersed every year from the neighboring forests that non-colonized current-year seedlings would develop ECM symbiosis within a year. A previous study in a heathland also reported that invading pine seedlings are able to remain non-mycorrhizal for a few years and await compatible ECM spores to colonize before starting growth (Collier and Bidartondo 2009).
ECM fungi observed in this volcanic site were dominated by Rhizopogon, Suillus, and Amphinema (Table 1). These ECM fungal lineages have been reported from pine seedlings at invasion forefronts in non-native regions (Policelli et al. 2019) as well as in a recently stabilized sand dune (Ashkannejhad and Horton 2006). Thus, propagules of these fungi should have good dispersal abilities. Indeed, Suillus spores can be dispersed more than 1 km by wind (Hynson et al. 2013; Peay et al. 2012), and probably further in the prevailing strong winds on this oceanic island. Furthermore, viable spores of suilloid and Amphinema spores can be dispersed up to hundreds of meters by mycophagous deer, as evidenced by successful ECM formation on pine seedlings with fecal matter inoculation (Ashkannejhad and Horton 2006; Nuñez et al. 2013). Kuchinoerabu-jima has a substantial population of Cervus nippon yakushimae (Ishida 2019), known to forage on litter and fungi (Agetsuma et al. 2011). Thus, they may contribute to the dispersal of ECM fungal spores from the surrounding remnant forest into the volcanic mudflow site, particularly hypogeous taxa such as Rhizopogon.
Compared to ECM colonization, there were much fewer cases of AM and DSE colonization, observed on 2 and 3 of 54 pine seedlings, respectively. This indicates that propagules of these fungi are less available to pine seedlings than ECM fungi at this volcanic site. In a secondary successional site after a forest fire, AM and DSE fungal colonization on regenerating pine seedlings was more frequent (Horton et al. 1998). Thus, while propagules of these fungi in soil could survive the fire, such propagules are not available in volcanic substrates. In the case of AM fungi, spore sizes are far larger than ECM fungi, which makes dispersal with wind to primary successional sites more difficult (Allen et al. 2005; Cázares et al. 2005). Furthermore, Wagg et al. (2008) suggested that AM fungi on Pinaceae, typically an ECM host plant, represent secondary colonization from hyphae of neighboring AM plants. Because pine seedlings in this volcanic mudflow site establish solitarily without neighboring AM plants, the absence of such secondary colonization may also partly account for the lower AM colonization. While little is known about the dispersal of DSE fungi, studies have suggested that fragmented mycelia and microsclerotia can be dispersed and serve as infection sources (Jumpponen and Trappe 1998; Yu et al. 2001). Furthermore, some studies have identified DSE fungal taxa within airborne fungal communities (Kauserud et al. 2005; Kivlin et al. 2014), probably in adhesion to soil particles (Jumpponen et al. 2017). In any case, such dispersal mechanisms should be infrequent at this primary successional site.
ECM colonization significantly increased nitrogen and phosphorus levels in needles of current-year seedlings; ECM seedlings had 2.6- to 2.7-fold higher nitrogen and 2.6- to 3.6-fold higher phosphorus levels than non-ECM seedlings. Suilloid and Amphinema species, both dominant in this volcanic site, develop extensive rhizomorphs (Agerer 2001), enabling efficient exploration of soil nutrients. Moreover, suilloid hyphae produce organic acids, solubilizing mineral nutrients from the rock surface (Casarin et al. 2004; Courty et al. 2010; van Schöll et al. 2008). Increased shoot/root ratio by ECM colonization would also indicate improved nutrient absorbing abilities with the extensive ECM mycelial systems, as seedlings can invest more carbon into shoots (Colpaert et al. 1996; Wallander 2000). Extensive rhizomorphs formed by these fungi also promote water absorption and transport (Duddridge et al. 1980; Parke et al. 1983), potentially contributing to higher survival rates under the frequent drought stress in volcanic substrates. Considering the dominance of these ECM fungi in the naturally established seedlings and the enhanced nutrient status by their colonization, they should play critical roles in pine seedling establishment in this primary successional site. While ECM fungal species on pioneer trees vary among volcanic sites (e.g., generalists such as Laccaria and Inocybe on Salix on Mts. Fuji and Usu [Nara et al. 2003; Obase et al. 2007] and specialists such as Alpova on Alnus seedlings on Izu-Oshima [Ishikawa and Nara 2023]), positive effects of ECM fungi on the establishment of pioneer seedlings are consistently supported (Dickie et al. 2013).
Compared to ECM fungi, colonization by AM or DSE fungi was far less frequent, even in 1- to 2-year-old seedlings. Moreover, the effects of AM and DSE fungi on seedling nutrient status remained nonsignificant and marginally significant, respectively. These findings indicate that AM and DSE fungi have negligible roles in pine seedling establishment at this volcanic site. In experimental conditions, AM fungal colonization has been shown to increase the foliar phosphorus concentration of Douglas fir seedlings (Smith et al. 1998); however, no previous studies have reported positive effects of AM colonization on typically ECM-host species under natural conditions. While direct evidence of DSE fungal functions in seedling establishment processes in the field is also unavailable, mineralization and solubilization of organic compounds by extracellular enzymes produced by DSE fungi could potentially benefit seedlings (Knapp and Kovács 2016; Mandyan and Jumpponen 2005; Newsham 2011). As host responses vary with DSE/mycorrhizal colonization ratios (Mandyan and Jumpponen 2005; Reininger and Sieber 2012; Xie et al. 2021), further colonization by DSE fungi with the development of soil propagule banks may potentially contribute to seedling establishment in the future.
In conclusion, we confirmed the colonization of AM and DSE fungi on naturally established pine seedlings in a primary successional site after the volcanic eruption, although their contribution to seedling nutrient status and growth remained insignificant with the extremely low frequencies of the colonized seedlings. In contrast, all 1- to 2-year-old seedlings and about 1/3 of the current-year seedlings were colonized by ECM fungi, dominated by Amphinema spp. and suilloid fungi with the extensive mycelial exploration type. Leaf nitrogen and phosphorus contents of the current-year seedlings colonized by these ECM fungi were far more than double that of the uncolonized seedlings of the same age. Our results indicate that these ECM fungi, not AM and DSE fungi, play the primary role as symbiotic microbes in the seedling establishment of the pioneer pine in this volcanic site.
Data availability
No datasets were generated or analysed during the current study.
References
Agerer R (1991) Characterization of Ectomycorrhiza. In: Norris J, Read D, Varma A (eds) Techniques for the study of Mycorrhiza. Academic, London, pp 25–73
Agerer R (2001) Exploration type of ectomycorrhizae. Mycorrhiza 11:107–114. https://doi.org/10.1007/s005720100108
Agetsuma N, Agetsuma-Yanagihara Y, Takafumi H (2011) Food habits of Japanese deer in an evergreen forest: litter-feeding deer. Mamm Biol 76:201–207. https://doi.org/10.1016/j.mambio.2010.04.002
Allen MF (1987) Re-establishment of mycorrhizas on Mount St Helens: Migration vectors. Trans Br Mycol Soc 88:413–417. https://doi.org/10.1016/s0007-1536(87)80019-0
Allen MF, Crisafulli C, Friese CF, Jeakins SL (1992) Re-formation of mycorrhizal symbioses on Mount St Helens, 1980–1990: interactions of rodents and mycorrhizal fungi. Mycol Res 96:447–453. https://doi.org/10.1016/S0953-7562(09)81089-7
Allen MF, Crisafulli CM, Morris SJ et al (2005) Mycorrhizae and Mount St. Helens: story of a symbiosis. In: Dale VH, Swanson FJ, Crisafulli CM et al (eds) Ecological responses to the 1980 eruption of Mount St. Helens. Springer, New York, pp 221–231
Andrade-Linares DR, Franken P (2013) Fungal endophytes in plant roots: taxonomy, colonization patterns, and functions. In: Aroca R (ed) Symbiotic endophytes. Springer, Berlin, pp 311–334
Ashkannejhad S, Horton TR (2006) Ectomycorrhizal ecology under primary succession on coastal sand dunes: interactions involving Pinus contorta, suilloid fungi and deer. New Phytol 169:345–354. https://doi.org/10.1111/j.1469-8137.2005.01593.x
Brundrett MC (2017) Global Diversity and Importance of Mycorrhizal and nonmycorrhizal plants. In: Tedersoo L (ed) Biogeography of Mycorrhizal Symbiosis. Springer, Cham, pp 533–556
Casarin V, Plassard C, Hinsinger P, Arvieu JC (2004) Quantification of ectomycorrhizal fungal effects on the bioavailability and mobilization of soil P in the rhizosphere of Pinus pinaster. New Phytol 163:177–185. https://doi.org/10.1111/j.1469-8137.2004.01093.x
Cázares E, Trappe JM, Jumpponen A (2005) Mycorrhiza-plant colonization patterns on a subalpine glacier forefront as a model system of primary succession. Mycorrhiza 15:405–416. https://doi.org/10.1007/s00572-004-0342-1
Collier FA, Bidartondo MI (2009) Waiting for fungi: the ectomycorrhizal invasion of lowland heathlands. J Ecol 97:950–963. https://doi.org/10.1111/j.1365-2745.2009.01544.x
Colpaert JV, van Laere A, van Assche JA, Jan V, Colpaert A, van LJA A (1996) Carbon and nitrogen allocation in ectomycorrhizal and non-mycorrhizal Pinus sylvestris L. seedlings. Tree Physiol 16:787–793. https://doi.org/10.1093/treephys/16.9.787
Colwell RK, Chao A, Gotelli NJ et al (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5:3–21. https://doi.org/10.1093/jpe/rtr044
Courty PE, Buée M, Diedhiou AG et al (2010) The role of ectomycorrhizal communities in forest ecosystem processes: new perspectives and emerging concepts. Soil Biol Biochem 42:679–698. https://doi.org/10.1016/j.soilbio.2009.12.006
Della Monica IF, Saparrat MCN, Godeas AM, Scervino JM (2015) The co-existence between DSE and AMF symbionts affects plant P pools through P mineralization and solubilization processes. Fungal Ecol 17:10–17. https://doi.org/10.1016/j.funeco.2015.04.004
Dickie IA, Koide RT, Fayish AC (2001) Vesicular-arbuscular mycorrhizal infection of Quercus rubra seedlings. In: New Phytologist. pp 257–264
Dickie IA, Martínez-García LB, Koele N et al (2013) Mycorrhizas and mycorrhizal fungal communities throughout ecosystem development. Plant Soil 367:11–39. https://doi.org/10.1007/s11104-013-1609-0
Duddridge J, Malibari A, Read D (1980) Structure and function of mycorrhizal rhizomorphs with special reference to their role in water transport. Nature 287:834–836. https://doi.org/10.1038/287834a0
Fukuchi S, Obase K, Tamai Y et al (2011) Vegetation and colonization status of mycorrhizal and endophytic fungi in plant species on acidic barren at crater basin of volcano Esan in Hokkaido, Japan. Eurasian J Res 14:1–11. http://hdl.handle.net/2115/47114
Geshi N, Itoh J (2018) Pyroclastic density currents associated with the 2015 phreatomagmatic eruption of the Kuchinoerabujima volcano. Earth Planets and Space 70:119. https://doi.org/10.1186/s40623-018-0881-x
Gooden B, Thompson ER, French K (2020) Do native plant associations with arbuscular mycorrhizal fungi and dark septate endophytes differ between reconstructed and remnant coastal dunes? Plant Ecol 221:757–771. https://doi.org/10.1007/s11258-019-00959-4
Horton TR (2017) Spore dispersal in ectomycorrhizal fungi at fine and regional scales. In: Tedersoo L (ed) Biogeography of Mycorrhizal Symbiosis. Springer, Cham, pp 61–78
Horton TR, Cázares E, Bruns TD et al (1998) Ectomycorrhizal, vesicular-arbuscular and dark septate fungal colonization of bishop pine (Pinus muricata) seedlings in the first 5 months of growth after wildfire. Mycorrhiza 8:11–18. https://doi.org/10.1007/s005720050205
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Hynson NA, Merckx VSFT, Perry BA, Treseder KK (2013) Identities and distributions of the co-invading ectomycorrhizal fungal symbionts of exotic pines in the Hawaiian Islands. Biol Invasions 15:2373–2385. https://doi.org/10.1007/s10530-013-0458-3
Iida K, Hayasaka D, Suzuki Y, Uchida T, Sawahata T, Hashimoto K (2021) Legacy of pre-eruption vegetation affects ground-dwelling arthropod communities after different types of volcanic disturbance. Ecol Evol 11:9110–9122. https://doi.org/10.1002/ece3.7755
Ishida H (2019) Species composition and species richness of Machilus Thunbergii secondary forests on Kuchinoerabujima Island, Japan. Veg Sci 36:71–79. https://doi.org/10.15031/vegsci.36.71
Ishikawa A, Nara K (2023) Primary succession of ectomycorrhizal fungi associated with Alnus sieboldiana on Izu-Oshima Island, Japan. Mycorrhiza 33:187–197. https://doi.org/10.1007/s00572-023-01112-w
Jumpponen A (2001) Dark septate endophytes - are they mycorrhizal? Mycorrhiza 11:207–211. https://doi.org/10.1007/s005720100112
Jumpponen A, Trappe JM (1998) Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol 140:295–310. https://doi.org/10.1046/j.1469-8137.1998.00265.x
Jumpponen A, Herrera J, Porras-Alfaro A, Rudgers J (2017) Biogeography of root-associated fungal endophytes. In: Tedersoo L (ed) Biogeography of Mycorrhizal Symbiosis. Springer, Cham, pp 195–222
Kamijo T, Kitayama K, Sugawara A et al (2002) Primary successional of the warm-temperate broad-leaved forest on a volcanic island Miyake-Jima, Japan. Folia Geobot 37:71–91. https://doi.org/10.1007/BF02803192
Kauserud H, Lie M, Stensrud Ø, Ohlson M (2005) Molecular characterization of airborne fungal spores in boreal forests of contrasting human disturbance. Mycologia 97:1215–1224. https://doi.org/10.3852/mycologia.97.6.1215
Kivlin SN, Winston GC, Goulden ML, Treseder KK (2014) Environmental filtering affects soil fungal community composition more than dispersal limitation at regional scales. Fungal Ecol 12:14–25. https://doi.org/10.1016/j.funeco.2014.04.004
Knapp DG, Kovács GM (2016) Interspecific metabolic diversity of root-colonizing endophytic fungi revealed by enzyme activity tests. FEMS Microbiol Ecol 92:fiw190. https://doi.org/10.1093/femsec/fiw190
Kõljalg U, Larsson KH, Abarenkov K et al (2005) UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol 166:1063–1068. https://doi.org/10.1111/j.1469-8137.2005.01376.x
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 65:339–349. https://doi.org/10.1111/j.1574-6941.2008.00531.x
Lindner RC, Harley CP (1942) A rapid method for the determination of nitrogen in plant tissue. Science (1979) 96:565–566. https://doi.org/10.1126/science.96.2503.565
Mandyan K, Jumpponen A (2005) Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud Mycol 53:173–189. https://doi.org/10.3114/sim.53.1.173
Matsuda Y, Noguchi Y, Ito S (2009) Ectomycorrhizal fungal community of naturally regenerated Pinus thunbergii seedlings in a coastal pine forest. J for Res 14:335–341. https://doi.org/10.1007/s10310-009-0140-x
Mayerhofer MS, Kernaghan G, Harper KA (2013) The effects of fungal root endophytes on plant growth: a meta-analysis. Mycorrhiza 23:119–128. https://doi.org/10.1007/s00572-012-0456-9
Molina R, Massicotte H, Trappe JM (1992) Specificity phenomena in mycorrhizal symbiosis: community-ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning: an integrative plant-fungal process. Chapman and Hall, New York, pp 357–423
Nara K (2006) Pioneer dwarf willow may facilitate tree succession by providing late colonizers with compatible ectomycorrhizal fungi in a primary successional volcanic desert. New Phytol 171:187–198. https://doi.org/10.1111/j.1469-8137.2006.01744.x
Nara K, Nakaya H, Wu B et al (2003) Underground primary succession of ectomycorrhizal fungi in a volcanic desert on Mount Fuji. New Phytol 159:743–756. https://doi.org/10.1046/j.1469-8137.2003.00844.x
Newsham KK (2011) A meta-analysis of plant responses to dark septate root endophytes. New Phytol 190:783–793. https://doi.org/10.1111/j.1469-8137.2010.03611.x
Nilsson RH, Anslan S, Bahram M et al (2019) Mycobiome diversity: high-throughput sequencing and identification of fungi. Nat Rev Microbiol 17:95–109. https://doi.org/10.1038/s41579-018-0116-y
Nuñez MA, Hayward J, Horton TR et al (2013) Exotic mammals disperse exotic fungi that promote invasion by exotic trees. PLoS ONE 8:e66832. https://doi.org/10.1371/journal.pone.0066832
Obase K, Tamai Y, Yajima T, Miyamoto T (2007) Mycorrhizal associations in woody plant species at the Mt. Usu Volcano, Japan. Mycorrhiza 17:209–215. https://doi.org/10.1007/s00572-006-0097-y
Obase K, Cha JY, Lee JK et al (2009) Ectomycorrhizal fungal communities associated with Pinus thunbergii in the eastern coastal pine forests of Korea. Mycorrhiza 20:39–49. https://doi.org/10.1007/s00572-009-0262-1
Ohsawa M (1984) Differentiation of vegetation zones and species strategies in the subalpine region of Mt. Fuji Vegetatio 57:15–52. https://doi.org/10.1007/BF00031929
Parke EL, Linderman RG, Black CH (1983) The role of ectomycorrhizas in drought tolerance of Douglas fir seedlings. New Phytol 95:83–95. https://doi.org/10.1111/j.1469-8137.1983.tb03471.x
Paz C, Öpik M, Bulascoschi L et al (2021) Dispersal of arbuscular mycorrhizal fungi: evidence and insights for Ecological studies. Microb Ecol 81:283–292. https://doi.org/10.1007/s00248-020-01582-x
Peay KG, Schubert MG, Nguyen NH, Bruns TD (2012) Measuring ectomycorrhizal fungal dispersal: macroecological patterns driven by microscopic propagules. Mol Ecol 21:4122–4136. https://doi.org/10.1111/j.1365-294X.2012.05666.x
Policelli N, Bruns TD, Vilgalys R, Nuñez MA (2019) Suilloid fungi as global drivers of pine invasions. New Phytol 222:714–725. https://doi.org/10.1111/nph.15660
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reininger V, Sieber TN (2012) Mycorrhiza reduces adverse effects of dark septate endophytes (DSE) on growth of conifers. PLoS ONE 7:e42865. https://doi.org/10.1371/journal.pone.0042865
Rodriguez JB, Self JR, Soltanpour PN (1994) Optimal conditions for phosphorus analysis by the ascorbic acid-molybdenum blue method. Soil Sci Soc Am J 58:866–870. https://doi.org/10.2136/sssaj1994.03615995005800030034x
Rodriguez RJ, White JF, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182:314–330. https://doi.org/10.1111/j.1469-8137.2009.02773.x
Rognes T, Flouri T, Nichols B et al (2016) VSEARCH: A versatile open source tool for metagenomics. PeerJ. https://doi.org/10.7717/peerj.2584
Scheiner D (1976) Determination of ammonia and Kjeldahl nitrogen by indophenol method. Water Res 10:31–36. https://doi.org/10.1016/0043-1354(76)90154-8
Smith S, Read D (2008) Mycorrhizal Symbiosis, 3rd Edition. Academic Press, Cambridge
Smith J, Johnson K, Cázares E (1998) Vesicular mycorrhizal colonization of seedlings of Pinaceae and Betulaceae after spore inoculation with Glomus intraradices. Mycorrhiza 7:279–285. https://doi.org/10.1007/s005720050193
Tameguri T, Nakamichi H, Yamamoto K et al (2016) Disaster research of Kuchinoerabujima eruptions in 2014–2015. Disaster Prev Res Inst 59:85–90
Tedersoo L, Jairus T, Horton BM et al (2008) Strong host preference of ectomycorrhizal fungi in a tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180:479–490. https://doi.org/10.1111/j.1469-8137.2008.02561.x
Tejesvi MV, Ruotsalainen AL, Markkola AM, Pirttilä AM (2010) Root endophytes along a primary succession gradient in northern Finland. Fungal Divers 41:125–134. https://doi.org/10.1007/s13225-009-0016-6
Teramoto Y, Shimokawa E, Ezaki T et al (2017) Influence of volcanic activity on vegetation succession and growth environment on the hillslope of Sakurajima volcano in southern Kyushu, Japan. J Res (Harbin) 28:309–317. https://doi.org/10.1007/s11676-016-0312-4
Teste FP, Laliberté E (2018) Plasticity in root symbioses following shifts in soil nutrient availability during long-term ecosystem development. J Ecol 107:633–649. https://doi.org/10.1111/1365-2745.13103
Teste FP, Jones MD, Dickie IA (2020) Dual-mycorrhizal plants: their ecology and relevance. New Phytol 225:1835–1851. https://doi.org/10.1111/nph.16190
Toju H, Yamamoto S, Sato H et al (2013) Community composition of root-associated fungi in a Quercus-dominated temperate forest: codominance of mycorrhizal and root-endophytic fungi. Ecol Evol 3:1281–1293. https://doi.org/10.1002/ece3.546
Tsuyuzaki S, Hase A, Niinuma H (2005) Distribution of different mycorrhizal classes on Mount Koma, northern Japan. Mycorrhiza 15:93–100. https://doi.org/10.1007/s00572-004-0304-7
van der Putten WH, Bardgett RD, Bever JD et al (2013) Plant-soil feedbacks: the past, the present and future challenges. J Ecol 101:265–276. https://doi.org/10.1111/1365-2745.12054
van Schöll L, Kuyper TW, Smits MM et al (2008) Rock-eating mycorrhizas: their role in plant nutrition and biogeochemical cycles. Plant Soil 303:35–47. https://doi.org/10.1007/s11104-007-9513-0
Venables WN, Ripley BD (2002) Modern applied statistics with S-plus, 4th Edition. Springer, New York
Wagg C, Pautler M, Massicotte HB, Peterson RL (2008) The co-occurrence of ectomycorrhizal, arbuscular mycorrhizal, and dark septate fungi in seedlings of four members of the Pinaceae. Mycorrhiza 18:103–110. https://doi.org/10.1007/s00572-007-0157-y
Wallander H (2000) Uptake of P from apatite by Pinus sylvestris seedlings colonised by different ectomycorrhizal fungi. Plant Soil 218:249–256. https://doi.org/10.1023/A:1014936217105
Wardle DA, Bonner KI, Barker GM (2002) Linkages between plant litter decomposition, litter quality, and vegetation responses to herbivores. Funct Ecol 16:585–595. https://doi.org/10.1046/j.1365-2435.2002.00659.x
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, pp 315–322
Wu B, Hogetsu T, Isobe K, Ishii R (2007) Community structure of arbuscular mycorrhizal fungi in a primary successional volcanic desert on the southeast slope of Mount Fuji. Mycorrhiza 17(6):495–506. https://doi.org/10.1007/s00572-007-0114-9
Xie L, Bi Y, Ma S et al (2021) Combined inoculation with dark septate endophytes and arbuscular mycorrhizal fungi: synergistic or competitive growth effects on maize? BMC Plant Biol 21:498. https://doi.org/10.1186/s12870-021-03267-0
Yamamoto S, Sato H, Tanabe AS et al (2014) Spatial segregation and aggregation of ectomycorrhizal and root-endophytic fungi in the seedlings of two Quercus species. PLoS ONE 9:e96363. https://doi.org/10.1371/journal.pone.0096363
Yu T, Nassuth A, Peterson RL (2001) Characterization of the interaction between the dark septate fungus phialocephala fortinii and Asparagus officinalis roots. Can J Microbiol 47:741–753. https://doi.org/10.1139/cjm-47-8-741
Acknowledgements
We thank Mori Kibube, Dr. Motohiro Kawanishi, Dr. Takuo Sawahata, and Kohei Yamada for their cooperation in the field survey. We also thank Yakushima Town (Kagoshima Prefecture) and the Ministry of the Environment, Japan, for the permissions of field survey.
Funding
Open Access funding provided by The University of Tokyo. This work was supported by JSPS KAKENHI grant number 19H03003, and JST SPRING, Grant Number JPMJSP2108.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AI. The first draft of the manuscript was written by AI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishikawa, A., Hayasaka, D. & Nara, K. Effects of root-colonizing fungi on pioneer Pinus thunbergii seedlings in primary successional volcanic mudflow on Kuchinoerabu Island, Japan. Mycorrhiza 34, 57–67 (2024). https://doi.org/10.1007/s00572-024-01142-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-024-01142-y