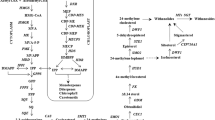
Abstract
It is becoming increasingly evident that the formation of arbuscular mycorrhiza (AM) enhances secondary metabolite production in shoots. Despite mounting evidence, relatively little is known about the underlying mechanisms. This study suggests that increase in artemisinin concentration in Artemisia annua colonized by Rhizophagus intraradices is due to altered trichome density as well as transcriptional patterns that are mediated via enhanced jasmonic acid (JA) levels. Mycorrhizal (M) plants had higher JA levels in leaf tissue that may be due to induction of an allene oxidase synthase gene (AOS), encoding one of the key enzymes for JA production. Non-mycorrhizal (NM) plants were exogenously supplied with a range of methyl jasmonic acid concentrations. When leaves of NM and M plants with similar levels of endogenous JA were compared, these matched closely in terms of shoot trichome density, artemisinin concentration, and transcript profile of artemisinin biosynthesis genes. Mycorrhization increased artemisinin levels by increasing glandular trichome density and transcriptional activation of artemisinin biosynthesis genes. Transcriptional analysis of some rate-limiting enzymes of mevalonate and methyl erythritol phosphate (MEP) pathways revealed that AM increases isoprenoids by induction of the MEP pathway. A decline in artemisinin concentration in shoots of NM and M plants treated with ibuprofen (an inhibitor of JA biosynthesis) further confirmed the implication of JA in the mechanism of artemisinin production.




Similar content being viewed by others
References
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Allen SE (1989) Chemical analysis of ecological materials, 2nd edn. Blackwell, Oxford
Bennett CR, Wallsgrove RM (1994) Secondary metabolites in plant defence mechanisms. New Phytol 127:617–633
Biermann BJ, Linderman RG (1981) Quantifying vesicular arbuscular mycorrhizae: a proposed method towards standardization. New Phytol 89:63–67
Brown GD, Sy LK (2007) In vivo transformations of artemisinic acid in Artemisia annua plants. Tetrahedron 63:9548–9566
Bruinsma M, Posthumus MA, Mumm R, Mueller MJ, Van Loon JJA, Dicke M (2009) Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: effect of time and dose, and comparison with induction by herbivores. J Exp Bot 60:2575–2587
Caretto S, Quarta A, Durante M, Nisi R, Paolis AD, Blando F, Mita G (2011) Methyl jasmonate and miconazole differently affect arteminisin production and gene expression in Artemisia annua suspension cultures. Plant Biol 13:51–58
Chappell J, Wolf F, Proulx J, Cuellar R, Saunders C (1995) Is there action catalyzed by 3-Hydroxy-3-Methylglutaryl Coenzyme A reductase a ratelimiting step for isoprenoid biosynthesis in plants? Plant Physiol 109:1337–1343
Copetta A, Lingua G, Berta G (2006) Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L var. Genovese Mycorrhiza 16:485–494
Cordoba E, Salmi M, Leon P (2009) Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J Exp Bot 60:2933–2943
Dixon RA (2001) Natural products and plant disease resistance. Nature 411:834–847
Ferreira JFS (2007) Nutrient deficiency in the production of artemisinin, dihydroartemisinic acid, and artemisinic acid in Artemisia annua L. J Agric Food Chem 55:1686–1694
Floß DS, Hause B, Lange PR, Kuster H, Strack D, Walter MH (2008) Knock-down of the MEP pathway isogene 1-deoxy-d-xylulose 5-phosphate synthase 2 inhibits formation of arbuscular mycorrhiza-induced apocarotenoids, and abolishes normal expression of mycorrhiza-specific plant marker genes. Plant J 56:86–100
Giovannetti M, Mosse B (1980) Evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:498–500
Gupta ML, Prasad A, Ram M, Kumar S (2002) Effect of the vesicular arbuscular mycorrhizal (VAM) fungus Glomus fasciculatum on the essential oil yield related characters and nutrient acquisition in the crops of different cultivars of menthol mint (Mentha arvensis) under field conditions. Biores Technol 81:77–79
Hans J, Hause B, Fester T, Strack D, Walter MH (2004) Cloning, characterization and immunolocalization of a mycorrhizal-inducible 1-deoxy-D-xylulose 5-phosphate isomerase in arbuscule-containing cells of maize. Plant Physiol 134:614–624
Hause B, Maier W, Miersch O, Kramell R, Strack D (2002) Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol 130:1213–1220
Hause B, Schaarschmidt S (2009) The role of jasmonates in mutualistic symbioses between plants and soil-born microorganisms. Phytochemistry 70:1589–1599
Henkes GJ, Thorpe MR, Minchin PEH, Schurr U, Rose USR (2008) Jasmonic acid treatment to part of the root system is consistent with simulated leaf herbivory, diverting recently assimilated carbon towards untreated roots within an hour. Plant Cell Environ 31:1229–1236
Howe GA, Lee GI, Itoh A, Li L, DeRocher AE (2000) Cytochrome P450-dependent metabolism of oxylipins in tomato. Cloning and expression of allene oxide synthase and fatty acid hydroperoxide lyase. Plant Physiol 123:711–724
Kapoor R, Giri B, Mukerji KG (2002a) Glomus macrocarpum: a potential bioinoculant improves essential oil quality and concentration in Dill (Anethum graveolens L.) and Carum (Trachyspermum ammi (Linn.) Sprague). World J Microbiol Biotechnol 18:459–463
Kapoor R, Giri B, Mukerji KG (2002b) Mycorrhization of coriander (Coriandrum sativum L) to enhance the concentration and quality of essential oil. J Sci Food Agric 82:339–342
Kapoor R, Giri B, Mukerji KG (2004) Improved growth and essential oil yield and quality in Foeniculum vulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. Biores Technol 93:307–311
Kapoor R, Chaudhary V, Bhatnagar AK (2007) Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 17:581–587
Karnovsky MJ (1965) A formaldehyde–glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137–138
Khaosaad T, Vierheilig H, Nell M, Zitterl-Eglseer K, Novak J (2006) Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp Lamiaceae). Mycorrhiza 16:443–446
Landgraf R, Schaarschmidt S, Hause B (2012) Repeated leaf wounding alters the colonization of Medicago truncatula roots by beneficial and pathogenic microorganisms. Plant Cell Environ 35:1344–1357
Laudert D, Weiler EW (1998) Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signalling. Plant J 15:675–684
León-Morcillo RJ, Ángel J, Martín-Rodríguez, Vierheilig H, Ocampo JA, García-Garrido JM (2012) Late activation of the 9-oxipilin pathway during arbuscular mycorrhizal formation in tomato and its and its regulation by jasmonate signalling. J Exp Bot 63:3545–3558
Liu S, Tian N, Li J, Huang J, Liu Z (2009) Isolation and identification of novel genes involved in artemisinin production from flowers of Artemisia annua using suppression subtractive hybridization and metabolite analysis. Planta Med 75:1542–1547
Loomis WD, Corteau R (1972) Essential oil biosynthesis. Recent Adv Phytochem 6:147–185
Lopez-Raez JA, Verhage A, Fernandez I, Garcia JM, Azcon-Aguilar C, Flors V, Pozo MJ (2010) Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J Exp Bot 61:2589–2601
Lu X, Zhang F, Jiang W, Lin X, Chen Y, Shen Q, Wang T, Wu S, Sun X, Tang K (2012) Characterization of the first specific jasmonate biosynthetic pathway gene allene oxide synthase from Artemisia annua. Mol Biol Rep 39:2267–2274
Lu X, Zhang F, Shen Q, Jiang W, Pan Q, Lv Z, Yan T, Fu X, Wang Y, Qian H, Tang K (2014) Over expression of allene oxide cyclase improves the biosynthesis of artemisinin in Artemisia annua L. Plos ONE 9:e91741. doi:10.1371/journal.pone.0091741
Maes L, Nieuwerburgh FCWV, Zhang Y, Reed DW, Pollier J, Vande-Casteele SRF, Inzé D, Covello PS, Deforce DLD, Gossens A (2011) Dissection of the phytohormonal regulation of trichome formation and biosynthesis of the antimalarial compound artemisinin in Artemisia annua plants. New Phytol 189:176–189
Mandal S, Evelin H, Giri B, Singh VP, Kapoor R (2013) Arbuscular mycorrhiza enhances the production of stevioside andrebaudioside-A in Stevia rebaudiana via nutritional and non-nutritional mechanisms. Appl Soil Ecol 72:187–194
Matsuura H, Aoi A, Satou C, Nakaya M, Masuta C, Nabeta K (2009) Simultaneous UPLC/MS analysis of endogenous jasmonic acid, salicylic acid and their related compounds. Plant Growth Regul 57:293–301
Maucher H, Hause B, Feussner I, Ziegler J, Wasternack C (2000) Allene oxide synthases of barley (Hordeum vulgare cv. Salome): tissue specific regulation in seedling development. Plant J 21:199–213
Meixner C, Ludwig-Müller J, Miersch O, Gresshoff P, Staehelin C, Vierheilig H (2005) Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts 1007. Planta 222:709–715
Mercke P, Bengtsson M, Bouwmeester HJ, Posthumus MA, Brodelius PE (2000) Molecular cloning, expression, and characterization of amorpha-4, 11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch Biochem Biophys 381:173–180
Morandi D (1996) Occurrence of phytoalexins and phenolic compounds in endomycorrhizal interactions, and their potential role in biological control. Plant Soil 185:241–251
Moreno–Fortunato I, Avato P (2008) Plant development and synthesis of essential oils in micropropagated and mycorrhiza inoculated plants of Origanum vulgare L. ssp. Hirtum (Link) Ietswaart. Plant Cell Tiss Org Cult 93:139–149
Pauwels L, Inzé D, Goossens A (2009) Jasmonate-inducible gene: what does it mean? Trends Plant Sci 14:87–91
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc 5:158–161
Qu H, Christensen KB, Fretté XC, Tian F, Rantanen J, Christensen LP (2010) A novel hybrid chromatography–crystallization process for the isolation and purification of a natural pharmaceutical ingredient from a medicinal herb. Org Process Res Dev 14:585–591
Ram M, Khan MA, Jha P, Khan S, Kiran U, Ahmad MM, Javed S, Abdin MZ (2010) HMG-CoA reductase limits artemisinin biosynthesis and accumulation in Artemisia annua L. plants. Acta Physiol Plant 32:859–866
Rapparini F, Llusia J, Penuelas J (2008) Effect of arbuscular mycorrhizal (AM) colonization on terpene emission and content of Artemisia annua L. Plant Biol 10:108–122
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Schramek N, Wang H, Römisch-Margl W, Keil B, Radykewicz T, Winzenhörlein B, Beerhues L, Bacher A, Rohdich F, Gershenzon J, Liu B, Eisenreich W (2010) Artemisinin biosynthesis in growing plants of Artemisia annua. A 13CO2 study. Phytochemistry 71:179–187
Shan C, Liang Z (2010) Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyroncristatum leaves under water stress. Plant Sci 178:130–139
Strack D, Fester T (2006) Isoprenoid metabolism and plastid reorganization in arbuscular mycorrhizal roots. New Phytol 172:22–34
Stumpe M, Carsjens JG, Stenzel I, Gobel C, Lang I, Pawlowski K, Hause B, Feussner I (2005) Lipid metabolism in arbuscular mycorrhizal roots of Medicago truncatula. Phytochemistry 66:781–791
Teoh KH, Polichuk DR, Reed DW, Covello PS (2009) Molecular cloning of analdehyde dehydrogenase implicated in artemisinin biosynthesis in Artemsia annua. Botany 87:635–642
Torelli A, Trotta A, Acerbi L, Arcidiacono G, Berta G, Branca C (2000) IAA and ZR content in leek (Allium porrum L.) as influenced by P nutrition and arbuscular mycorrhizae, in relation to plant development. Plant Soil 226:29–35
Toussaint JP, Smith FA, Smith SE (2007) Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza 17:291–297
Towler MJ, Weathers PJ (2007) Evidence of artemisinin production from IPP stemming from both mevalonate and non-mevalanate pathways. Plant Cell Rep 26:2129–2136
Treutter D (2006) Significance of flavonoids in plant resistance: a review. Environ Chem Lett 4:147–157
Verpoorte R, Memelink J (2002) Engineering secondary metabolite production in plants. Curr Opin Biotech 13:181–187
Vierheilig H, Piche Y (2002) Signalling in arbuscular mycorrhiza: facts and hypotheses. In: Buslig B, Manthey J (eds) Flavonoids in cell function. Academic, New York, pp 23–39
Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell11:1337–1349
Wallaart TE, Bouwmeester HJ, Hille J, Poppinga L, Maijers NCA (2001) Amorpha-4, 11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 212:460–465
Walter MH, Fester T, Strack D (2000) Arbuscular mycorrhizal fungi induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with the accumulation of the ‘yellow pigment’ and other apocarotenoids. Plant J 21:571–578
Wang H, Ma C, Li Z, Ma L, Wang H, Ye H, Xu G, Liu B (2010) Effects of exogenous methyl jasmonate on artemisinin biosynthesis and secondary metabolite in Artemisia annua L. Ind Crop Prod 31:214–218
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review. Ann Bot 111:1021–1058
Weathers PJ, Elkholy S, Wobbe KK (2006) Artemisinin: the biosynthetic pathway and its regulation in Artemisia annua, a terpenoid–rich species. In Vitro Cell Dev Biol Plant 42:309–317
Yoshida Y, Sano R, WadaT, Takabayashi J, Okada K (2009) Jasmonic acid control of GLABRA3 links inducible defense and trichome patterning in Arabidopsis. Development 136:1039–1048
Yu ZX, Lia JX, Yanga CQ, Hua WL, Wanga LJ, Chen XY (2012) The jasmonate-responsive AP2/ERF transcription factors AaERF1 and AaERF2 positively regulate artemisinin biosynthesis in Artemisia annua L. Mol Plant 5:353–365
Zhang YY, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJH, Ross ARS, Covello PS (2008) The molecular cloning of artemisinic aldehyde 11(13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem 283:21501–21508
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotech Adv 23:283–333
Acknowledgments
The research work was partially funded by the Department of Biotechnology, University Grants commission, Delhi, India. Shantanu Mandal and Shivangi Upadhyay gratefully acknowledge the Council of Scientific and Industrial Research for Research Fellowships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Induction of genes (presented in rectangular box) of mevalonate and artemisinin biosynthesis pathways in Artemisia annua plants subjected to P fertilizer, exogenous treatment of 200 μM MeJA under high and low P and mycorrhizal inoculation. Thickness of arrows indicates the significant changes in transcription levels compared to control plants of the genes coding for the enzyme cited, as determined by qRT-PCR analysis; = denotes no changes in gene expression. HMGR 3-hydroxy-3-methylglutaryl-CoA reductase; DXS 1-deoxyxylulose 5-phosphate synthase; DXR 1-deoxyxylulose 5-phosphate reductoisomerase; ADS Amorpha-4, 11-diene synthase; CYP cytochrome P 450 CYP71AV1; DBR2 double bond reductase 2; ALDH1 aldehyde dehydrogenase 1. (PPT 298 kb)
Fig. S2
Formation of arbuscules (a) and vesicles (b) in the cortical region of A. annua roots. (PPTX 219 kb)
Fig. S3
Effect of G. intraradices inoculation and exogenous application of IBU on relative frequency of arbuscules and vesicles in the cortical region of A. annua roots. (PPTX 131 kb)
Fig. S4
Scanning electron micrographs of leaves of Artemisia annua plants showing effect of G. intraradices inoculation, different exogenous concentrations of methyl jasmonate (μM MeJA) under low and high phosphorus, and exogenous application of ibuprofen (500 μM IBU) on glandular trichome density in control (a), 50 P (b), mycorrhizal (c), 100 MeJA (d), 200 MeJA (e), 300 MeJA (f), 100 MeJA + 50P (g), 200 MeJA + 50P (h), 500 IBU (i), 200 MeJA + 500 μM IBU (j), 300 MeJA + 50P (K), and M + 500 IBU (l). Arrow indicates the glandular trichome. C control, M G. intraradices inoculation, P phosphorus, MeJA methyl jasmonates, IBU ibuprofen. (PPT 3728 kb)
Fig. S5
Effect of G. intraradices inoculation, P fertilizer, and different concentrations (0, 100, 200, and 300 μM) of exogenously applied methyl jasmonate (MeJA) under low and high phosphorus on expression levels of Amorpha-4, 11-diene synthase (ADS), Cytochrome P 450 CYP71AV1 (CYP), and aldehyde dehydrogenase 1 (ALDH1) gene in leaves of Artemisia annua plants. C = NM + 0P + 0 MeJA, M = 0P + 0 MeJA. (PPT 239 kb)
Rights and permissions
About this article
Cite this article
Mandal, S., Upadhyay, S., Wajid, S. et al. Arbuscular mycorrhiza increase artemisinin accumulation in Artemisia annua by higher expression of key biosynthesis genes via enhanced jasmonic acid levels. Mycorrhiza 25, 345–357 (2015). https://doi.org/10.1007/s00572-014-0614-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-014-0614-3