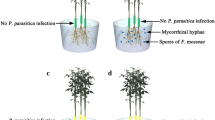
Abstract
Arbuscular mycorrhizal fungi (AMF) trigger beneficial effects on their hosts, but it is unknown how plants modulate their defense responses during root colonization of AMF and the symbiotic benefits are initiated. The purpose of this study was to analyze the root mycorrhizal colonization process of trifoliate orange and the responsive patterns of plant growth, root peroxide hydrogen (H2O2), antioxidant enzymes and their encoding gene expression, and sugar, lipid and phosphate transporter protein gene expression at 7‒56 days of inoculation (doi) with Funneliformis mosseae (Fm). Fm developed appressoriums on the root surface at 7 doi, followed by abundant arbuscules in root cortical cells at 28 doi, intracellular vesicles at 42 doi, and root mycorrhizal colonization rate of 41.54% at 56 doi. Plant growth improvement by Fm started at 28 doi. The immune defense response of roots was initiated at 7 doi, as evidenced by the increase of H2O2 levels and superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activity, as well as the up-regulation of PtMn-SOD, PtCu/Zn-SOD, PtPOD, and PtCAT expression, which lasted until 14 doi. Starting at 28 doi, a sugar transporter gene (PtSWEET), a lipid transporter gene (PtSTR), and a phosphate transporter gene (PtPT6) were initiated to be up-regulated, followed by the up-regulation of PtSTR2, PtPT3, and PtPT5 at 42 doi and PtFe-SOD at 56 doi. Arbuscule formation and plant growth improvement together at 28 doi suggested that arbuscules trigger improved growth responses of host plants. This study also reveals the initiation of host immune defense response and function in early root AMF colonization.
Graphical Abstract

Similar content being viewed by others
Introduction
Citrus, one of the widely grown fruit trees worldwide, is heavily dependent on arbuscular mycorrhizal fungi (AMF) to facilitate its access to nutrients and water from the soil due to its shallow root systems and few root hairs [1]. AMF roles in citrus plants have garnered a lot of interest [2]. AMF are a widely distributed group of soil fungi that can establish symbiosis with most terrestrial plants, including citrus [3]. AMF can promote growth performance, nutrient acquisition, and stress tolerance of the host plant, as well as crop yield and quality [4,5,6,7,8].
AMF colonization of plant roots starts with the germination of fungal spores in the soil, followed by the continuous growth of hyphae [9]. Under the action of signaling molecules, fungal hyphae contact the surface of root epidermal cells and/or root hairs to form an appressorium, and enter the roots, where fungal hyphae colonize the cortical cells of roots, some of which continuously remifying and fill a cortical cell to form a complex tree-like structure, the arbuscule [9,10,11]. The tip expansion of intraradical hyphae in AMF of some genera excluding Gigaspora and Scutellospora forms vesicles within cortical cells [9]. Hydrogen peroxide (H2O2) is an important signaling molecule in plants that broadly regulates plant growth and development, responds to stresses, and is involved in plant–microbe interactions [12]. According to Liu et al. [13], H2O2 levels in white clover plants increased significantly at the beginning of microbial inoculation and then decreased at the later stages, showing a signaling of H2O2 in response to microbial colonization in roots. H2O2 burst is necessary and sufficient to induce immune responses [14]. Antioxidant enzymes and their encoding genes, as a natural plant defense system, induce a transient defense response in the early stages of AMF‒host plant interaction [15]. Antioxidant enzyme activities are increased significantly at the beginning of root AMF colonization and then decreased in the later stages of AMF growth as the symbiosis develops [16]. Therefore, the response patterns of H2O2, antioxidant enzymes, and their encoding genes can reveal the defense response mechanisms of plants to microbial colonization.
Mutual sensing of signals and the exchange of signaling molecules between AMF and the host plant initiate the establishment of symbionts [17]. Arbuscular mycorrhizae are manifested by a bidirectional exchange at the symbiotic interface, where the host plant provides carbon sources (fatty acids and sugars) to the arbuscular mycorrhizal fungus, while the arbuscular mycorrhizal fungus provides the host plant with nutrients, especially phosphorus (P) [18, 19]. Sugar transport and distribution in plants requires the involvement of sugar transporter proteins such as SWEET (sugar will eventually be exported transporters) [20]. Inoculation with AMF in citrus increased the expression of CsSWEET gene in fruits [21]. Multiple SWEET gene expression was up-regulated in AMF‒host plants (e.g., Medicago truncatula and soybean) [22, 23]. During AMF colonization process, lipids are transported from plant roots to AMF [24] as its main source of carbon [25]. It has been reported that lipid transport proteins (STR/STR2) were involved in mycorrhizal symbiosis in alfalfa [26]. Jiang et al. [27] found that AMF induced lipids synthesis in plants, and lipids entered AMF mainly through STR/STR2, establishing arbuscular mycorrhizae. Yang et al. [28] also reported that inoculation with AMF in trifoliate orange (Poncirus trifoliata; a rootstock used in citrus industry) enhanced host P uptake, mainly through phosphate trasnporter genes (PtPT3, PtPT5, and PtPT6). Thus, sugars, lipids, and PT genes play important roles in the symbiosis of AMF and plants, but the underlying mechanisms involved are very complex and need to be studied in depth.
The aim of this study was to further shed light on the mechanism of symbiosis between AMF and trifoliate orange by analyzing the changes in plant growth performance, H2O2 levels, antioxidant enzyme activities, and expression of antioxidant enzyme genes and symbiosis-associated genes during the process of root AMF colonization.
Materials and methods
Preparation of AMF inoculum
An arbuscular mycorrhizal fungus Funneliformis mosseae (BGC XZ02A) was chosen, because the fungus has been demonstrated to have positive effects on trifoliate orange [29]. This fungus was trapped using white clover as the host plant for about 10 weeks under potted conditions, and the inoculum consisted of fungal colonized root segments, spores (22 spores/g), hyphae, and growth substrates, stored at 4 °C, and used within three months.
Plant culture and experimental design
In March 2022, seeds were removed from trifoliate orange fruits, treated with 10% NaOH to remove pectin from the seed surface, disinfected with 70% alcohol for 10 min, and rinsed three times with distilled water before being placed in pre-autoclaved (121 °C, 0.11 MPa, 2 h) sand (< 2 mm in the diameter) for their germination, where environmental conditions were 30 °C/23 °C (day/night temperature, 16 h / 8 h), with a constant relative air humidity of 75% and a light intensity of 1500 Lux.
After one month, uniformly sized seedlings with four leaves were transplanted into pots (16 × 11 × 15 cm) pre-supplied with 2.5 kg of autoclaved soil-sand mixture (3: 1, v/v). Meanwhile, 150 g of mycorrhizal fungal inocula was placed around roots of trifoliate orange seedlings as the inoculated treatment, whereas the uninoculated treatment also received an equivalent amount of autoclaved mycorrhizal fungal inocula plus 2 mL of inoculum filtrate through a 20-µm nylon mesh.
Treated seedlings were placed in a controlled greenhouse as described by Cao et al. [30]. This experiment was conducted between May 19 and July 13, 2022. The plants were harvested at 7, 14, 21, 28, 42, and 56 days of inoculation (doi), with 4 pots per treatment harvested each time for a total of 48 pots. The experiment, therefore, consisted of a total of two treatments, each with twenty four replicates.
Determination of plant growth and root mycorrhizal colonization
On the day of each harvest, plant height was measured. After harvest, the taproot length was determined using vernier calipers, and the shoot and root biomass was weighed. Subsequently, root segments with 1‒2 cm long were cut and stained with the protocol of Phillips and Hayman [31] with 0.05% trypan blue in lactophenol for 30 s. Root mycorrhizal colonization was observed under a microscope. Root length colonization rate (%) = colonized root length / observed total root length × 100.
Determination of root H2O2 concentrations
Root H2O2 concentrations were determined as per the protocol outlined by Velikova et al. [32]. The 0.20 g of fresh sample was ground into a homogenate with 5 mL of 0.1% trichloroacetic acid in an ice bath and centrifuged at 10,000 × g for 15 min. The 1 mL of supernatant was reacted with 1 mL of 10 mmol/L phosphate buffer (pH 7.0) and 2 mL of 1 mol/L KI, and their absorbance values were recorded at 390 nm.
Determination of root antioxidant enzyme activities
Superoxide dismutase (SOD) activity was determined according to the method described by Wu [33]. The reaction solution consisted of 50 µL of the enzyme extract, 300 µL of 130 mmol/L L-methionine, 300 µL of 750 µmol/L nitroblue tetrazolium, 300 µL of 100 µmol/L EDTA-Na2, 300 µL of 20 µmol/L riboflavin, and 250 µL of distilled water. CAT activity was measured as per the protocol described by He et al. [34]. A 4-mL reaction solution consisted of 0.2 mL of the enzyme extract, 2.0 mL of 0.1 mmol/L phosphate buffer (pH 7.8), and 1.8 mL of distilled at 25 °C for 3 min, followed by the addition of 0.3 mL of 0.1 mol/L H2O2. Peroxidase (POD) activity was assayed using the procedure outlined by Chance and Maehly [35]. A 5-mL mixture contained 2.9 mL phosphate buffers, 1.0 mL of 0.1 mol/L H2O2, 1.0 mL of 0.05 mol/L guaiacol, and 0.1 mL of the enzyme extract.
Analysis of relative expression of genes
Root total RNA was extracted by the MiniBEST plant RNA kit (No. 9769; TaKaRa, Dalian, China). The RNA integrity was detected by 1.0% agarose gel electrophoresis, and the concentration of the extracted RNA was calculated by A260/A280 ratio. The qualified RNA was reverse transcribed to cDNA using a PrimeScript™ RT reagent kit with gDNA Eraser (RR047A; Takara). Five antioxidant enzyme genes (PtFe-SOD, PtMn-SOD, PtCu/Zn-SOD, PtPOD, and PtCAT) [34], a sugar transporter protein gene (PtSWEET) [36], two fatty acid transporter protein genes (PtSTR and PtSTR2) [26], and three phosphorus transporter protein genes (PtPT3, PtPT5, and PtPT6) [28] were selected based on previous studies. The genes were identified through the NCBI database (www.ncbi.nlm.nih.gov) and the genome-wide of trifoliate orange (http://citrus.hzau.edu.cn). The Primer Premier 5.0 software was used to design primer sequences of selected genes for qRT-PCR (Additional file 1: Table S1). The cDNA was used as the template. qRT-PCR was performed on an Fast Real-time PCR System (7900HT, ABI, Nanjing, China). The β-actin was employed as an internal reference gene. Each gene had three biological replicates, with three technical replicates per biological replicate. The relative expression of genes was calculated according to the method of Livak and Schmittgen [37], normalized to the gene of the no-Fm treatment.
Data analysis
All data analysis was performed in the SAS software (v8.1), where one-way analysis of variance and LSD tests were used at the 0.05 level for significant differences among treatments. The SigmaPlot (v10.0) was used for figure production.
Results
Changes in root length AMF colonization
At 7 doi, Fm was found at the root surface, showing a branching pattern and the formation of appressorium (Fig. 1a). At 14 doi, mycorrhizal hyphae had colonized into the roots and formed intraradical hyphae. At 21 doi, well-developed intraradical hyphae were formed in the roots, with obvious branching and visible arbuscules. At 28 doi, intraradical hyphae formed a large number of arbuscules within the cells. At 42 doi, a large number of intercellular vesicles formed by the apical expansion of hyphae could be clearly observed. At 56 doi, a large number of both intraradical hyphae and intercellular vesicles were clearly observed. The mycorrhizal colonization rate of Fm-inoculated roots ranged from 2.93% at 7 doi to 41.54% at 56 doi, and the root length colonization rate increased with the increase of Fm inoculation time (Fig. 1b).
The colonization process a and change in root length colonization rate b of Funneliformis mosseae in trifoliate orange seedlings. Data (means ± SD, n = 4) with different letters on the bar indicate significant (p ≤ 0.05) differences between treatments. A arbuscule, doi days of inoculation, Eh extraradical hyphae, Ih intraradical hyphae, V vesicles
Changes in plant growth performance
The growth performance of trifoliate orange seedlings changed significantly with the extension of the days of F. mosseae inoculation (Fig. 2a). At 7‒21 doi, plant height, taproot length, and shoot and root biomass did not differ significantly between Fm- and no-Fm-inoculated treatments (Fig. 2b–e). Starting from 28 doi, the growth performance in Fm-inoculated seedlings was better than that in no-Fm-inoculated seedlings. The plant height of Fm-inoculated seedlings was significantly higher than that of no-Fm-inoculated seedlings by 42.73%, 71.64%, and 84.21% at 28, 42, and 56 doi, respectively. Similarly, shoot biomass was increased by 64.71%, 68.00%, and 96.97% under Fm- versus no-Fm-inoculated treatment at 28, 42, and 56 doi, respectively, along with 54.55%, 81.25%, and 66.67% significantly higher root biomass in Fm-inoculated seedlings than no-Fm-inoculated seedlings, respectively. The taproot length of Fm-inoculated seedlings showed significant changes from 42 doi, increasing by 31.16% and 24.03% at 42 and 56 doi, respectively, compared with no-Fm-inoculated seedlings.
Changes in plant growth performance (a), plant height (b), taproot length (c), shoot biomass (d), and root biomass (e) of trifoliate orange seedlings after inoculation with Funneliformis mosseae. Data (means ± SD, n = 4) with different letters on the bar indicate significant (p ≤ 0.05) differences between treatments
Changes in root H2O2 levels
Compared with no-Fm inoculation, H2O2 levels in roots of Fm-inoculated seedlings were significantly increased only at 7 and 14 doi by 63.47% and 34.73%, respectively, along with no significant difference at 21‒56 doi (Fig. 3).
Changes in root antioxidant enzyme activities
Compared with no-Fm inoculation, Fm inoculation significantly increased root SOD activity at 7 and 56 doi by 60.00% and 29.38%, respectively (Fig. 4a). There was no significant difference in SOD activity between the two treatments from 14 doi to 42 doi. Root POD and CAT activities were significantly elevated at 7, 14, and 56 doi after Fm inoculation, with 69.46%, 43.26%, and 49.54% increase in POD activity and 199.22%, 93.89%, and 64.46% increase in CAT activity, respectively, plus no significant changes at 21‒42 doi (Fig. 4b, c).
Changes in superoxide dismutase (SOD) (a), peroxidase (POD) (b), and catalase (CAT) (c) activities in roots of trifoliate orange seedlings after inoculation with Funneliformis mosseae. Data (means ± SD, n = 4) with different letters on the bar indicate significant (p ≤ 0.05) differences between treatments
Changes in the expression of root antioxidant enzyme genes
Compared with no-Fm inoculation, Fm inoculation did not significantly affect root PtFe-SOD expression at 7‒42 doi, but up-regulated root PtFe-SOD expression (1.46 folds) at 56 doi (Fig. 5a). Fm inoculation also up-regulated root PtMn-SOD expression at 7 and 14 doi by 2.43 and 1.78-fold, respectively, compared with no-Fm treatment, along with no significant difference at the subsequent 21‒56 doi (Fig. 5b). PtCu/Zn-SOD expression just got significantly up-regulated (1.60-fold) at 7 doi by Fm versus no-Fm inoculation, plus no significant change at 14‒56 doi (Fig. 5c). Compared with no-Fm inoculation, Fm inoculation distinctly up-regulated PtPOD expression at 7, 14, and 56 doi by 2.43-, 3.36-, and 1.85-fold, respectively, as well as PtCAT expression at 7, 14, and 56 doi by 4.06-, 3.46-, and 2.14-fold, respectively, accompanied by no significant difference at 21‒42 doi (Fig. 5d, e).
Changes in relative expression of PtFe-SOD (a), PtMn-SOD (b), PtCu/Zn-SOD (c), PtPOD (d), and PtCAT (e) genes in roots of trifoliate orange seedlings after inoculation with Funneliformis mosseae. Data (means ± SD, n = 3) with different letters on the bar indicate significant (p ≤ 0.05) differences between treatments
Changes in the expression of root SWEET and STR genes
The expression of root PtSWEET was not initiated by Fm inoculation at 7‒21 doi, whereas the expression of root PtSWEET was up-regulated at 28, 42, and 56 doi by 1.50-, 2.00-, and 2.17-fold, respectively, compared with no-Fm inoculation (Fig. 6a). Similarly, at 7‒21 doi, Fm inoculation also did not affect the expression of PtSTR and PtSTR2 in roots (Fig. 6b, c). Starting from 28 doi, PtSTR expression was up-regulated by Fm inoculation by 1.33 folds at 28 doi, 1.45 folds at 42 doi, and 2.30 folds at 56 doi, respectively, compared with no-Fm inoculation. PtSTR2 expression was up-regulated only at 42 and 56 doi under Fm- versus no-Fm-inoculation conditions by 1.49- and 2.12-fold, respectively.
Changes in the expression of root PT genes
Fm inoculation did not significantly alter root PtPT3 and PtPT5 expression at 7‒28 doi and root PtPT6 expression at 7‒21 doi (Fig. 7a–c). Root PtPT3 and PtPT5 expression was up-regulated by Fm inoculation by 2.46- and 2.30-fold at 42 doi and by 3.22- and 4.76-fold at 56 doi, respectively. Root PtPT6 expression was up-regulated by Fm inoculation at 28, 42, and 56 doi by 1.59-, 1.86-, and 2.13-fold, respectively.
Discussion
The present study showed that Fm was able to contact roots of trifoliate orange and form appressorium at 7 doi, and the mycorrhizal colonization rate increased with time, reaching 41.54% at 56 doi. At 7‒56 doi, roots of trifoliate orange went through four stages: the formation of appressorium at 7 doi, further expansion of hyphae within mycorrhizal roots at 14‒21 doi, formation of arbuscules at 28 doi, and formation of vesicles and numerous intraradical hyphae at 42‒56 doi. At 7‒21 doi, Fm contacted with root surface to form colonization points and appressorium, penetrated epidermal cells into cortical cells to form intraradical hyphae, and then branched to form a developed hyphal network, which was consistent with the results of Sheng et al. [38] in Pinellia ternata plants. At 28 doi, the hyphae in roots branched continuously, forming arbuscules and filling the cell. Arbuscules are important sites for nutrient exchange between plant cells and AMF [13, 39, 40], where arbuscules are ensheathed by a host membrane, termed the periarbuscular membrane, which facilitates nutrient exchange [39, 41]. This indicates the functional initiation of arbuscular mycorrhizae in trifoliate orange at 28 doi. In general, the formation of arbuscules precedes the formation of vesicles in some Glomus species [42]. Therefore, we found that the apical expansion of hyphae formed intercellular vesicles at 42 doi. Vesicles contain lipid-like droplets that function as nutrient stores, and AMF can use the nutrients stored in the vesicles when mycorrhizal metabolism is reduced [43]. Subsequently there was a large number of intraradical hyphae as well as vesicles in roots at 56 doi, showing the maturation of arbuscular mycorrhizae.
AMF contribute to the growth and development of the host plant after forming a symbiosis in roots [44]. The present study showed that Fm inoculation produced a significantly positive effect on plant height (r = 0.82, p < 0.01), taproot length (r = 0.70, p < 0.01), shoot (r = 0.79, p < 0.01) and root biomass (r = 0.75, p < 0.01) starting from 28 doi (the stage of arbuscule formation), indicating that AMF colonization triggered a positive effect on plant growth of the host, in correlation with the formation of arbuscules in root cortical cells. In white clover, the positive effect of Paraglomus occultum on the improvement of shoot and root biomass also occurred at 20 doi, accompanied by the formation of arbuscules [13]. Similar improvement in host growth by AMF was also reported in sugarcane and drought-stressed trifoliate orange [4, 45]. The mycelium network formed by AMF expands the contact between plant roots and soil, thus promoting plant growth and development [46]. In contrast, before 28 doi, Fm inoculation did not significantly improve growth performance of trifoliate orange, because it takes some time for AMF to colonize the host plant and form a symbiotic relationship. It remains to be further determined whether the host supplies more photosynthetic products to root mycorrhizae for their growth in the early stage of mycorrhizal formation [47].
Plant H2O2 is dramatically increased after microbial infestation, which is a defense response of the host plant to microbial infestation [48]. The results of the present study showed that root H2O2 levels were significantly increased at 7 and 14 doi of Fm inoculation, and then no significant change started at 21 doi. This is in agreement with the findings of Song and Song [49] in alfalfa after root colonization of Glomus intraradices. Fester and Hause [43] also reported the increase in root H2O2 levels after inoculation of Medicago truncatula with G. intraradices, especially when the mycelium started to penetrate root cortical cells and during arbuscular formation. This suggests that roots of trifoliate orange initiated an immune defense response at 7‒14 doi in response to Fm colonization through elevated H2O2 levels.
Antioxidant enzymes are activated as a defense system during the early stages of AMF colonization of host plants and then inactivated as the symbiosis continues to develop [15, 50, 51]. In this study, activities of root antioxidant enzymes (SOD, POD, and CAT) were significantly increased at the beginning of Fm inoculation (7 doi), while root POD and CAT activities continued to be elevated at 14 doi as well, which was consistent with changes in root H2O2 levels. Significantly elevated CAT and POD activities were also observed in alfalfa during root early colonization of AMF [49]. Interestingly, at 56 doi, Fm inoculation again significantly raised root SOD, POD, and CAT activities. Lokhandwala et al. [52] found in a meta-analysis that AMF inoculation increased antioxidant enzyme activities of host plants by 16%, regardless of stress or not. The increase in antioxidant enzyme activities is a response of plant immune defense in the early stages of AMF colonization [53]. In the later stages of root AMF colonization, AMF enhance the host’s antioxidant capacity to resist oxidative damage [54]. In addition, the expression of stress-responsive genes is significantly up-regulated during the early stages of microbial infection of plants [55]. In the present study, the expression of PtMn-SOD, PtCu/Zn-SOD, PtPOD, and PtCAT gene was distinctly increased at 7 and 14 doi following Fm colonization, further indicating that trifoliate orange recognized root colonization of Fm and activated defense responses, triggering the host plant to generate an immune response at the early stage of mycorrhizal fungal colonization [56, 57]. After being recognized as a beneficial fungus, the defense system was removed [49], and thus no change in the antioxidant defense system was found between inoculated versus uninoculated plants. Additionally, PtFe-SOD, PtPOD, and PtCAT expression was again up-regulated at 56 doi of Fm. This is in agreement with the findings of Li et al. [58] who reported that AMF inoculation resulted in up-regulation of CsFe-SOD, CsPOD, and CsCAT expression in field citrus, suggesting enhanced antioxidant potential of the host plant at the late stage of AMF colonization. In addition, in the early stage of root colonization of Fm, the response of different PtSOD genes to mycorrhizal colonization was variable, with PtMn-SOD and PtCu/Zn-SOD responding first and PtFe-SOD responding later. The intrinsic mechanism is not well defined. Van Camp et al. [59] also found that Fe-SOD was closely related to endosymbioint. At 7 doi, SOD activity in roots was significantly increased, along with the up-regulation expression of PtMn-SOD and PtCu/Zn-SOD and no change in PtFe-SOD expression. Similarly, at 56 doi, SOD activity in roots was significantly increased, along with the up-regulation expression of PtFe-SOD. This indicated that SOD activity changes under mycorrhizal inoculation conditions are associated with PtSOD gene species at different times. The inconsistent results in the enzyme activity and gene expression may be due to differences in transription and translation after gene expression and the distribution and functions of these SOD isoenzymes in plant organelles. Kim et al. [60] also proposed that the down-regulated expression of a SOD type can cause changes in the expression of other SOD types.
In addition, the establishment of symbiotic associations relies on bidirectional nutrient exchange, such as sugars, lipids, and PT gene expression [61]. This study showed that PtSWEET gene was significantly up-regulated from 28 doi of Fm, which was accompanied by the formation of arbuscules. This suggests that the host plant began providing sugars to the Fm at 28 doi. Arbuscules are sites of of nutrient exchange between plants and AMF [62]. Several SWEET genes were up-regulated in potato, alfalfa, and soybean with mycorrhizal formation [19, 22, 63], among which the expression of SWEET1b in alfalfa was up-regulated in arbuscule-containing cells [19]. The localization of PtSWEET protein in mycorrhizal root cells needs further study. AMF induce lipid synthesis in plants, and plants’ lipids enter AMF through STR and STR2 proteins located in the periarbuscular membrane as the main carbon source of nutrients [27, 64, 65]. This study showed that PtSTR expression was up-regulated from 28 doi, while PtSTR2 was up-regulated from 42 doi, implying that the host has supplied fungal partners with lipids at 28 doi, of which PtSTR was preferentially initiated.
An important function of mycorrhizal mycorrhizae is to up-regulate the expression of host PT genes to promote P uptake by the host [66]. For example, StPT3 in potato, MtPT4 in alfalfa, and OsPT11 in rice were identified to import phosphate released by AMF from the symbiotic interface into plant cells to increase plant P levels [67, 68]. In this study, PtPT6 expression was up-regulated in Fm-inoculated plants from 28 doi, while PtPT3 and PtPT5 started to be up-regulated only at 42 doi, implying that the mycorrhiza traveled to promote host P uptake at this time, accompanied by the preferential initiation of PtPT6. Mycorrhizal extraradical hyphae take up soil inorganic phosphate and transport it within the intraradical hyphae as polyphosphate particles, which are hydrolyzed upon arrival at the fungus-root cell interface (arbuscules) and translocated within the plant as H2PO4− [69]. P exchange occurs during arbuscular formation, so that the response of PtPTs expression was initiated only after 28 doi.
Conclusion
During root colonization of Fm in trifoliate orange, root defense systems (such as H2O2, SOD, POD, and CAT and their corresponding encoding genes) were initiated at 7‒14 doi and subsequently maintained unchanged, compared to no-Fm (Fig. 8a). At 28 doi, massive formation of arbuscule in the roots was accompanied by growth improvement, up-regulated expression of PtSWEET and PtSTR and subsequent up-regulated expression of PtPTs (Fig. 8b), suggesting the initiation of bidirectional nutrient functions. These results reveal the defense response of the host plant to mycorrhizal fungal colonization and the establishment of a symbiotic association, and also provide a clear understanding of the exchange of nutrients between AMF and the host plant.
A model diagram regarding root responses to AMF colonization at 7‒56 doi. Here, a showed the initiation of root antioxidant defense in response to AMF colonization at 7‒14 doi; b indicated the initiation of SWEETs, PTs, and STRs at 28‒56 doi, after arbuscule formation, possibly accompanied by nutrient exchange between the two partners
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References:
Menge JA, Johnson ELV, Platt RG. Mycorrhizal dependency of several citrus cultivars under three nutrient regimes. New Phytol. 1978;81:553–9.
Wu QS, Srivastava AK, Zou YN, Malhotra SK. Mycorrhizas in citrus: beyond soil fertility and plant nutrition. Ind J Agric Sci. 2017;87:427–43.
Bennett AE, Groten K. The costs and benefits of plant–arbuscular mycorrhizal fungal interactions. Annu Rev Plant Biol. 2022;73:649–72.
Juntahum S, Jongrungklang N, Kaewpradit W, Ekprasert J, Boonlue S. Improved physiological performances of sugarcane during maturation and ripening phase by inoculation of arbuscular mycorrhizal fungi. Sugar Tech. 2021;23:336–42.
Ahammed GJ, Shamsy R, Liu A, Chen S. Arbuscular mycorrhizal fungi-induced tolerance to chromium stress in plants. Environ Pollut. 2023;327: 121597.
Liu MY, Li QS, Ding WY, Dong LW, Deng M, Chen JH, Wu QS. Arbuscular mycorrhizal fungi inoculation impacts expression of aquaporins and salt overly sensitive genes and enhances tolerance of salt stress in tomato. Chem Biol Technol Ag. 2023;10:5.
Shi J, Wang X, Wang E. Mycorrhizal symbiosis in plant growth and stress adaptation: from genes to ecosystems. Annu Rev Plant Biol. 2023;74:569–607.
Zhou LJ, Wang Y, Alqahtani MD, Wu QS. Positive changes in fruit quality, leaf antioxidant defense system, and soil fertility of Beni-Madonna tangor citrus (Citrus nanko × C. amakusa) after field AMF lnoculation. Horticulturae. 2023;9:1324.
Peterson R, Massicotte H, Melvilla L. Mycorrhizas: anatomy and cell biology. Otawa: NRC Research Press; 2004.
Genre A, Chabaud M, Timmers T, Bonfante P, Barker DG. Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell. 2005;17:3489–99.
MacLean AM, Bravo A, Harrison MJ. Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell. 2017;29:2319–35.
Pusztahelyi T, Holb IJ, Pócsi I. Secondary metabolites in fungus-plant interactions. Front Plant Sci. 2015;6:573.
Liu XQ, Xie MM, Hashem A, Abd-Allah EF, Wu QS. Arbuscular mycorrhizal fungi and rhizobia synergistically promote root colonization, plant growth, and nitrogen acquisition. Plant Growth Regul. 2023;100:691–701.
Hückelhoven R, Kogel KH. Reactive oxygen intermediates in plant-microbe interactions: Who is who in powdery mildew resistance? Planta. 2003;216:891–902.
Nath M, Bhatt D, Prasad R, Gill SS, Anjum NA, Tuteja N. Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front Plant Sci. 2016;7:1574.
Blilou I, Bueno P, Ocampo JA, García-Garrido JM. Induction of catalase and ascorbate peroxidase activities in tobacco roots inoculated with the arbuscular mycorrhizal Glomus mosseae. Mycol Res. 2000;104:722–5.
Ji X, Xia Y, Zhang H, Cui JL. The microscopic mechanism between endophytic fungi and host plants: From recognition to building stable mutually beneficial relationships. Microbiol Res. 2022;216: 127056.
Gutjahr C, Parniske M. Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu Rev Cell Dev Biol. 2013;29:593–617.
An JY, Zeng T, Ji CY, Graaf SD, Zheng ZJ, Xiao TT, Deng XX, Xiao XY, Bisseling T, Limpens E, Pan ZY. A Medicago truncatula SWEET transporter implicated in arbuscule maintenance during arbuscular mycorrhizal symbiosis. New Phytol. 2019;224:396–408.
Breia R, Conde A, Badim H, Fortes AM, Gerós H, Granell A. Plant SWEETs: from sugar transport to plant–pathogen interaction and more unexpected physiological roles. Plant Physiol. 2021;186:836–52.
Li QS, Srivastava AK, Zou YN, Wu QS. Field inoculation responses of arbuscular mycorrhizal fungi versus endophytic fungi on sugar metabolism associated changes in fruit quality of Lane late navel orange. Sci Horti. 2023;308: 111587.
Zhao SP, Chen A, Chen CJ, Li CC, Xia R, Wang XX. Transcriptomic analysis reveals the possible roles of sugar metabolism and export for positive mycorrhizal growth responses in soybean. Physiol Plant. 2019;166:712–28.
Cope KR, Kafle A, Yakha JK, Pfeffer PE, Strahan GD, Garcia K, Subrananian S, Bücking H. Physiological and transcriptomic response of Medicago truncatula to colonization by high-or low-benefit arbuscular mycorrhizal fungi. Mycorrhiza. 2022;32:281–303.
Drigo B, Kowalchuk GA, Knapp BA, Pijl AS, Boschker HT, Van Veen JA. Impacts of 3 years of elevated atmospheric CO2 on rhizosphere carbon flow and microbial community dynamics. Global Change Biol. 2013;19:621–36.
Luginbuehl LH, Menard GN, Kurup S, Van Erp H, Radhakrishnan GV, Breakspear A, Oldroyd GE, Eastmond PJ. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science. 2017;356:1175–8.
Zhang Q, Blaylock LA, Harrison MJ. Two Medicago truncatula half-ABC transporters are essential for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Cell. 2010;22:1483–97.
Jiang Y, Wang WX, Xie QY, Liu N, Liu LX, Wang DP, Zhang XW, Yang C, Chen XY, Tang DZ, Wang E. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017;356:1172–5.
Yang L, Zou YN, Tian ZH, Wu QS, Kuča K. Effects of beneficial endophytic fungal inoculants on plant growth and nutrient absorption of trifoliate orange seedlings. Sci Hortic. 2021;277: 109815.
Wu QS, Liu CY, Zhang DJ, Zou YN, He XH, Wu QH. Mycorrhiza alters the profile of root hairs in trifoliate orange. Mycorrhiza. 2016;26:237–47.
Cao JL, He WX, Zou YN, Wu QS. An endophytic fungus, Piriformospora indica, enhances drought tolerance of trifoliate orange by modulating the antioxidant defense system and composition of fatty acids. Tree Physiol. 2023;43:452–66.
Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55:158–61.
Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59–66.
Wu QS. Experimental guideline in plant physiology. Beijing: China Agricultural Press; 2019.
He JD, Zou YN, Wu QS, Kuča K. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci Hortic. 2020;262: 108745.
Chance B, Maehly AC. Assay of catalases and peroxidases. Method Enzymol. 1955;2:773–5.
Zheng Q, Tang Z, Xu Q, Deng X. Isolation, phylogenetic relationship and expression profiling of sugar transporter genes in sweet orange (Citrus sinensis). Plant Cell Tiss Org. 2014;119:609–24.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and 2−∆∆Ct method. Methods. 2001;25:402–8.
Sheng CL, Guo QS, Liu ZY, Zhu SG, Liu YX. Infection progress of arbuscular mycorrhizae on tissue-cultured plantlets of Pinellia ternata. Chin J Chin Mater Med. 2011;32:93–6.
Bonfante P, Genre A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat Commun. 2010;1:48.
Wang F. Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit Rev Env Sci Tec. 2017;47:1901–57.
Wipf D, Krajinski F, van Tuinen D, Recorbet G, Courty PE. Trading on the arbuscular mycorrhiza market: from arbuscules to common mycorrhizal networks. New Phytol. 2019;223:1127–42.
Dodd JC, Boddington CL, Rodriguez A, Gonzalez-Chavez C, Mansur I. Mycelium of arbuscular mycorrhizal fungi (AMF) from different genera: form, function and detection. Plant Soil. 2000;226:131–51.
Fester T, Hause G. Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza. 2005;15:373–9.
Choi J, Summers W, Paszkowski U. Mechanisms underlying establishment of arbuscular mycorrhizal symbioses. Annu Rev Phytopathol. 2018;56:135–60.
Zhang F, Zou YN, Wu QS, Kuča K. Arbuscular mycorrhizas modulate root polyamine metabolism to enhance drought tolerance of trifoliate orange. Environ Exp Bot. 2020;171: 103962.
Bona E, Cantamessa S, Massa N, Manassero P, Marsano F, Copetta A, Lingua G, D’Agostino G, Gamalero E, Berta G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza. 2016;27:1–11.
Goicoechea N, Baslam M, Erice G, Irigoyen JJ. Increased photosynthetic acction limain alfalfa associated with arbuscular mycorrhizal fungi (AMF) and cultivated in greenhouse under elevated CO2. J Plant Physiol. 2014;171:1774–81.
Gray VM. The Role of the C:N:P stoichiometry in the carbon balance dynamics of the legume-AMF-rhizobium tripartite symbiotic association. In: Maheshwari D.K. Plant Growth and Health Promoting Bacteria. Springer-Verlag, Germany, pp. 2010:387−416.
Song F, Song G, Dong A, Kong X. Regulatory mechanisms of host plant defense responses to arbuscular mycorrhiza. Acta Ecol Sin. 2011;31:322–7.
Liu JY, Maldonado-Mendoza I, Lopez-Meyer M, Cheung F, Town CD, Harrison MJ. Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J. 2007;50:529–44.
Van Wees SCM, Van der Ent S, Pieterse CMJ. Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol. 2008;11:443–8.
Lokhandwala A, Hoeksema JD. Priming by arbuscular mycorrhizal fungi of plant antioxidant enzyme production: a meta-analysis. Annu Plant Rev. 2019;2:1069–84.
Kiirika LM, Schmitz U, Colditz F. The alternative Medicago truncatula defense proteome of ROS-defective transgenic roots during early microbial infection. Fron Plant Sci. 2014;5:341.
Avio L, Sbrana C, Giovannetti M, Frassinetti S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci Hortic. 2017;224:265–71.
Kapulnik Y, Volpin H, Itzhaki H, Ganon D, Galili S, David R, Shaul O, Elad Y, Chet I, Okon Y. Suppression of defence responses in mycorrhizal alfalfa and tobacco roots. New Phytol. 1996;133:59–64.
Zamioudis C, Pieterse CMJ. Modulation of host immunity by beneficial microbes. Mol Plant Microbe In. 2012;25:139–50.
Pandey P, Irulappan V, Bagavathiannan MV, Senthil-Kumar M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front Plant Sci. 2017;8:537.
Li QS, Xie YC, Rahman MM, Hashem A, Abd Allah EF, Wu QS. Arbuscular mycorrhizal fungi and endophytic fungi activate leaf antioxidant defense system of lane late navel orange. J Fungi. 2022;8:282.
Van Camp W, Bowler C, Villarroel R, Tsang EW, Van Montagu M, Inze D. Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. P Natl Acad Sci. 1990;87:9903–7.
Kim YC, Miller CD, Anderson AJ. Transcriptional regulation by iron of genes encoding iron-and manganese-superoxide dismutases from Pseudomonas putida. Gene. 1999;239:129–35.
Salvioli di Fossalunga A, Novero M. To trade in the field: the molecular determinants of arbuscular mycorrhiza nutrient exchange. Chem Biol Technol Ag 2019;6:12.
Treseder KK, Allen EB, Egerton-Warburton LM, Hart MM, Klironomos JN, Maherali H, Tedersoo L. Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: a trait-based predictive framework. J Ecol. 2018;106:480–9.
Manck-Götzenberger J, Requena N. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front Plant Sci. 2016;7:487.
Bravo A, Brands M, Wewer V, Dörmann P, Harrison MJ. Arbuscular mycorrhiza-specific enzymes FatM and RAM 2 fine-tune lipid biosynthesis to promote development of arbuscular mycorrhiza. New Phytol. 2017;214:1631–45.
Roth R, Paszkowski U. Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr Opin Plant Biol. 2017;39:50–6.
Benedetto A, Magurno F, Bonfante P, Lanfranco L. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza. 2005;15:620–7.
Küster H. The Medicago truncatula transcriptome database MtExpress: genome-wide expression profiles at your fingertips. Plant Cell Physiol. 2021;62:1359–61.
Sisaphaithong T, Yanase M, Mano T, Tanabe S, Minami E, Tanaka A, Hata S, Kobae Y. Localized expression of the Dwarf14-like2a gene in rice roots on infection of arbuscular mycorrhizal fungus and hydrolysis of rac-GR24 by the encoded protein. Plant Signal Behav. 2021;16:2009998.
Smith SE, Gianinazzi-Pearson V, Koid R, Cairney JWG. Nutrient transport in mycorrhiza: structure, physiology and consequences for efficiency of the symbiosis. Plant Soil. 1994;159:103–13.
Acknowledgements
The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R355), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
This work was supported by the Scientific and Technological Innovation Team of Outstanding Young Scientists, Hubei Provincial Department of Education (T201604). The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R355), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
XQL and ZL conducted the experiment and made data curation. YNZ and QSW designed the experiment. XQL wrote the original manuscript. QSW and MDA revised the manuscript. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interest regarding the publication of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Specific primer sequences of genes used for qRT-PCR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, XQ., Liu, Z., Zou, YN. et al. Defense responses and symbiotic functional initiation in trifoliate orange‒arbuscular mycorrhizal fungi interaction. Chem. Biol. Technol. Agric. 11, 3 (2024). https://doi.org/10.1186/s40538-023-00526-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00526-0