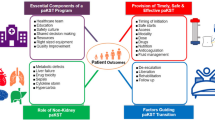
Abstract
Background
In the past decade, there have been substantial advances in our understanding of pediatric AKI. Despite this progress, large gaps remain in our understanding of pharmacology and nutritional therapy in pediatric AKI.
Methods
During the 26th Acute Disease Quality Initiative (ADQI) Consensus Conference, a multidisciplinary group of experts reviewed the evidence and used a modified Delphi process to achieve consensus on recommendations for gaps and advances in care for pharmacologic and nutritional management of pediatric AKI. The current evidence as well as gaps and opportunities were discussed, and recommendations were summarized.
Results
Two consensus statements were developed. (1) High-value, kidney-eliminated medications should be selected for a detailed characterization of their pharmacokinetics, pharmacodynamics, and pharmaco-“omics” in sick children across the developmental continuum. This will allow for the optimization of real-time modeling with the goal of improving patient care. Nephrotoxin stewardship will be identified as an organizational priority and supported with necessary resources and infrastructure. (2) Patient-centered outcomes (functional status, quality of life, and optimal growth and development) must drive targeted nutritional interventions to optimize short- and long-term nutrition. Measures of acute and chronic changes of anthropometrics, body composition, physical function, and metabolic control should be incorporated into nutritional assessments.
Conclusions
Neonates and children have unique metabolic and growth parameters compared to adult patients. Strategic investments in multidisciplinary translational research efforts are required to fill the knowledge gaps in nutritional requirements and pharmacological best practices for children with or at risk for AKI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 26th Acute Disease Quality Initiative (ADQI) served as a call to action for comprehensive care in the treatment of pediatric acute kidney injury (AKI) and disease. While in the last decade there have been significant advances in our understanding of pediatric AKI management and therapeutics, the roles of pharmacology and nutrition have been largely neglected. This ADQI meeting acknowledged the essential and unique roles pharmacotherapy and nutrition therapy play in treating children with AKI and disease or those who are at risk for developing AKI.
Pharmacotherapeutics and nutrition are disciplines which are inextricably linked in pediatric patients and share common challenges. Serum creatinine is the most common metric to assess kidney function for medication management and is impacted by nutritional status. Disease states and medications that can contribute to AKI such as shock and vasoactive drugs may alter regional blood flow and therefore nutrient utilization. Furthermore, both pharmacology and nutrition have been neglected topics of pediatric AKI research. As an example, research into the optimal method to dose medication and nutrition therapy is limited. Standard antibiotic doses used in pediatric critically ill children may lead to subtherapeutic or supratherapeutic exposure [1]. Therapeutic drug monitoring of medications is rare and developmentally tailored dosing guidance is limited [2]. Hospitalized children with AKI experience the highest rates of energy and protein deficits [3]. The resultant high prevalence of malnutrition is associated with substantial mortality in children with AKI [4]. Suboptimal nutritional management may contribute to high rates of muscle protein breakdown, metabolic derangements, poor fluid and electrolyte stewardship, and reduced rehabilitative capabilities necessary for survivors. The growth and developmental challenges that underlie every aspect of targeted medication and nutrition therapy in pediatric patients, particularly those with AKI and acute kidney disease (AKD), are understudied.
Herein, we address these inter-related topics by focusing on the specific challenges faced while caring for children with AKI, and propose future research goals to provide optimal individualized care.
Methods
The 26th ADQI Consensus Conference, the first ADQI devoted to pediatric AKI, was held in Napa, CA, USA, in November 2021, as described [5]. The consensus-building process was informed by review of articles by workgroup members identified through a PubMed search. A modified Delphi method was used to reach consensus, using evidence-based literature, whenever possible. A summary of the consensus conference has been published [5]. Within this article, we provide a summary of the current knowledge of pharmacotherapy and nutritional therapy in pediatric AKI and the existing knowledge gaps, and detail recommendations for next steps.
Results
Critical recommendations of the panel focused on a need to improve our understanding and treatment of (1) pharmacotherapy and (2) nutrition therapy in pediatric AKI by addressing the significant knowledge gaps therein (Table 1). These consensus recommendations are as follows:
-
Pharmacotherapy: High-value, kidney-eliminated medications should be selected for a detailed characterization of their pharmacokinetics, pharmacodynamics, and pharmaco-“omics” in sick children across the developmental continuum. This will allow for the optimization of real-time modeling with the goal of improving patient care. Nephrotoxin stewardship will be identified as an organizational priority and supported with necessary resources and infrastructure.
-
Nutrition therapy: Patient-centered outcomes (functional status, quality of life, and optimal growth and development) must drive targeted nutritional interventions to optimize short- and long-term nutrition. Measures of acute and chronic changes of anthropometrics, body composition, physical function, and metabolic control should be incorporated into nutritional assessments.
To fully address the current state of evidence leading this consensus recommendation and opportunities for future research advancement, we sought to answer the two questions developed during the ADQI Consensus Conference.
Question 1: What actions promote the safe and effective use of kidney eliminated and nephrotoxic medications in children?
Pharmacokinetic changes in critically ill pediatric patients have been understudied
Critically ill patients experience changes in the absorption, distribution, metabolism, and elimination of medications [6]. While enteral administration of medications is often the preferred route, factors may affect the adequacy of absorption including changes in gastric pH, decreased perfusion, delayed gastric emptying or dysmotility, bowel compromise, or altered anatomy. Subcutaneous, intramuscular, and transdermal absorption may be altered by perfusion changes and subcutaneous edema, both of which are common in critical illness. For these reasons, intravenously administered drugs offer the most predictable therapeutic effect in the critically ill. Vascular permeability in shock and heart failure may expand volume of distribution and decrease the plasma concentrations of drugs and minerals [7]. Altered protein binding from vascular permeability, decreased protein synthesis, and catabolism change the free fraction of drugs. Metabolism and elimination through the liver may be affected by altered blood flow or enzymatic activity. Kidney function may be increased or decreased in critical illness which affects drug elimination [8]. Disease states common in critical illness, including sepsis and shock, are associated with an increased risk of AKI. A proportion of critically ill patients experience the opposite problem—supraphysiologic kidney function—so-called “augmented renal clearance” which has been linked to decreased drug exposure and treatment failure in adults [9]. However, there has been limited investigation into this syndrome in pediatric patients. Some of these pharmacokinetic changes are obvious (e.g., decreased albumin concentration suggesting altered protein binding) whereas others are assumed, but not readily verified (e.g., changes to liver enzymatic activity).
AKI further changes pharmacokinetics
AKI can modify both pharmacokinetics and pharmacodynamics. While drug elimination via the kidney is altered, so too are other aspects of the pharmacokinetic profile including the volume of distribution, plasma protein binding, and non-kidney metabolism. Patients with AKI often experience an expanded volume of distribution of hydrophilic drugs due to the increase in total body water [6]. Fluid overload is common in critically ill patients and impacts drug dosing [10]. Although newer technology is available to directly assess blood volume, its viability for the pediatric and neonatal population is uncertain, and rarely is the tool used clinically to tailor pharmacotherapy. In severe AKI, the presence of uremic molecules may displace drugs from protein bindings sites.
AKI may also impact non-kidney elimination pathways. Experimental models have identified alterations to metabolism in the presence of AKI, but these have been insufficiently corroborated in humans. Studies also report decreased activity of transporter proteins (such as organic anion transporters in the proximal tubule) which could lead to increased drug exposure [11]. All of these challenges can be further accentuated when patients are on any form of continuous kidney replacement therapy (CKRT), where the kinetics of medication and nutrition therapy are rarely studied [12, 13]. In patients on CKRT, dosage, timing, and duration of medications merit careful attention and are areas in need of further investigation.
Extrapolating drug dosing in pediatric patients
Little is known about optimal dosing of medication and nutrition therapy for hospitalized pediatric patients. Drugs are mainly designed for and tested in adults, and mostly in non-critically ill patients. Pediatric extrapolation of medication and nutrition therapy dosing was proposed and supported to an extent by regulatory agencies based on three underlying assumptions: (1) similar disease progressions in the two populations, (2) similar responses to interventions, and (3) similar exposure–response relationships [14]. However, this decision-making was based on several decades old literature and clinical practice. As our technology has improved and our care has become more sophisticated, the knowledge gap has widened. For example, the physiology and pharmacokinetics of an extremely premature 24-week neonate weighing 500 g were not anticipated as part of the pediatric drug dosing extrapolation. Additionally, developmental changes in drug clearance are not accounted for in the weight-based dose recommendations [15].
Most data extrapolated from adult studies may not be appropriate for this patient population, especially during critical illness. Pediatric dosing is based on a decision tree model, asking if drugs have (1) similar progression and/or responses, and (2) similar exposure and/or response [14, 16]. To improve the efficiency of pharmacology studies in infants and children, opportunistic sampling and sparse sampling, noninvasive matrices (e.g., hair, exhaled breath, saliva), and microvolume assays have been suggested [17, 18]. In opportunistic and sparse sampling methods, a single procedure is performed to limit the burden of phlebotomy for research. This may include alignment of specimen collection with standard of care procedures (e.g., blood draw), or recovery of residual specimens drawn for clinical care for research purposes. These techniques limit the burden of research both for the patients and for the families, and for the research community, a priority of great importance in the pediatric and neonatal populations. Drawing the right balance between sufficient sampling and patient burden, including low recruitment, to clinical studies is one of the challenges faced in this field.
Developmental changes impact drug disposition and response
Developmental changes enhance complexity to the perturbed pharmacokinetics in critical illness [19]. Across the continuum of pediatric patients, body composition, organ size, organ function, and gene expression vary, leading to alterations in cellular function and metabolic activity. For example, total body water changes dramatically in the first months of life, from 85% in neonates to 60% by 6 months of age. This change is accompanied by increases in protein mass and muscle [20]. Furthermore, some tissues may be more sensitive to effects of medication early in life. Drug metabolizing enzyme activity and alterations in kidney function also evolve over time [1].
Evaluating drug disposition in critically ill pediatric patients, especially neonates, requires a comprehensive assessment of developmental factors that augment pharmacokinetic complexity. Estimating kidney function in neonates with critical illness is poorly described [21]. Although kidney function develops with maturation, the biomarkers utilized for kidney assessment have inadequate performance in neonates, especially those who are premature or critically ill [22]. In the first days of life, neonatal serum creatinine, the traditional mainstay of endogenous filtration markers, reflects maternal serum creatinine. As kidney function matures after birth, serum creatinine slowly declines and GFR increases [23]. Neonates also have less muscle, and therefore less creatinine production, which needs to be accounted for in GFR estimation [15]. Accurately tracking an infant on this dynamic curve of GFR maturation is not yet achievable at the bedside [24]. Furthermore, premature neonates may have ongoing post-natal nephrogenesis, but glomerulogenesis may be abnormal in this population [25].
Nephrotoxin burden is large in pediatric patients
Nephrotoxin exposure is a significant predictor of AKI in pediatric and neonatal patients in both the hospital and community [26, 27]. Up to one-fourth of the medications administered to hospitalized patients may be nephrotoxic and can result in potentially avoidable adverse drug events [28]. The odds of AKI in pediatric patients increased when exposed to nephrotoxins (OR 1.7, 95% CI 1.04–2.9) [26]. While it is both impractical, and likely inappropriate, to eliminate the use of all nephrotoxic therapies, the benefit must be weighed against the risk.
Nephrotoxin exposure is uniquely challenging in pediatrics given the biologic variable of development on the risk for drug toxicity. Developmental factors may affect AKI risk in neonates, as a potentiator or a mediator. An individual’s glomerular filtration rate gradually increases from birth, reaching adult values at approximately 2 years of age. During this transition, the risk of nephrotoxicity may be increased, particularly with substances eliminated by the kidney [23]. In neonates, the immaturity of the tubules in early life may play a role in the mechanism for nephrotoxins with injury that increases with increasing intracellular concentration (e.g., aminoglycosides) [19]. Concurrently, a neonate’s inability to maximally concentrate their urine may predispose to intravascular volume depletion which increases the nephrotoxic potential of medications impacting kidney hemodynamics [23]. Sex differences have been seen in nephrotoxin-associated AKI [29]. These may need to be accounted for with the hormonal changes that occur postnatally, and throughout childhood and adolescence.
Nephrotoxin burden, defined as the cumulative or aggregate exposure to nephrotoxins, is a major risk factor in pediatric AKI [30]. Both the duration of drug exposure and the nephrotoxic potential of the drug influence overall burden. Risk is potentiated with exposure to multiple nephrotoxins simultaneously [26]. Exposure to multiple nephrotoxins is common in neonates with critical illness, especially those born prematurely [31]. Studies have shown that the risk of AKI increases with use of simultaneous nephrotoxins, with those exposed to three or more medications being at greater risk of developing higher stage AKI than those with lower levels of exposure [26]. In a French pharmacovigilance study, concomitant administration of ≥ 2 nephrotoxins was associated with 66% of AKI cases [32]. In adults, each additional nephrotoxin used in the post-AKI period is associated with a 1.3-fold higher risk of new or progressive CKD even after adjustment for relevant confounders. For individuals with > 4 nephrotoxins, there is a 2.1-fold higher risk of CKD in 3 years [33]. Nephrotoxic risk is often cumulative, and pediatric patients (especially critically ill children and neonates) are at high risk of exposure to multiple nephrotoxins simultaneously. Evidence of long-term risk for CKD is beginning to emerge [34].
Pharmacovigilance and nephrotoxin stewardship programs are necessary to improve outcomes in pediatrics
Best practices for decreasing the incidence, severity, and complications associated with nephrotoxin exposure, particularly when multiple nephrotoxins are used concurrently, are still evolving. While standardized approaches to pediatric patients have been developed [35], and the suggested KDIGO bundle includes nephrotoxin stewardship [36], neither of these approaches have been formally tested for benefit in children. Stewardship in general integrates key principles including individual and institutional commitment, data, resources, and tools including to facilitate implementation of best practices. One pediatric-specific model of nephrotoxin stewardship is the NINJA (Nephrotoxic Injury Negated by Just in Time Action) program. In patients at high risk for nephrotoxic medication-associated AKI, daily serum creatinine monitoring is recommended [37]. With this relatively simple intervention, there was a 25% reduction in AKI rate and 42% reduction in AKI days per 100 nephrotoxin medication exposure in the first year. This comes from rapid AKI recognition and nephrotoxic medication discontinuation. By 3 years, the results were sustained with a 31% AKI intensity decrease and a 64% AKI rate decrease. These nephrotoxin programs have been expanded to neonates, and demonstrated decreased risk of nephrotoxic medication exposure while maintaining a high serum creatinine surveillance rate [31]. Importantly for the smallest neonates, the Baby NINJA program did not increase the need for blood transfusions [38]. Further potential extensions include developing reflex serum creatinine monitoring for high-risk patients and local medication list tailoring [39].
The success of the nephrotoxin stewardship programs depends on engaged physicians, supportive hospital leadership, active discussion, and multidisciplinary engagement with physicians and pharmacists [40]. Sustained QI requires a culture change throughout the multidisciplinary team. Despite growing evidence that quality programs are effective at improving nephrotoxin stewardship in pediatric patients, best practices for dissemination and implementation of these programs require further characterization, especially in neonates.
Research recommendations to address existing gaps
Despite a recent growth in our understanding of pharmacokinetics and pharmacodynamics, there remain large gaps in our understanding (Table 1). We recommend that high-value, kidney-eliminated medications should be selected for a detailed characterization of their pharmacokinetics, pharmacodynamics, and pharmaco-“omics” in sick children across the developmental continuum, to allow optimization of real-time modeling towards developing improvements in patient care. Selection of these medications will necessarily be determined by the frequency of use, therapeutic window, and context of the evaluation. As an example, a detailed assessment of a frequently used kidney-eliminated chemotherapy agent in sick children could provide substantial benefits for safety and effectiveness. Another case is the development of a deeper understanding of the pharmacology of kidney eliminated anti-epileptics will help with therapeutic tailoring over a child’s development. Furthermore, we recommend that nephrotoxin stewardship should be identified as an organizational priority and supported with necessary resources and infrastructure.
Question 2: What are the nutritional targets in children with acute kidney injury and disease and how are they defined?
Optimal nutritional management works in concert with pharmacologic management
Malnutrition and protein energy wasting is common in hospitalized children with AKI, with a prevalence rate of 30–55% in children receiving CKRT [4, 41,42,43]. Nutritional management may prevent worsening nutritional status in children with AKI, persistent AKI, and AKD. Inability to provide adequate nutrition has often been cited as a significant factor in the decision to initiate CKRT in the pediatric population. Initiation of CKRT can improve fluid management and may prevent worsening nutritional status [3, 44]. However, there are few studies which evaluate the effect of nutritional therapy on outcomes beyond mortality.
The Pediatric Renal Nutrition Taskforce, an international collaborative of pediatric dietitians and nephrologists, recently published clinical practice recommendations for the nutritional management of children with AKI [45]. These recommendations were supported by an extensive literature review and Delphi process. Moreover, they were designed to provide clarity to the prescription of nutrition therapies and improve patient outcomes in children with AKI. Optimal nutritional management allows for control of metabolic derangements, control of fluid balance, and supported recovery of tissue repair and immune function. Despite the potential that nutritional stewardship holds, there is scarce evidence to support nutrition interventions to improve outcomes in children with AKI/AKD. Fluid overload has an independent and synergistic effect on the outcomes of critically ill children with AKI [46]. Nutritional fluids are modifiable and can be optimized to improve fluid control prior to worsening AKI while positively influencing the frequency of metabolic abnormalities in children with AKI. These clinical practice recommendations work in concert with the research recommendations to help identify key areas needed for advancement of nutritional therapy in children AKI/AKD.
Age-related nutrient requirements impact nutrition risk
The dynamic nature of AKI, AKD, and recovery within the developmental and growth spectrum of a pediatric patient limits the accuracy of extrapolative nutrition interventions in clinical practice. Malnutrition and protein energy wasting has been independently associated with mortality in children receiving CKRT [4]. Yet, mortality is a crude end point measurement as malnutrition has multiple contributory mechanisms and outcomes that may be bidirectionally confounded by AKI medical management and dialytic modalities. The effect of age and its influence on developmental stage needs to be considered as malnutrition disproportionally affects younger children [47].
AKI and critical illness affect the metabolic response and goals to nutrition therapy
The physiological targets and goals for nutritional management change based on the severity of AKI/AKD, nutritional status, and age. Acutely, nutritional targets should focus on normalization of electrolytes and minerals as well as volume control through proper stewardship of nutritional fluids. Once these initial targets are achieved, the patient’s therapeutic nutrition goals should shift towards supporting rehabilitative processes including promoting improved metabolic function, immune function, tissue repair, and mitigating lean mass loss and functional decline. In addition to these nutritional targets, it is critical to understand the effects of AKI/AKD on nutritional status. Key nutritional principles to consider in this paradigm are included in Table 2.
Nutrient kinetics and medication exposure alter macronutrient need in children with AKI
Specific macronutrient targets are extremely limited as no calorimetric or nutrient kinetic studies have been reported on children with AKI. Catabolism compounded with nutritional deficits are frequently reported in children [48] and adults [49] with AKI. Historically, CKRT was considered a contraindication to indirect calorimetry given potential influence of bicarbonate clearance. Data support a potential underestimation of energy expenditure in adults and thus inappropriate prescription of energy [50]. Additionally, extrapolation from both critically ill pediatric [51] and adult AKI [52] data confirms a shift in substrate utilization due to inflammatory driven metabolic dysfunction resulting in increased beta oxidation.
Medication and dialysis fluid exposure compound non-nutritional energy and substrate exposure, most notably in the form of dextrose and citrate-based anticoagulation, leading to the equivalent of 500 additional calories daily in adults [53]. Net calorie loss can also occur if utilizing glucose free replacement solutions [53]. Excessive or suboptimal provision of energy and substrate may lead to prolonged recovery time. The need for individualized energy prescriptions may hinge on the technological advancements required to make indirect calorimetry a more utilized tool in hospitalized children. Additional research should focus on utilizing indirect calorimetry or other techniques, such as doubly labeled water, with careful emphasis on the need to study the effect of dialysis and medication-related fluid exposure in children with AKI.
Baseline protein requirements are higher in children than in adults given the need for development and growth. Increased muscle protein breakdown in AKI/AKD drives negative nitrogen balances [54]. Attenuation of skeletal muscle loss through adequate nutrition support is critical in supporting tissue repair, rehabilitation, and improved functional outcomes in survivors. In acute intermittent dialytic therapies and conservative management, protein needs are often extrapolated from CKD or maintenance dialytic therapy yet steady-state energy and protein metabolism are primary assumptions that should not be assumed in acute or critical illness. The metabolic consequences of underfeeding may also subsequently lead to increased catabolism due to redirection of protein as a substrate for energy.
More work is needed to understand each child’s unique nutritional needs in the setting of AKI. Pediatric CKRT studies consistently report losses equivalent to ~ 10–20% of amino acids provided via nutrition support [41, 55]. Most amino acid concentrations appear to stabilize after 5 days of initiation of CKRT [55]. However, carnitine, a key nutrient in long-chain fatty acid metabolism, has been reported as a critical amino acid of concern in children receiving CKRT with secondary carnitine deficiency reported in 100% of patients by the third week of CKRT [56]. Secondary carnitine deficiency has the potential to negatively influence rehabilitative processes given its effects on cardiac and skeletal muscle. Thus, specific dosing targets of protein or specific amino acids in children with AKI and AKD remain undetermined, but should be dosed based on nutrition-related outcome metrics.
Critical illness and AKI further modify electrolyte, mineral, vitamin, and trace element requirements
Optimal management of micronutrients in children with AKI and AKD is even more elusive. Electrolyte abnormalities are hallmark complications of AKI, but are dynamic and dependent on the child’s clinical status, medications, and CKRT-dialytic modality. The most commonly reported mineral abnormalities in children receiving CKRT are hypophosphatemia and hypomagnesemia [57]. Adequate electrolyte and mineral control may have long-term rehabilitation effects. Improved phosphate levels (an essential requirement for energy production) in adults receiving CKRT is associated with fewer ventilator days [58]. Studies reporting nutritional intake and AKI rarely report electrolyte or mineral intakes from nutrition and pharmacological sources. Therapeutic targets for electrolytes and minerals should be adjusted based on monitoring levels.
Little is known also regarding vitamin and trace element needs in children with AKI and AKD. There have been two studies of micronutrient balance in children receiving CKRT which evaluated folate, selenium, zinc, copper, chromium, and manganese, and found negative balances of folate and selenium were potentially present within the first week of CKRT [55, 59]. Vitamin C has received less attention despite its water soluble nature and likelihood of losses in effluent [60]. Recent studies have raised the question if CKRT, AKI, or critical illness contributes to larger variation in plasma levels of micronutrients in metabolic disturbances. It is also possible that CKRT duration and primary diagnosis (e.g., burns, multi-organ failure) pose the greater risk for micronutrient-deficient states [61].
Lastly, there is limited evidence that micronutrient supplementation improves patient outcomes in any hospitalized child. Micronutrient monitoring is not standard practice and often not attained until overt physical manifestations of a deficiency are present. Laboratory values of most micronutrients can be affected by inflammation, movement between plasma and intracellular compartments, decreased sequestration, and increased exposure to reactive oxygen species [62]. The ideal circumstance would be a baseline assessment of micronutrient values before critical illness and a trend over time, particularly in patients with prolonged illness. Rarely will this be available; thus, differentiating between the physiological response to illness versus deficient states becomes extremely difficult. Ideally, future research regarding micronutrient needs, intake, and outcomes likely lies with a multidisciplinary approach to identify the appropriate provision of micronutrients. An integrative approach is needed, encompassing pre-clinical, epidemiological, and translational models including well-designed prospective studies, particularly evaluating micronutrient metabolism changes and balance during periods of high oxidative stress and inflammation.
Feeding modality may mitigate adverse outcomes but requires further study
Enteral feeding is recommended in critically ill children in order to maintain gut integrity and mitigate gut atrophy. Enteral protein intake has been associated with lower 60-day mortality compared to parenteral (PN) amino acids in critically ill children [63]. In adults receiving CKRT, low protein intake has been associated with increased mortality, which is primarily delivered enterally [49]. This association of protein intake supporting improved mortality has not been found in children with AKI. Most studies analyzing protein losses in CKRT are in children receiving PN in which clearance and metabolic dysfunction due to development and growth may not be similar [41, 55]. Additionally, the PEPaNIC (early versus late parenteral nutrition in the pediatric ICU) trial in critically ill children showed that withholding PN for the first week of an ICU admission decreased the incidence of new infections and improved time to recovery when compared to supplemental PN [64]. In early PN, the dose of amino acids was associated with increased infections and longer dependency on mechanical ventilation for critically ill children [65].
It is theorized that in the acute phase of critical illness, catabolism may be part of a larger adaptive and beneficial process of autophagy which may initially be protective. However, enteral nutrition has been reported as a barrier to achieving nutritional goals in children receiving CKRT [66]. High rates of gastrointestinal dysmotility in critically ill children with AKI compared to those without AKI likely limit the ability to initiate or advance enteral feeding [67]. Thus, PN may be necessary to achieve nutrition targets in children with AKI, especially those who are malnourished. The nutritional effects of feeding the gut are likely much more nuanced and need further investigation.
Advancement of nutritional therapy relies on identification of appropriate outcomes
The principles of nutrition therapy in children with AKI, persistent AKI and AKD, should promote nutrient balance to support normalization, recovery, and rehabilitation in survivors. Thus, outcome metrics should reflect these end goals to allow for appropriate comparisons of nutritional interventions. An evaluation of nutritional intervention efficacy is also dependent on our ability to accurately diagnose phenotypic nutritional morbidities (e.g., malnutrition, kidney cachexia, frailty, and sarcopenia). Lastly, it is difficult to adequately identify nutritional outcome metrics when follow-up in AKI survivorship in pediatrics is scarce. Nutrition support and mobilization have been shown to attenuate muscle loss and promote muscle protein synthesis in critically ill children [68]. AKI PICU survivors have high rates of poor functional outcomes and experience increased technological dependence after hospital discharge [43]. Ideally, monitoring variables should include those that would optimize the rehabilitative process, allowing for the recuperation of functional status, improvement in lean mass and muscle strength, promotion of muscle protein synthesis, and reversal of metabolic alterations such as insulin resistance (Table 3).
Research recommendations to address existing gaps
Patient-centered outcomes such as functional status, quality of life, and optimal growth and development must drive the targeted nutritional interventions, optimizing short- and long-term nutrition, incorporating measures of acute and chronic changes of anthropometrics, body composition, physical function, and metabolic control (Table 4).
Conclusion
With advancing CKRT technologies and as the boundaries of viability have been pushed in the neonatal intensive care unit, pharmacology and nutrition in pediatric AKI have been understudied. Further research advancements in the field of pediatric AKI must focus on the unique aspects of development as a biological variable, a detailed characterization of high value, kidney-eliminated medications, and patient-centered outcomes to drive targeted nutritional interventions.
References
Hartman SJF, Brüggemann RJ, Orriëns L, Dia N, Schreuder MF, de Wildt SN (2020) Pharmacokinetics and target attainment of antibiotics in critically ill children: a systematic review of current literature. Clin Pharmacokinet 59:173–205. https://doi.org/10.1007/s40262-019-00813-w
Cies JJ, Moore WS 2nd, Enache A, Chopra A (2018) beta-lactam therapeutic drug management in the PICU. Crit Care Med 46:272–279. https://doi.org/10.1097/CCM.0000000000002817
Kyle UG, Akcan-Arikan A, Orellana RA, Coss-Bu JA (2013) Nutrition support among critically ill children with AKI. Clin J Am Soc Nephrol 8:568–574. https://doi.org/10.2215/CJN.05790612
Castillo A, Santiago MJ, Lopez-Herce J, Montoro S, Lopez J, Bustinza A, Moral R, Bellon JM (2012) Nutritional status and clinical outcome of children on continuous renal replacement therapy: a prospective observational study. BMC Nephrol 13:125. https://doi.org/10.1186/1471-2369-13-125
Goldstein SL, Akcan-Arikan A, Alobaidi R, Askenazi DJ, Bagshaw SM, Barhight M, Barreto E, Bayrakci B, Bignall ONR, Bjornstad E, Brophy PD, Chanchlani R, Charlton JR, Conroy AL, Deep A, Devarajan P, Dolan K, Fuhrman DY, Gist KM, Gorga SM, Greenberg JH, Hasson D, Ulrich EH, Iyengar A, Jetton JG, Krawczeski C, Meigs L, Menon S, Morgan J, Morgan CJ, Mottes T, Neumayr TM, Ricci Z, Selewski D, Soranno DE, Starr M, Stanski NL, Sutherland SM, Symons J, Tavares MS, Vega MW, Zappitelli M, Ronco C, Mehta RL, Kellum J, Ostermann M, Basu RK; Pediatric ADQI Collaborative (2022) Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi consensus statement. JAMA Netw Open 5:e2229442. https://doi.org/10.1001/jamanetworkopen.2022.29442
Rodieux F, Wilbaux M, van den Anker JN, Pfister M (2015) Effect of kidney function on drug kinetics and dosing in neonates, infants, and children. Clin Pharmacokinet 54:1183–1204. https://doi.org/10.1007/s40262-015-0298-7
Batchelor HK, Marriott JF (2015) Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol 79:395–404. https://doi.org/10.1111/bcp.12267
Dhont E, Van Der Heggen T, De Jaeger A, Vande Walle J, De Paepe P, De Cock PA (2020) Augmented renal clearance in pediatric intensive care: are we undertreating our sickest patients? Pediatr Nephrol 35:25–39. https://doi.org/10.1007/s00467-018-4120-2
Udy AA, Lipman J, Jarrett P, Klein K, Wallis SC, Patel K, Kirkpatrick CM, Kruger PS, Paterson DL, Roberts MS, Roberts JA (2015) Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit Care 19:28. https://doi.org/10.1186/s13054-015-0750-y
Alobaidi R, Basu RK, DeCaen A, Joffe AR, Lequier L, Pannu N, Bagshaw SM (2020) Fluid accumulation in critically ill children. Crit Care Med 48:1034–1041. https://doi.org/10.1097/ccm.0000000000004376
Philips BJ, Lane K, Dixon J, Macphee I (2014) The effects of acute renal failure on drug metabolism. Expert Opin Drug Metab Toxicol 10:11–23. https://doi.org/10.1517/17425255.2013.835802
Shaw AR, Mueller BA (2017) Antibiotic dosing in continuous renal replacement therapy. Adv Chronic Kidney Dis 24:219–227. https://doi.org/10.1053/j.ackd.2017.05.004
Berger MM, Broman M, Forni L, Ostermann M, De Waele E, Wischmeyer PE (2021) Nutrients and micronutrients at risk during renal replacement therapy: a scoping review. Curr Opin Crit Care 27:367–377. https://doi.org/10.1097/MCC.0000000000000851
Dunne J, Rodriguez WJ, Murphy MD, Beasley BN, Burckart GJ, Filie JD, Lewis LL, Sachs HC, Sheridan PH, Starke P, Yao LP (2011) Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics 128:e1242-1249. https://doi.org/10.1542/peds.2010-3487
Anderson BJ, Holford NH (2009) Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 24:25–36. https://doi.org/10.2133/dmpk.24.25
Food and Drug Administration (2014) General clinical pharmacology considerations for pediatric studies for drugs and biological products: guidance for industry. U.S Department of Health and Human Services, Center for Drug Evaluation and Research (CDER). https://www.fda.gov/media/90358/download. Accessed 11 September 2023
Balevic SJ, Cohen-Wolkowiez M (2018) Innovative study designs optimizing clinical pharmacology research in infants and children. J Clin Pharmacol 58(Suppl 10):S58–S72. https://doi.org/10.1002/jcph.1053
Tang Girdwood SC, Tang PH, Murphy ME, Chamberlain AR, Benken LA, Jones RL, Stoneman EM, Kaplan JM, Vinks AA (2021) Demonstrating feasibility of an opportunistic sampling approach for pharmacokinetic studies of β-lactam antibiotics in critically ill children. J Clin Pharmacol 61:565–573. https://doi.org/10.1002/jcph.1773
Charlton JR, Harer MW, Swan C, Nielsen R (2019) Immature megalin expression in the preterm neonatal kidney is associated with urinary loss of vitamin carrier proteins. Pediatr Res 85:405–411. https://doi.org/10.1038/s41390-018-0261-z
Dotta A, Chukhlantseva N (2012) Ontogeny and drug metabolism in newborns. J Matern Fetal Neonatal Med 25(Suppl 4):83–84. https://doi.org/10.3109/14767058.2012.715463
Wilhelm-Bals A, Combescure C, Chehade H, Daali Y, Parvex P (2020) Variables of interest to predict glomerular filtration rate in preterm newborns in the first days of life. Pediatr Nephrol 35:703–712. https://doi.org/10.1007/s00467-019-04257-z
Abitbol CL, Seeherunvong W, Galarza MG, Katsoufis C, Francoeur D, Defreitas M, Edwards-Richards A, Master Sankar Raj V, Chandar J, Duara S, Yasin S, Zilleruelo G (2014) Neonatal kidney size and function in preterm infants: what is a true estimate of glomerular filtration rate? J Pediatr 164:1026-1031.e1022. https://doi.org/10.1016/j.jpeds.2014.01.044
Iacobelli S, Guignard JP (2021) Maturation of glomerular filtration rate in neonates and infants: an overview. Pediatr Nephrol 36:1439–1446. https://doi.org/10.1007/s00467-020-04632-1
Smeets NJL, IntHout J, van der Burgh MJP, Schwartz GJ, Schreuder MF, de Wildt SN (2022) Maturation of GFR in term-born neonates: an individual participant data meta-analysis. J Am Soc Nephrol 33:1277–1292. https://doi.org/10.1681/ASN.2021101326
Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE (2004) Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7:17–25. https://doi.org/10.1007/s10024-003-3029-2
Moffett BS, Goldstein SL (2011) Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol 6:856–863. https://doi.org/10.2215/CJN.08110910
Charlton JR, Boohaker L, Askenazi D, Brophy PD, D’Angio C, Fuloria M, Gien J, Griffin R, Hingorani S, Ingraham S, Mian A, Ohls RK, Rastogi S, Rhee CJ, Revenis M, Sarkar S, Smith A, Starr M, Kent AL; Neonatal Kidney Collaborative (2019) Incidence and risk factors of early onset neonatal AKI. Clin J Am Soc Nephrol 14:184–195. https://doi.org/10.2215/CJN.03670318
Taber SS, Mueller BA (2006) Drug-associated renal dysfunction. Crit Care Clin 22:357–374. https://doi.org/10.1016/j.ccc.2006.02.003
Boddu R, Fan C, Rangarajan S, Sunil B, Bolisetty S, Curtis LM (2017) Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am J Physiol Renal Physiol 313:F740–F755. https://doi.org/10.1152/ajprenal.00049.2017
Kane-Gill SL (2021) Nephrotoxin stewardship. Crit Care Clin 37:303–320. https://doi.org/10.1016/j.ccc.2020.11.002
Stoops C, Stone S, Evans E, Dill L, Henderson T, Griffin R, Goldstein SL, Coghill C, Askenazi DJ (2019) Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action): reduction of nephrotoxic medication-associated acute kidney injury in the neonatal intensive care unit. J Pediatr 215:e226. https://doi.org/10.1016/j.jpeds.2019.08.046
Pierson-Marchandise M, Gras V, Moragny J, Micallef J, Gaboriau L, Picard S, Choukroun G, Masmoudi K, Liabeuf S, French National Network of Pharmacovigilance Centres (2017) The drugs that mostly frequently induce acute kidney injury: a case - noncase study of a pharmacovigilance database. Br J Clin Pharmacol 83:1341–1349. https://doi.org/10.1111/bcp.13216
Schreier DJ, Rule AD, Kashani KB, Mara KC, Kane-Gill SL, Lieske JC, Chamberlain AM, Barreto EF, ACT Study Team (2022) Nephrotoxin exposure in the 3 years following hospital discharge predicts development or worsening of chronic kidney disease among acute kidney injury survivors. Am J Nephrol 53:273–281. https://doi.org/10.1159/000522139
Menon S, Kirkendall ES, Nguyen H, Goldstein SL (2014) Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr 165:e522. https://doi.org/10.1016/j.jpeds.2014.04.058
Goswami E, Ogden RK, Bennett WE, Goldstein SL, Hackbarth R, Somers MJG, Yonekawa K, Misurac J (2019) Evidence-based development of a nephrotoxic medication list to screen for acute kidney injury risk in hospitalized children. Am J Health Syst Pharm 76:1869–1874. https://doi.org/10.1093/ajhp/zxz203
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120:c179-184. https://doi.org/10.1159/000339789
Goldstein SL, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, Seid M, Ashby M, Foertmeyer N, Brunner L, Lesko A, Barclay C, Lannon C, Muething S (2013) Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics 132:e756–e767. https://doi.org/10.1542/peds.2013-0794
Gavigan HW, Slagle CL, Krallman KA, Poindexter BB, Hooper DK, Goldstein SL (2021) Blood transfusion rates in Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action)-a single-center experience. Pediatr Nephrol 36:1901–1905. https://doi.org/10.1007/s00467-020-04917-5
Yonekawa KE, Zhou C, Haaland WL, Wright DR (2019) Nephrotoxin-related acute kidney injury and predicting high-risk medication combinations in the hospitalized child. J Hosp Med 14:462–467. https://doi.org/10.12788/jhm.3196
Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, Kirkendall ES (2016) A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 90:212–221. https://doi.org/10.1016/j.kint.2016.03.031
Lion RP, Vega MR, Smith EO, Devaraj S, Braun MC, Bryan NS, Desai MS, Coss-Bu JA, Ikizler TA, Akcan Arikan A (2022) The effect of continuous venovenous hemodiafiltration on amino acid delivery, clearance, and removal in children. Pediatr Nephrol 37:433–441. https://doi.org/10.1007/s00467-021-05162-0
Vega MW, Juarez M, Lee JY, Srivaths P, Williams E, Akcan Arikan A (2018) Quality improvement bedside rounding audits enhance protein provision for pediatric patients receiving continuous renal replacement therapy. Pediatr Crit Care Med 19:1054–1058. https://doi.org/10.1097/PCC.0000000000001698
Smith M, Bell C, Vega MW, Tufan Pekkucuksen N, Loftis L, McPherson M, Graf J, Akcan Arikan A (2022) Patient-centered outcomes in pediatric continuous kidney replacement therapy: new morbidity and worsened functional status in survivors. Pediatr Nephrol 37:189–197. https://doi.org/10.1007/s00467-021-05177-7
Murphy HJ, Cahill JB, Twombley KE, Kiger JR (2018) Early continuous renal replacement therapy improves nutrition delivery in neonates during extracorporeal life support. J Ren Nutr 28:64–70. https://doi.org/10.1053/j.jrn.2017.06.008
Vega MRW, Cerminara D, Desloovere A, Paglialonga F, Renken-Terhaerdt J, Walle JV, Shaw V, Stabouli S, Anderson CE, Haffner D, Nelms CL, Polderman N, Qizalbash L, Tuokkola J, Warady BA, Shroff R, Greenbaum LA (2023) Nutritional management of children with acute kidney injury-clinical practice recommendations from the Pediatric Renal Nutrition Taskforce. Pediatr Nephrol 38:3559–3580. https://doi.org/10.1007/s00467-023-05884-3
Gist KM, Selewski DT, Brinton J, Menon S, Goldstein SL, Basu RK (2020) Assessment of the independent and synergistic effects of fluid overload and acute kidney injury on outcomes of critically ill children. Pediatr Crit Care Med 21:170–177. https://doi.org/10.1097/PCC.0000000000002107
Carvalho-Salemi J, Salemi JL, Wong-Vega MR, Spooner KK, Juarez MD, Beer SS, Canada NL (2018) Malnutrition among hospitalized children in the United States: changing prevalence, clinical correlates, and practice patterns between 2002 and 2011. J Acad Nutr Diet 118:40-51.e47. https://doi.org/10.1016/j.jand.2017.02.015
Mehta NM, Bechard LJ, Dolan M, Ariagno K, Jiang H, Duggan C (2011) Energy imbalance and the risk of overfeeding in critically ill children. Pediatr Crit Care Med 12:398–405. https://doi.org/10.1097/PCC.0b013e3181fe279c
Kritmetapak K, Peerapornratana S, Srisawat N, Somlaw N, Lakananurak N, Dissayabutra T, Phonork C, Leelahavanichkul A, Tiranathanagul K, Susantithapong P, Loaveeravat P, Suwachittanont N, Wirotwan TO, Praditpornsilpa K, Tungsanga K, Eiam-Ong S, Kittiskulnam P (2016) The impact of macro-and micronutrients on predicting outcomes of critically ill patients requiring continuous renal replacement therapy. PLoS One 11:e0156634. https://doi.org/10.1371/journal.pone.0156634
Jonckheer J, Demol J, Lanckmans K, Malbrain M, Spapen H, De Waele E (2020) MECCIAS trial: metabolic consequences of continuous veno-venous hemofiltration on indirect calorimetry. Clin Nutr 39:3797–3803. https://doi.org/10.1016/j.clnu.2020.04.017
Coss-Bu JA, Klish WJ, Walding D, Stein F, Smith EO, Jefferson LS (2001) Energy metabolism, nitrogen balance, and substrate utilization in critically ill children. Am J Clin Nutr 74:664–669. https://doi.org/10.1093/ajcn/74.5.664
Hellerman M, Sabatino A, Theilla M, Kagan I, Fiaccadori E, Singer P (2019) Carbohydrate and lipid prescription, administration, and oxidation in critically ill patients with acute kidney injury: a post hoc analysis. J Ren Nutr 29:289–294. https://doi.org/10.1053/j.jrn.2018.09.002
Nystrom EM, Nei AM (2018) Metabolic support of the patient on continuous renal replacement therapy. Nutr Clin Pract 33:754–766. https://doi.org/10.1002/ncp.10208
Wang XH, Mitch WE (2014) Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 10:504–516. https://doi.org/10.1038/nrneph.2014.112
Zappitelli M, Juarez M, Castillo L, Coss-Bu J, Goldstein SL (2009) Continuous renal replacement therapy amino acid, trace metal and folate clearance in critically ill children. Intensive Care Med 35:698–706. https://doi.org/10.1007/s00134-009-1420-9
Sgambat K, Clauss S, Moudgil A (2021) Effect of levocarnitine supplementation on myocardial strain in children with acute kidney injury receiving continuous kidney replacement therapy: a pilot study. Pediatr Nephrol 36:1607–1616. https://doi.org/10.1007/s00467-020-04862-3
Santiago MJ, Lopez-Herce J, Urbano J, Bellon JM, del Castillo J, Carrillo A (2009) Hypophosphatemia and phosphate supplementation during continuous renal replacement therapy in children. Kidney Int 75:312–316. https://doi.org/10.1038/ki.2008.570
Thompson Bastin ML, Stromberg AJ, Nerusu SN, Liu LJ, Mayer KP, Liu KD, Bagshaw SM, Wald R, Morris PE, Neyra JA (2022) Association of phosphate-containing versus phosphate-free solutions on ventilator days in patients requiring continuous kidney replacement therapy. Clin J Am Soc Nephrol 17:634–642. https://doi.org/10.2215/CJN.12410921
Pasko DA, Churchwell MD, Btaiche IF, Jain JC, Mueller BA (2009) Continuous venovenous hemodiafiltration trace element clearance in pediatric patients: a case series. Pediatr Nephrol 24:807–813. https://doi.org/10.1007/s00467-008-1083-8
Ostermann M, Summers J, Lei K, Card D, Harrington DJ, Sherwood R, Turner C, Dalton N, Peacock J, Bear DE (2020) Micronutrients in critically ill patients with severe acute kidney injury - a prospective study. Sci Rep 10:1505. https://doi.org/10.1038/s41598-020-58115-2
Ben-Hamouda N, Charriere M, Voirol P, Berger MM (2017) Massive copper and selenium losses cause life-threatening deficiencies during prolonged continuous renal replacement. Nutrition 34:71–75. https://doi.org/10.1016/j.nut.2016.09.012
Marino LV, Valla FV, Beattie RM, Verbruggen S (2020) Micronutrient status during paediatric critical illness: a scoping review. Clin Nutr 39:3571–3593. https://doi.org/10.1016/j.clnu.2020.04.015
Mehta NM, Bechard LJ, Zurakowski D, Duggan CP, Heyland DK (2015) Adequate enteral protein intake is inversely associated with 60-d mortality in critically ill children: a multicenter, prospective, cohort study. Am J Clin Nutr 102:199–206. https://doi.org/10.3945/ajcn.114.104893
Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, Debaveye Y, Vlasselaers D, Desmet L, Casaer MP, Garcia Guerra G, Hanot J, Joffe A, Tibboel D, Joosten K, Van den Berghe G (2016) Early versus late parenteral nutrition in critically ill children. N Engl J Med 374:1111–1122. https://doi.org/10.1056/NEJMoa1514762
Vanhorebeek I, Verbruggen S, Casaer MP, Gunst J, Wouters PJ, Hanot J, Guerra GG, Vlasselaers D, Joosten K, Van den Berghe G (2017) Effect of early supplemental parenteral nutrition in the paediatric ICU: a preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir Med 5:475–483. https://doi.org/10.1016/S2213-2600(17)30186-8
Wong Vega M, Juarez Calderon M, Tufan Pekkucuksen N, Srivaths P, Akcan Arikan A (2019) Feeding modality is a barrier to adequate protein provision in children receiving continuous renal replacement therapy (CRRT). Pediatr Nephrol 34:1147–1150. https://doi.org/10.1007/s00467-019-04211-z
Lopez-Herce J, Santiago MJ, Sanchez C, Mencia S, Carrillo A, Vigil D (2008) Risk factors for gastrointestinal complications in critically ill children with transpyloric enteral nutrition. Eur J Clin Nutr 62:395–400. https://doi.org/10.1038/sj.ejcn.1602710
Malagaris I, Herndon DN, Polychronopoulou E, Rontoyanni VG, Andersen CR, Suman OE, Porter C, Sidossis LS (2019) Determinants of skeletal muscle protein turnover following severe burn trauma in children. Clin Nutr 38:1348–1354. https://doi.org/10.1016/j.clnu.2018.05.027
Mehta NM, Corkins MR, Lyman B, Malone A, Goday PS, Carney LN, Monczka JL, Plogsted SW, Schwenk WF, American Society for Parenteral and Enteral Nutrition Board of Directors (2013) Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr 37:460–481. https://doi.org/10.1177/0148607113479972
Fiaccadori E, Cremaschi E (2009) Nutritional assessment and support in acute kidney injury. Curr Opin Crit Care 15:474–480. https://doi.org/10.1097/MCC.0b013e328332f6b2
Jonckheer J, Vergaelen K, Spapen H, Malbrain M, De Waele E (2019) Modification of nutrition therapy during continuous renal replacement therapy in critically ill pediatric patients: a narrative review and recommendations. Nutr Clin Pract 34:37–47. https://doi.org/10.1002/ncp.10231
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
MV: no conflicts of interest.
MS: medical advisory board of Nuwellis and supported by K12TR004415.
PD: consultant for BioPorto Inc.
PB: no conflicts of interest.
DS: no conflicts of interest.
AAA: Bioporto, Baxter, Medtronic, research grants paid to institution.
RB: consultant/advisory member: Bioporto, Biomerieux, Seastar Therapeutics, Potrero, Baxter Acute Therapies.
SG: Baxter/Gambro Renal Products; SeaStar Medical, Inc.; Medtronic, CHF Solutions, La Jolla Pharma, ExThera Inc.; BioPorto Diagnostics, Inc.; RAIDAR Health; Fresenius, Inc.; MediBeacon, Inc.; Otsuka, Inc.
JRC: co-owner of Sindri Technologies, LLC, consultant for Medtronics, investor in ZorroFlow (unrelated), supported by P50DK096373, R21DK134104, R56DK110622.
EB: Consults for Wolters-Kluwer (unrelated).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wong Vega, M., Starr, M.C., Brophy, P.D. et al. Advances in pediatric acute kidney injury pharmacology and nutrition: a report from the 26th Acute Disease Quality Initiative (ADQI) consensus conference. Pediatr Nephrol 39, 981–992 (2024). https://doi.org/10.1007/s00467-023-06178-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06178-4