Abstract
Pediatric acute kidney support therapy (paKST) programs aim to reliably provide safe, effective, and timely extracorporeal supportive care for acutely and critically ill pediatric patients with acute kidney injury (AKI), fluid and electrolyte derangements, and/or toxin accumulation with a goal of improving both hospital-based and lifelong outcomes. Little is known about optimal ways to configure paKST teams and programs, pediatric-specific aspects of delivering high-quality paKST, strategies for transitioning from acute continuous modes of paKST to facilitate rehabilitation, or providing effective short- and long-term follow-up. As part of the 26th Acute Disease Quality Initiative Conference, the first to focus on a pediatric population, we summarize here the current state of knowledge in paKST programs and technology, identify key knowledge gaps in the field, and propose a framework for current best practices and future research in paKST.
Similar content being viewed by others
Introduction
Pediatric acute kidney support therapy (paKST) has become an important part of the care of acutely and critically ill neonates, infants, and children. The goal of a paKST program is to reliably provide safe, effective, and timely extracorporeal supportive care that optimizes both hospital-based and lifelong outcomes for pediatric patients with acute kidney injury (AKI), inadequate clearance of toxins, and/or fluid and electrolyte derangements in the context of organ failures and critical illness. While there is a significant body of literature describing populations of pediatric patients who receive kidney support therapy (KST) and their outcomes [1,2,3,4,5], much less is known about optimal structures to configure paKST teams and programs, pediatric-specific aspects of delivering high-quality paKST, strategies for transitioning away from continuous modes to intermittent or chronic modes or to dialysis-independent rehabilitative states, or the best approach to providing intermediate- and long-term follow-up which will enable early detection of subacute and chronic sequelae after paKST that may affect health and well-being over the child’s lifespan. As a result, paKST varies greatly across pediatric hospitals both in the composition and functioning of paKST programs and in the technical aspects and processes of paKST delivery. In addition, utilizing paKST platforms for non-kidney-related diseases is an active area of investigation with the potential to improve outcomes in severe and complex disease processes refractory to standard interventions.
The Acute Disease Quality Initiative (ADQI, formerly known as Acute Dialysis Quality Initiative) methodology and the overall consensus recommendations of the 26th ADQI have been published previously [6, 7]. A detailed description of the ADQI methodology is also available at www.adqi.net. The 26th ADQI Conference convened international experts on pediatric AKI, including nephrologists, intensivists, pharmacists, dieticians, a patient representative, and an expert in pediatric social determinants of health. Each member of the subgroup on paKST engaged with pre-conference work developing a list of preliminary questions and objectives and performing a systematic literature review around these key questions. Key questions and recommendations were rigorously refined during the in-person 26th ADQI meeting using input from the entire group of conference participants as well as subgroup breakout sessions and were put into final form in post-conference subgroup meetings.
Here, we summarize the current state of knowledge regarding paKST program structure, function, and technology, identify key knowledge gaps in this field, and propose a framework for current best practices and future inquiry in paKST. Our group identified more than 50 unresolved questions in paKST. We distilled those questions to ask: How can paKST be used to improve care and outcomes in children? We categorized our questions into four key areas (Fig. 1): (1) the essential components of a paKST program; (2) the provision of timely, safe, and effective paKST; (3) the factors guiding paKST de-escalation and liberation; (4) the role of paKST for non-kidney indications. We expand on the first three of these concepts below. The fourth was included in the 26th ADQI consensus report [7], but its more detailed treatment is beyond the scope of this report and will be addressed at an appropriate length separately.
Building pediatric acute kidney support therapy programs
Pediatric acute kidney support therapy program requirements
Well-functioning programs are fundamental to the reliable and consistent delivery of high-quality, complex procedures such as paKST. Specific components of a paKST program may vary by location and health care setting, and each program will need to determine the model that best suits its particular setting, patient population, and available KSTs. The model depends on institutional resources available (i.e., dialysis, nephrology, and intensive care unit (ICU)/critical care teams) and may be nephrology led, ICU led, or a hybrid of those.
The paKST program should be led by physician and nurse directors who can engage stakeholders and develop a multi-disciplinary team to.
-
Create a culture of safety and transparency
-
Secure resources of protected time, personnel, and equipment
-
Obtain and maintain paKST equipment and supplies
-
Track patient- and process-specific quality improvement (QI) data that will enable teams to understand and improve their processes and outcomes.
A list of suggested metrics is presented in Table 1, recognizing that this is neither an exhaustive list of potentially useful domains nor likely to be immutable over time. Individual programs may find some of them useful and others less so, and programs may find different metrics useful at different times. The principle that assessment practices should be matched to local needs is vital to a thoughtful and effective QI approach.
The program’s medical and nursing directors should be well versed in bedside paKST care and have protected time dedicated to program development, education, and quality improvement. The paKST multidisciplinary leaders engage physicians, nurses, advance practice providers (including physician assistants and nurse practitioners), and the wider nephrology and critical care teams involved in the provision of paKST. Additional team members include pharmacists, nutritionists, social workers, surgeons and interventional radiologists, quality improvement/data specialists, and hospital administration personnel. The paKST team should meet regularly to provide updates; address potential safety events; budget for personnel and capital resources; develop education strategies; review and revise guidelines, policies, and procedures; and review center-specific quality data which will drive future projects and improvements.
Programs must choose where paKST care will be provided within the hospital, which may include dedicated hospital units or areas, the neonatal, pediatric, and cardiac intensive care units (ICU), and operating rooms. They must also choose what types of paKST they will provide—acute peritoneal dialysis (PD), acute intermittent hemodialysis (IHD), and/or acute continuous kidney support therapy modes—and whether they will provide paKST in tandem with other extracorporeal supportive therapies, including extracorporeal membrane oxygenation (ECMO), apheresis therapies, or extracorporeal liver support therapies. Updated and specific policies and procedures need to be in place for these services and must be easily accessible to all stakeholders.
In situations in which a hospital or team cannot provide safe and effective paKST, which may often occur with the smallest children and neonates, the program must develop communication channels, criteria, and processes to transfer care to centers (or units within their own center) that can provide such care [8].
An evidence-based approach to KST program-building is lacking. Key areas for investigation include development of resource- and needs-assessment tools, creation of communication aids within and among treatment teams, and establishment of educational approaches for on-boarding of new team members, maintenance of competency, and dissemination of new information or practice changes. Programs will need sufficient fluidity and responsiveness to adapt to changing circumstances and pressures, and as such will need an on-going QI framework to assess program performance and opportunities.
Quality improvement initiatives in acute KST in neonates and children
Acute KSTs are highly technical and complex purification procedures that can be performed using the peritoneal membrane or through access to the blood. Services, equipment, and quality of care differ across centers. Unnecessary practice variations stemming from differences in operational models, knowledge gaps, and/or failure to adopt and implement best practices (i.e., how KST is prescribed, monitored, and delivered) lead to suboptimal outcomes [9, 10]. High-quality acute KST demands assurance systems to assess whether practices and interventions are occurring as intended to ensure optimal care delivery and outcomes. To date, lack of agreed-upon benchmarks to evaluate the processes of paKST delivery has hindered the establishment of a comprehensive quality control system [11, 12]. While the preponderance of KST research examines the application of therapy (i.e., patient selection, timing, modality, dose, and anticoagulation), there are limited data exploring the organizational structures or processes by which care is being delivered, leading to a lack of consensus for acceptable performance standards [11, 13]. Appropriate selection and integration of QI efforts into clinical practice will facilitate improvements in reliability and standardization of care. Efforts at reducing practice variability and developing new standards in pediatrics are emerging but have not yet been broadly accepted or adopted [11, 13,14,15,16,17].
As there are currently no available consensus benchmarks for optimal paKST, development and implementation of a quality framework should take high priority in research and QI efforts. As highlighted by the pediatric commentary to the 22nd ADQI [14], areas of particular interest include, but are not limited to.
-
paKST program structures and formal training programs
-
Treatment delivery benchmarks for circuit priming practices, delivered dose, fluid management, circuit life, and anticoagulation
-
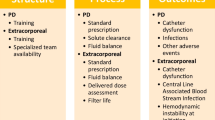
paKST-specific adverse events and outcomes, such as hemodynamic instability at circuit initiation, bleeding, transfusion requirements, catheter malfunction, and catheter-associated blood stream infections
QI guidelines for the neonatal and infant population may be of special importance in light of the specific challenges of paKST equipment and prescription in very small, physiologically immature critically ill patients. Assessments of hemodynamics at circuit initiation and long-term growth and development, especially in cases where paKST requirement is prolonged, are particularly important.
Validation of these QI standards can then be used to establish minimum acceptable performance criteria against which a paKST program can measure itself, driving further programmatic improvement [14].
Optimizing pediatric acute kidney support therapy delivery
Timing of initiation in paKST
The basic questions: Is earlier KST in AKI better for patient outcomes? How early is early enough?—remain largely unanswered for the pediatric population. The field of critical care nephrology has been shaped by the recognition that the development of kidney injury and, in particular, the need for KST is associated with profound morbidity and mortality risk in critically ill patients. The potential impacts of the metabolic and fluid balance derangements resulting from AKI are rational targets for therapeutic intervention, leading to questions regarding the potential benefits of early KST for preventing, reducing, or correcting said metabolic and fluid abnormalities.
Evidence for timing of initiation in pediatrics is fairly sparse based on retrospective and/or observational data, but consistently demonstrates poorer outcomes in children with more severe kidney injury and/or fluid accumulation at the time of KST initiation in the ICU, on ECMO, or after cardiac surgery [18,19,20]. Inferences from recently conducted meta-analyses and multicenter randomized controlled trials in adults [21,22,23,24,25,26,27,28] are limited due to developmental physiologic differences and case mix. Focusing on AKI staging to define early/late KST [21, 24, 26, 27] introduces bias due to limitations of creatinine-based assessments of kidney function.
These uncertainties call for a personalized approach in deciding the optimal timing of initiation of KST for AKI to determine whether individuals with specific disease processes (such as cardiac surgery, sepsis, and acute respiratory distress syndrome) or clinical conditions (e.g., based on illness severity scores or organ dysfunction scores) would benefit from early KST.
Clinical thresholds of fluid balance/fluid overload, kidney injury biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL) and TIMP-2*IGFBP-7, or a combination of clinical, functional, and/or biochemical markers (e.g., the renal angina index (RAI), the fluid overload kidney injury score (FOKIS), and the furosemide stress test (FST) [29,30,31,32]) may provide better tools for decisions on timing of initiation of KST and is currently an active area of research. Notably, in spite of decades of accumulated data demonstrating the correlation between fluid overload and poor outcomes, acknowledged in practice guidelines such as those for pediatric sepsis and ECMO management [33, 34], studies for which the primary indication for KST initiation is a threshold of fluid overload do not exist.
The challenges associated with developing a cohort of pediatric patients that is large enough to address any one of these questions in a randomized controlled trial are formidable, and novel approaches are needed. Moreover, clinically impactful outcomes besides mortality (e.g., duration of mechanical ventilation, ICU and hospital length of stay, early mobility, and global functional outcomes) are essential in future trials in order to drive widespread interest in and adoption of identified best practices.
Pediatric acute KST modality
Continuous KST modes are widely used in acute settings, but little is known about particular practice patterns within the field. A recent modified Delphi study queried paKST prescribers and found that continuous venovenous hemodiafiltration (CVVHDF) was the most commonly employed modality [35], but data on the associations of different modalities with patient outcomes are lacking. Whether continuous venovenous hemodialysis (CVVHD) or continuous venovenous hemofiltration (CVVH) are more beneficial than CVVHDF in certain disease states or patient populations or settings is unknown. Likewise, it is unknown whether there is a specific ratio of convective versus dialytic clearance that is most beneficial in acutely and critically ill pediatric patients or in specific clinical scenarios. Modes that combine some of the features of continuous KST and HD, such as prolonged intermittent kidney replacement therapy (PIKRT), may offer advantages in resource utilization and cost, coordination of testing and procedures, gradual liberation from KST, and/or patient rehabilitation efforts that have not yet been elucidated [36]. Similarly, well-established, less expensive modes such as PD may be preferable in some situations outside of post-cardiac surgery and resource-limited care, such as newly diagnosed kidney failure or other conditions in which a longer course of dialysis dependence is anticipated. The clinical and contextual factors that dictate choice of one modality over another are largely unknown and warrant further investigation.
Hemofilter selection in pediatric acute KST
In the treatment of AKI and fluid overload, the choice of hemofilter for continuous paKST has been guided by a few general principles: (1) the surface area of the filter should match as closely as possible to the patient’s body surface area; (2) the extracorporeal priming volume and blood flow rates for the filter set should be as minimal as possible; (3) the chosen filter should provide good clearance characteristics for small- and middle-sized particles; and (4) the membrane should be as biocompatible as possible, limiting the likelihood of bradykinin-release syndrome upon circuit initiation. The challenges in obtaining a filter that met all of these criteria, especially for our smallest and sickest patients, have led to a number of adaptations in clinical use, as well as on-going efforts to design and implement right-sized KST machines and filters specifically for that population. The current state of those efforts is represented in Table 2.
Outcomes in specific disease states, however, may be modifiable if the filter choices available offer the ability to match disease pathogenesis to clearance characteristics. This idea has been particularly tantalizing in patients with severe sepsis/septic shock and those with liver failure. Initial findings that the AN-69 filter may have the advantage of improved cytokine clearance in sepsis have been offset by the increased risk of bradykinin-release syndrome when this filter is used [37]. As a result, a number of filters have been designed and are currently being tested to achieve cytokine and/or endotoxin removal without inducing negative hemodynamic consequences; these include the oXiris membrane, the polymethylmethacrylate (PMMA) membrane, the CytoSorb cartridge, and the selective cytopheretic device. Similarly, charcoal and albumin-coated filters have been developed for liver failure patients in the hope of providing improved clearance of protein-bound solutes and toxins while awaiting clinical recovery or liver transplant. Tandem therapies combining paKST with apheresis potentially offer another pathway to improve outcomes with extracorporeal support in these patients. These issues will be the subject of an upcoming submission from our group, where they can be addressed in appropriate depth.
Pediatric acute KST dosing
Effluent volume remains the best method to assess dosing in paKST. The recommended dose of 2–3 L/1.73 m2/h of effluent was derived from expert opinion with little empiric basis when there was active debate in adult dosing between 25 and 45 ml/kg/h. Two large, randomized clinical trials demonstrated that higher doses are not superior to standard dosing [38], forming the basis for the current Kidney Disease Improving Global Outcomes (KDIGO) AKI guidelines establishing 20–25 ml/kg/h as the target delivered effluent dose in adults [39]. Similar studies are lacking in children. Analogous doses may lead to a higher intensity of treatment that compromises antimicrobial exposure and nutritional/mineral/vitamin adequacy. For example, in a 10-kg infant/child, an effluent rate of 2 L/1.73 m2/h corresponds to nearly 60 ml/kg/h, which is considered high-volume therapy in adults. High-volume therapy does not confer any clinical benefits in adults and may be associated with undesirable side effects such as more frequent or high-dose electrolyte replacement (e.g., phosphorus, calcium, potassium) and undetected but clinically important loss of micronutrients and lower antibiotic levels [40,41,42]. Effects of high-volume KST on other high-priority therapeutic agents, including steroids, immunosuppressive drugs, anticonvulsants, inotropes, sedatives, analgesics, and antivirals, are needed to inform both drug-dosing recommendations in KST and the risks associated with current paKST practices. The importance of addressing nutritional losses and their replacement in critically ill pediatric patients requiring KST cannot be overstated.
In addition, an understanding of KST dosing in tandem therapies such as ECMO and apheresis, and strategies to address medication doses during “down times”—both planned and inadvertent—impact the balance between prescribed and delivered dose. How this impacts safe and effective delivery of paKST is an area for ongoing investigation [43,44,45,46].
Circuit priming and initiation in paKST
paKST can be associated with significant clinical complications such as hypotension, hypothermia, bleeding, unplanned circuit loss, and thrombocytopenia [47]. Information on interventions that can be performed immediately before KST initiation to prevent undesirable events and avoid further compromise in an already fragile infant or child is sparse.
Many complications at KST initiation arise from the need for large extra-corporeal volumes (ECV) in relation to the patient’s total blood volume (TBV). Right-sized circuits with smaller filters and smaller ECV (Table 2) reduce the need for blood primes and limit hypotensive events around the time of initiation in small children [48,49,50,51]. Blood priming is generally recommended when the ECV is > 10–15% of the TBV in an effort to avoid acute hemodilution that may cause hemodynamic instability at circuit starts. No study has definitively established the appropriate threshold for blood prime in paKST. Blood primes increase the complexity of initiation and come with added risks, such as allosensitization and risks inherent to the use of stored blood which can cause hyperkalemia, hypocalcemia, and acidosis. The team must prepare for these issues proactively to prevent or minimize worsening hemodynamic instability. Various blood priming protocols exist to yield a more “physiologic” prime solution; safety, efficacy, and clinical consequences of these approaches need to be explored to determine which, if any, of them can be widely recommended and implemented [48, 49, 52,53,54,55]. In the meantime, the specific procedure for small children who may benefit from a blood prime initiation should be standardized for the individual program and not dictated by the rounding providers/team.
Vascular access for paKST
Well-functioning vascular access is a prerequisite for effective delivery of paKST, and temporary central venous catheters (CVCs) specifically designed for dialysis are the most utilized type of vascular access in children. Patient size and hemodynamic stability, underlying disease processes, expected type and duration of paKST, and center preferences and resources may all play a role in the choice and functional characteristics of vascular access. If paKST is likely to be needed for weeks, placement of a tunneled catheter may be preferable. In neonates requiring paKST, a tunneled double-lumen catheter that was cut to the desired length decreased complication rates compared to historic temporary catheters [56]. Two single-lumen catheters have been successfully used in small patients [57, 58].
Despite careful selection and placement, malfunctioning of vascular access—kinking/bending, leakage, thrombosis, infection, or high turbulence in inadequately sized catheters—contributes significantly to circuit failure necessitating a circuit/filter change [59]. Although pediatric recommendations for catheter size are available [60], optimal catheter sizing based on patient weight or height has not been established in pediatrics.
Catheter-related thrombosis/stenosis merits particular attention. Loss of vascular access—including permanent changes that preclude future use of that vein for permanent vascular or dialysis access—embolic events such as pulmonary embolism, and catheter-related bloodstream infections all have serious impact on the disease course and outcomes. Patients with liver failure, sepsis, and other pro-thrombotic conditions, as well as patients with longer duration of paKST treatment and particular catheter sizes and locations are at increased risk of thrombosis [61]. Evidence in non-dialysis ultrasound-guided CVC in adults suggests that a catheter-to-internal vessel size ratio > 45% increases thrombosis/stenosis risk [62]. Current KDIGO guidelines recommend ultrasound-guided catheter placement but do not specify an optimal catheter-to-vessel ratio [39]. Clinicians must carefully balance the benefits of good catheter flow against the risk of inducing thrombosis with an outsize catheter when choosing the size of vascular access for KST. Surface-modified catheters designed to lessen the risk of thrombosis [63] have not been widely adopted in clinical use. A recent pediatric study demonstrated a thrombosis rate of 7.4% with the preponderance in the smallest patients: 5 of the 6 patients with thrombosis were neonates [64]. We recommend future studies to establish guidelines for catheter sizing and length across the spectrum of pediatric patient sizes as well as for catheter-related thrombosis surveillance to allow for future development of more appropriate catheters for use in this high-risk population.
Circuit anticoagulation in paKST
Effective circuit anticoagulation remains one of the key determinants of successful administration of KST. The four most common continuous kidney replacement therapy anticoagulation strategies are systemic unfractionated heparin, low molecular weight heparin, prostacyclin, and regional citrate anticoagulation (RCA); nafamostat—a synthetic serine protease inhibitor—is emerging as a first-choice circuit anticoagulant in some centers, most notably in Asia [65]. Regional strategies that have minimal systemic effects have gained traction due to lower rates of bleeding events [66]. RCA has been incorporated into some KST machines in a semi-automated fashion with citrate infusion rates modulated with device software. When systemic anticoagulation is necessary, unfractionated heparin is most frequently used, but the lack of correlation between heparin dose and standard clinical monitoring tests such as activated partial thromboplastin time and activated clotting time poses a significant challenge for care teams. Alternative measures such as anti-Xa levels and thromboelastography have not demonstrated an advantage over traditional measures of heparin effect [67]. Elucidation of the best available anticoagulation and monitoring strategy or strategies, development of novel ones, fully automated integration of anticoagulation delivery into paKST machines, and creation of antithrombogenic membranes/circuit elements are all important areas for future study. Agents such as bivalirudin, argatroban, and nafamostat need to be systematically tested in children.
Fluid removal strategies in paKST
Treatment and/or prevention of clinically important fluid overload along with the ability to accommodate nutritional, medication, and blood product administration needs are frequent indications for paKST. Fluid balance needs may differ from patient to patient and from day to day or hour to hour in individual patients, necessitating a flexible and responsive approach to mechanical fluid removal. The assessment of appropriate rates of fluid removal in paKST patients is challenging, as the clinician must balance the risks and benefits of rapid fluid removal versus those of intravascular volume depletion. Incorporation of insensible fluid loss and the compartmentalization of excess volume between intravascular and extravascular spaces are important considerations in prescribing fluid removal rates.
Even in the presence of apparently stable hemodynamics, new regional cardiac stunning is common, often occurs within the first 4 h of therapy, and may be related to high ultrafiltration rates among adult critically ill KST patients [68]. The role of integrated ultrafiltration monitoring systems in acute KST is not clear and deserves further exploration; hematocrit sensors are considered standard in intermittent hemodialysis treatment [69, 70] but have not been studied in continuous KST. In critically ill patients, invasive hemodynamic monitoring and laboratory testing, such as venous oxygen saturation, may provide real-time guidance on fluid removal strategies in paKST and should be incorporated into future trials. Rigorous studies of the acceptable and safe range of ultrafiltration rates in paKST are needed to guide decision making; however, development of other decision support tools may be needed to account for the dynamic needs over the acute illness course for individual paKST patients.
Clear communication among paKST prescribers, critical care teams, and paKST operators is vital to treatment success. Shared approaches and language relating to evaluation of fluid balance, overarching clinical concerns and goals, daily and hourly fluid balance targets, and contingency planning should be developed by each paKST program. Clear communication with patients, families, and caregivers should also be emphasized within this framework. Daily (and sometimes more often) evaluations of the appropriate fluid removal rate are appropriate.
The role of peritoneal dialysis in paKST
As the use of KST for AKI has increased among hospitalized children over the last two decades, the use of peritoneal dialysis (PD) has declined steadily within resource-abundant regions [71]. PD continues to have an important role in remote and/or resource-limited settings as well as in neonates after cardiac surgery. A 2013 meta-analysis did not demonstrate differences in outcomes between AKI patients treated with PD compared to blood-based KST modes [72]. PD offers many appealing aspects for paKST programs:
-
Suitability in patients of nearly all ages, sizes, and disease states, including those with severe bleeding diatheses (in order to avoid the need for anticoagulation) or in whom obtaining vascular access is otherwise not feasible or safe
-
Low cost—PD can be provided at three to five times less cost compared to blood-based acute KST [73]
-
Ready transition from acute, inpatient therapy to chronic or rehabilitative therapy, including outpatient therapy
The International Society of Peritoneal Dialysis has developed detailed guidelines for the use of PD in both adult and pediatric AKI [74]. Existing barriers and challenges to PD use in various healthcare settings, optimal ways to dose acute PD for both clearance and fluid management—which may have significant differences from chronic PD dosing strategies—impact of PD on non-kidney disease, especially respiratory failure, and the roles of PD modalities such as tidal PD and continuous-flow PD are all rich areas for exploration in paKST care.
Post-KST care for pediatric patients
Pediatric acute KST de-escalation and liberation
The question of when to stop or de-escalate KST may be second only to the question of when to start it in perplexity and importance. Available data demonstrate that, while kidney recovery may be incomplete, most children requiring dialysis for AKI will recover sufficiently to be dialysis independent [75, 76]. However, there is limited evidence to guide KST discontinuation. Current practices vary significantly and utilize assessment of hemodynamic stability, fluid balance status, urine output trends over time, and clinician estimates of the patient’s ability to maintain euvolemia and metabolic balance to make decisions about timing of discontinuation or transition to intermittent KST. A recent meta-analysis in adults identified 16 variables for predicting successful KST discontinuation; these variables can be categorized into physiologic parameters, such as hemodynamics and urine output, biochemical markers to evaluate glomerular filtration rate/kidney function, and novel kidney markers [77]. No comparable studies are available in children and given that there is no evidence to suggest that important differences between adults and children exist in this area, we recommend that future adult studies consider inclusion of pediatric patients where possible.
The consequences of too early or too late separation from KST among children with AKI are not yet defined. Clinically relevant outcomes such as end-of-therapy fluid balance, bloodstream infection rates, duration of mechanical ventilation, early mobility participation, sedative exposure, and ICU and hospital lengths of stay should be tracked and incorporated in paKST outcomes studies. As we increasingly appreciate the importance of early mobility and rehabilitation in critically ill patients [78], we will need to delineate ways in which KST needs and goals can harmonize with those initiatives. This may include earlier transition to intermittent modes of KST (3–4 h of HD or chronic PD) or designated prolonged (6–18 h) KST-free periods to facilitate rehabilitative therapies in appropriate patient populations. Markers that signal a high likelihood of success for discontinuation of KST—such as specific urine output thresholds (with or without diuretic challenge) or biochemical indicators—should be sought and implemented. Finally, for those patients who cannot readily liberate from KST, appropriate fluid and metabolic management strategies while kidney function remains impaired as well as strategies for transition to intermittent or chronic KST care—including both medical and administrative aspects of care—should be detailed.
Long-term monitoring and follow-up for paKST patients
Children who survive an episode of AKI requiring dialysis are at risk for adverse long-term outcomes such as chronic kidney disease, hypertension, and neurodevelopmental impairments, but only a minority receive pediatric nephrologist follow-up [75, 79,80,81]. It is unclear whether a subset of paKST patients at higher risk of these outcomes can be identified. Once known, the cadence with which these patients should receive follow-up evaluation and what tests those evaluations should contain should be delineated. Large longitudinal cohorts will be needed to answer these questions, allowing for development of clinical risk stratification tools to guide referrals and anticipatory guidance at the time of discharge from the index paKST event.
Pediatric KST in resource-limited settings
The delivery of paKST in resource-limited and low-income settings has been mentioned in this report but deserves particular emphasis here. Innovative structures and processes will be required to deliver high-quality paKST when personnel, equipment, and physical space are limited and the processes of repair and resupply are slow and/or uncertain. Research efforts should focus on developing needs-assessment tools that can be flexibly applied to various circumstances, educational programs that promote effective troubleshooting and technical expertise along with quality assurance skills, identifying and using off-site and/or technology-based information resources, and paKST delivery that can be used in a broad swath of clinical scenarios while allowing both equipment and personnel to be deployed efficiently. As mentioned above, PD offers a number of advantages—wide applicability, safety, low cost, and transition from acute to chronic care—that may be particularly germane in resource-limited settings. In some settings, however, paKST teams may find that application of HD or PIKRT, which allow one piece of equipment to be used for more than one patient in the course of a day, better serves their needs. These teams will need data with which to guide the development of their particular programs and paKST delivery; they will also need funding for research to provide that data locally and/or regionally.
Conclusion
Optimizing patient outcomes through provision of high-quality paKST requires programs and processes that are built on a robust evidence base, are right-sized for pediatric patients and their unique treatment specifications, are responsive to changing needs within individual patients as well as treatment environments, and are mindful of the broader context of acute illness and long-term care. Important work has been done in many of these areas to date, but fundamental questions remain. We have outlined key areas of paKST program building, clinical care, and follow-up that are ripe for exploration in both resource-abundant and resource-limited settings. Finding answers to these outstanding questions will drive the field forward and move us closer to better patient outcomes.
References
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20. https://doi.org/10.1056/nejmoa1611391
Jetton JG, Boohaker LJ, Sethi SK et al (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194. https://doi.org/10.1016/S2352-4642(17)30069-X
Fitzgerald JC, Basu RK, Akcan-Arikan A et al (2016) Acute kidney injury in pediatric severe sepsis: an independent risk factor for death and new disability. Crit Care Med 44:2241–2250. https://doi.org/10.1097/CCM.0000000000002007
Alten JA, Cooper DS, Blinder JJ et al (2021) Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the Multicenter Neonatal and Pediatric Heart and Renal Outcomes Network. Crit Care Med 49:e941–e951. https://doi.org/10.1097/CCM.0000000000005165
Beken S, Akbulut BB, Albayrak E et al (2021) Evaluation of neonatal acute kidney injury after critical congenital heart disease surgery. Pediatr Nephrol 36:1923–1929. https://doi.org/10.1007/s00467-020-04890-z
Kellum JA (2002) The Acute Dialysis Quality Initiative: methodology. Adv Ren Replace Ther 9:245–247. https://doi.org/10.1053/jarr.2002.35564
Goldstein SL, Akcan-Arikan A, Alobaidi R, Askenazi DJ et al (2022) Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi consensus statement. JAMA Netw Open 5:e2229442. https://doi.org/10.1001/jamanetworkopen.2022.29442
Askenazi DJ, Basu RK (2021) Kidney support therapy in the pediatric patient: unique considerations for a unique population. Semin Dial 34:530–536. https://doi.org/10.1111/sdi.12978
Rewa O, Mottes T, Bagshaw SM (2015) Quality measures for acute kidney injury and continuous renal replacement therapy. Curr Opin Crit Care 21:490–499. https://doi.org/10.1097/MCC.0000000000000262
Shen B, Xu J, Wang Y, Jiang W, Teng J, Ding X (2018) Continuous renal replacement therapy quality control and performance measures. Contrib Nephrol 194:134–145. https://doi.org/10.1159/000485611
Rewa OG, Tolwani A, Mottes T et al (2019) Quality of care and safety measures of acute renal replacement therapy: workgroup statements from the 22nd Acute Disease Quality Initiative (ADQI) consensus conference. J Crit Care 54:52–57. https://doi.org/10.1016/j.jcrc.2019.07.003
Connor MJ, Karakala N (2017) Continuous renal replacement therapy: reviewing current best practice to provide high-quality extracorporeal therapy to critically ill patients. Adv Chronic Kidney Dis 24:213–218. https://doi.org/10.1053/j.ackd.2017.05.003
Mottes TA, Goldstein SL, Basu RK (2019) Process based quality improvement using a continuous renal replacement therapy dashboard. BMC Nephrol 20:17. https://doi.org/10.1186/s12882-018-1195-8
Selewski DT, Askenazi DJ, Kashani K et al (2021) Quality improvement goals for pediatric acute kidney injury: pediatric applications of the 22nd Acute Disease Quality Initiative (ADQI) conference. Pediatr Nephrol 36:733–746. https://doi.org/10.1007/s00467-020-04828-5
Rewa OG, Eurich DT, Noel Gibney RT, Bagshaw SM (2018) A modified Delphi process to identify, rank and prioritize quality indicators for continuous renal replacement therapy (CRRT) care in critically ill patients. J Crit Care 47:145–152. https://doi.org/10.1016/j.jcrc.2018.06.023
Rewa OG, Villeneuve PM, Lachance P et al (2017) Quality indicators of continuous renal replacement therapy (CRRT) care in critically ill patients: a systematic review. Intensive Care Med 43:750–763. https://doi.org/10.1007/s00134-016-4579-x
Ruiz EF, Ortiz-Soriano VM, Talbott M et al (2020) Development, implementation and outcomes of a quality assurance system for the provision of continuous renal replacement therapy in the intensive care unit. Sci Rep 10:20616. https://doi.org/10.1038/s41598-020-76785-w
Cortina G, McRae R, Hoq M et al (2019) Mortality of critically ill children requiring continuous renal replacement therapy: effect of fluid overload, underlying disease, and timing of initiation. Pediatr Crit Care Med 20:314–322. https://doi.org/10.1097/PCC.0000000000001806
Selewski DT, Blatt CTT, NB, et al (2012) Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med 40:2694–2699
Barhight MF, Soranno D, Faubel S, Gist KM (2018) Fluid management with peritoneal dialysis after pediatric cardiac surgery. World J Pediatr Congenit Heart Surg 9:696–704. https://doi.org/10.1177/2150135118800699
Gaudry S, Hajage D, Schortgen F et al (2016) Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 375:122–133. https://doi.org/10.1056/nejmoa1603017
Combes A, Bréchot N, Amour J et al (2015) Early high-volume hemofiltration versus standard care for post-cardiac surgery shock the HEROICS study. Am J Respir Crit Care Med 192:1179–1190. https://doi.org/10.1164/rccm.201503-0516OC
Cui J, Tang D, Chen Z, Liu G (2018) Impact of early versus late initiation of renal replacement therapy in patients with cardiac surgery-associated acute kidney injury: meta-analysis with trial sequential analysis of randomized controlled trials. Biomed Res Int 2018:6942829. https://doi.org/10.1155/2018/6942829
Zarbock A, Kellum JA, Schmidt C et al (2016) Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN Randomized Clinical Trial. JAMA 315:2190–2199. https://doi.org/10.1001/jama.2016.5828
Gaudry S, Hajage D, Benichou N et al (2020) Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet 395:1506–1515. https://doi.org/10.1016/S0140-6736(20)30531-6
Gaudry S, Hajage D, Martin-Lefevre L et al (2021) Comparison of two delayed strategies for renal replacement therapy initiation for severe acute kidney injury (AKIKI 2): a multicentre, open-label, randomised, controlled trial. Lancet 397:1293–1300. https://doi.org/10.1016/S0140-6736(21)00350-0
Bagshaw SM, Wald R, Adhikari NKJ, Bellomo R et al (2020) Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med 383:240–251. https://doi.org/10.1056/nejmoa2000741
Li X, Liu C, Mao Z, Li Q, Zhou F (2021) Timing of renal replacement therapy initiation for acute kidney injury in critically ill patients: a systematic review of randomized clinical trials with meta-analysis and trial sequential analysis. Crit Care 25:15. https://doi.org/10.1186/s13054-020-03451-y
Basu RK, Kaddourah A, Goldstein SL et al (2018) Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: a multicentre, multinational, prospective observational study. Lancet Child Adolesc Health 2:112. https://doi.org/10.1016/S2352-4642(17)30181-5
Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL (2014) Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol 9:654–662. https://doi.org/10.2215/CJN.09720913
Akcan-Arikan A, Gebhard DJ, Arnold MA, Loftis LL, Kennedy CE (2017) Fluid overload and kidney injury score: a multidimensional real-time assessment of renal disease burden in the critically ill patient. Pediatr Crit Care Med 18:524–530. https://doi.org/10.1097/PCC.0000000000001123
Kakajiwala A, Kim JY, Hughes JZ et al (2017) Lack of furosemide responsiveness predicts acute kidney injury in infants after cardiac surgery. Ann Thorac Surg 104:1388–1394. https://doi.org/10.1016/j.athoracsur.2017.03.015
Weiss SL, Peters MJ, Alhazzani W, Agus MSD et al (2020) Surviving Sepsis Campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med 21:e52–e106. https://doi.org/10.1097/PCC.0000000000002198
Bridges BC, Dhar A, Ramanathan K, Steflik HJ et al (2022) Extracorporeal Life Support Organization guidelines for fluid overload, acute kidney injury, and electrolyte management. ASAIO J 68:611–618. https://doi.org/10.1097/MAT.0000000000001702
Fuhrman DY, Gist KM, Akcan-Arikan A (2023) Current practices in pediatric continuous kidney replacement therapy: a systematic review-guided multinational modified Delphi consensus study. Pediatr Nephrol 38:2817–2826. https://doi.org/10.1007/s00467-022-05864-z
Sinha R, Sethi SK, Bunchman T et al (2018) Prolonged intermittent renal replacement therapy in children. Pediatr Nephrol 33:1283–1296. https://doi.org/10.1007/s00467-017-3732-2
Monard C, Rimmele T, Ronco C (2019) Extracorporeal blood purification therapies for sepsis. Blood Purif 47:2–15. https://doi.org/10.1159/000499520
Schiffl H (2020) Intensity of renal replacement therapy and outcomes in critically ill patients with acute kidney injury: critical appraisal of the dosing recommendations. Ther Apher Dial 24:620–627. https://doi.org/10.1111/1744-9987.13471
Kellum JA, Lameire N, Aspelin P et al (2012) Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. https://doi.org/10.1038/kisup.2012.1
Berger MM, Broman M, Forni L, Ostermann M et al (2021) Nutrients and micronutrients at risk during renal replacement therapy: a scoping review. Curr Opin Crit Care 27:367–377. https://doi.org/10.1097/MCC.0000000000000851
Hoff BM, Maker JH, Dager WE, Heintz BH (2020) Antibiotic dosing for critically ill adult patients receiving intermittent hemodialysis, prolonged intermittent renal replacement therapy, and continuous renal replacement therapy: an update. Ann Pharmacother 54:43–55. https://doi.org/10.1177/1060028019865873
Li L, Li X, Xia Y, Chu Y et al (2020) Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front Pharmacol 11:786. https://doi.org/10.3389/fphar.2020.00786
Venkataraman R, Kellum JA, Palevsky P (2002) Dosing patterns for continuous renal replacement therapy at a large academic medical center in the United States. J Crit Care 17:246–250. https://doi.org/10.1053/jcrc.2002.36757
Monti G, Herrera M, Kindgen-Milles D, Marinho A et al (2007) The DOse REsponse Multicentre International Collaborative Initiative (DO-RE-MI). Contrib Nephrol 156:434–443. https://doi.org/10.1159/000102137
Tolwani AJ, Campbell RC, Stofan BS, Lai KR et al (2008) Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol 19:1233–1238. https://doi.org/10.1681/ASN.2007111173
Vesconi S, Cruz DN, Fumagalli R, Kindgen-Milles D et al (2009) Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Crit Care 13:R57. https://doi.org/10.1186/cc7784
Nishimi S, Sugawara H, Onodera C, Toya Y et al (2019) Complications during continuous renal replacement therapy in critically ill neonates. Blood Purif 47:74–80. https://doi.org/10.1159/000496654
Askenazi D, Ingram D, White S, Cramer M et al (2016) Smaller circuits for smaller patients: improving renal support therapy with Aquadex(TM). Pediatr Nephrol 31:853–860. https://doi.org/10.1007/s00467-015-3259-3
Liu ID, Ng K-H, Lau PY-W, Yeo W-S et al (2013) Use of HF20 membrane in critically ill unstable low-body-weight infants on inotropic support. Pediatr Nephrol 28:819–822. https://doi.org/10.1007/s00467-012-2394-3
Coulthard MG, Crosier J, Griffiths C, Smith J et al (2014) Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 29:1873–1881
Vidal E, Cocchi E, Paglialonga F, Ricci Z et al (2019) Continuous veno-venous hemodialysis using the cardio-renal pediatric dialysis emergency machine (TM): first clinical experiences. Blood Purif 47:149–155. https://doi.org/10.1159/000494437
Hackbarth RM, Eding D, Smith CG, Koch A et al (2005) Zero balance ultrafiltration (Z-BUF) in blood-primed CRRT circuits achieves electrolyte and acid-base homeostasis prior to patient connection. Pediatr Nephrol 20:1328–1333. https://doi.org/10.1007/s00467-005-1970-1
Pasko DA, Mottes TA, Mueller BA (2003) Pre dialysis of blood prime in continuous hemodialysis normalizes pH and electrolytes. Pediatr Nephrol 18:1177–1183. https://doi.org/10.1007/s00467-003-1258-2
Fernandez S, Santiago MJ, Gonzalez R, Urbano J et al (2019) Hemodynamic impact of the connection to continuous renal replacement therapy in critically ill children. Pediatr Nephrol 34:163–168
Yorgin P, Ludlow M, Chua A, Alexander S (2006) A technique for rapid exchange of continuous renal replacement therapy. Pediatr Nephrol 21:743–746. https://doi.org/10.1007/s00467-006-0050-5
Onwubiko C, Askenazi D, Ingram D, Griffin R et al (2021) Small tunneled central venous catheters as an alternative to a standard hemodialysis catheter in neonatal patients. J Pediatr Surg 56:2219–2223. https://doi.org/10.1016/j.jpedsurg.2021.03.047
El Masri K, Jackson K, Borasino S, Law M et al (2013) Successful continuous renal replacement therapy using two single-lumen catheters in neonates and infants with cardiac disease. Pediatr Nephrol 28:2383–2387. https://doi.org/10.1007/s00467-013-2578-5
Sutherland SM, Davis AS, Powell D, Tanaka J et al (2022) Kidney replacement therapy in low birth weight preterm newborns. Pediatrics 150:e2022056570. https://doi.org/10.1542/peds.2022-056570
Juncos LA, Chandrashekar K, Karakala N, Baldwin I (2021) Vascular access, membranes and circuit for CRRT. Semin Dial 34:406–415. https://doi.org/10.1111/sdi.12977
Sutherland SM, Alexander SR (2012) Continuous renal replacement therapy in children. Pediatr Nephrol 27:2007–2016. https://doi.org/10.1007/s00467-011-2080-x
Wall C, Moore J, Thachil J (2016) Catheter-related thrombosis: a practical approach. J Intensive Care Soc 17:160–167. https://doi.org/10.1177/1751143715618683
Spencer TR, Mahoney KJ (2017) Reducing catheter-related thrombosis using a risk reduction tool centered on catheter to vessel ratio. J Thromb Thrombolysis 44:427–434. https://doi.org/10.1007/s11239-017-1569-y
Meier P, Meier R, Turini P, Friolet R, Blanc E (2011) Prolonged catheter survival in patients with acute kidney injury on continuous renal replacement therapy using a less thrombogenic micropatterned polymer modification. Nephrol Dial Transplant 26:628–635. https://doi.org/10.1093/ndt/gfq449
Szeps I, Ostlund A, Norberg A, Flaring U, Andersson A (2021) Thromboembolic complications of vascular catheters used for pediatric continuous renal replacement therapy: prevalence in a single-center, retrospective cohort. Pediatr Crit Care Med 22:743–752. https://doi.org/10.1097/PCC.0000000000002754
Choi J-Y, Kang Y-J, Jang HM, Jung H-Y et al (2015) Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk. Medicine 94:e2392. https://doi.org/10.1097/MD.0000000000002392
Gattas DJ, Rajbhandari D, Bradford C, Buhr H et al (2015) A randomized controlled trial of regional citrate versus regional heparin anticoagulation for continuous renal replacement therapy in critically ill adults. Crit Care Med 43:1622–1629. https://doi.org/10.1097/CCM.0000000000001004
Kuhle S, Eulmesekian P, Kavanagh B, Massicotte P et al (2007) Lack of correlation between heparin dose and standard clinical monitoring tests in treatment with unfractionated heparin in critically ill children. Haematologica 92:554–557. https://doi.org/10.3324/haematol.10696
Slessarev M, Salerno F, Ball IM, McIntyre CW (2019) Continuous renal replacement therapy is associated with acute cardiac stunning in critically ill patients. Hemodial Int 23:325–332. https://doi.org/10.1111/hdi.12760
Merouani A, Kechaou W, Litalien C, Ducruet T, Jouvet P (2011) Impact of blood volume monitoring on fluid removal during intermittent hemodialysis of critically ill children with acute kidney injury. Nephrol Dial Transplant 26:3315–3319. https://doi.org/10.1093/ndt/gfq855
Garro R, Sutherland S, Bayes L, Alexander S, Wong C (2015) CRIT-LINE: a noninvasive tool to monitor hemoglobin levels in pediatric hemodialysis patients. Pediatr Nephrol 30:991–998. https://doi.org/10.1007/s00467-014-2986-1
Chanchlani R, Nash DM, McArthur E et al (2019) Secular trends in incidence, modality, and mortality with dialysis receiving AKI in children in Ontario a population-based cohort study. Clin J Am Soc Nephrol 14:1288–1296. https://doi.org/10.2215/CJN.08250718
Chionh CY, Soni SS, Finkelstein FO, Ronco C, Cruz DN (2013) Use of peritoneal dialysis in AKI: a systematic review. Clin J Am Soc Nephrol 8:1649–1660. https://doi.org/10.2215/CJN.01540213
Reznik V, Randolph G, Collins C et al (1993) Cost analysis of dialysis modalities for pediatric acute renal failure. J Int Soc Perit Dial 13:311–313
Nourse P, Cullis B, Finkelstein F et al (2021) ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 update. Perit Dial Int 41:139–157. https://doi.org/10.1177/0896860820982120
Askenazi DJ, Feig D, Graham N, Hui-Stickle S, Goldstein SL (2006) 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69:184–189. https://doi.org/10.1038/sj.ki.5000032
Paden ML, Warshaw BL, Heard ML, Fortenberry JD (2011) Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med 12:153–158. https://doi.org/10.1097/PCC.0b013e3181e2a596
Katulka RJ, Al Saadon A, Sebastianski M, Featherstone R et al (2020) Determining the optimal time for liberation from renal replacement therapy in critically ill patients: a systematic review and meta-analysis (DOnE RRT). Crit Care 24:50. https://doi.org/10.1186/s13054-020-2751-8
Pun BT, Balas MC, Barnes-Daly MA, Thompson JL et al (2019) Caring for critically ill patients with the ABCDEF Bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med 47:3–14. https://doi.org/10.1097/CCM.0000000000003482
Robinson CH, Jeyakumar N, Luo B, Wald R et al (2021) Long-term kidney outcomes following dialysis-treated childhood acute kidney injury: a population-based cohort study. J Am Soc Nephrol 32:2005–2019. https://doi.org/10.1681/ASN.2020111665
Pande C, Noll L, Serrano F et al (2020) Association of acute kidney injury with neurodevelopmental outcomes in infants undergoing surgery for congenital heart disease. J Am Coll Cardiol 75:631. https://doi.org/10.1016/s0735-1097(20)31258-4
Hessey E, Morissette G, Lacroix J et al (2018) Healthcare utilization after acute kidney injury in the pediatric intensive care unit. Clin J Am Soc Nephrol 13:685–692. https://doi.org/10.2215/CJN.09350817
The ADQI 26 workgroup
The following individuals contributed to the formulation and content of this work in accordance with their participation in the 26th Acute Disease Quality Initiative (ADQI XXVI):
Rashid Alobaidi1, Sean M. Bagshaw1, Matthew Barhight2, Erin Barreto3, O.N. Ray Bignall II4, Erica Bjornstad5, Patrick Brophy6, Jennifer Charlton7, Andrea L. Conroy8, Prasad Devarajan9, Kristin Dolan10, Dana Fuhrman11, Katja M. Gist9, Stephen M. Gorga12, Jason H. Greenberg13, Denise Hasson9, Emma Heydari1, Arpana Iyengar14, Jennifer Jetton15, Catherine Krawczeski4, Leslie Meigs15, Shina Menon16, Catherine Morgan1, Theresa Mottes2, Zaccaria Ricci17, David T. Selewski18, Danielle Soranno8, Natalja Stanski9, Michelle Starr8, Scott M. Sutherland19, Jordan Symons16, Marcelo Tavares20, Molly Vega21, Michael Zappitelli22, Claudio Ronco23, Ravindra L. Mehta24, John Kellum25, Marlies Ostermann.26
1Alberta Health Sciences University, Edmonton, Alberta, Canada.
2Northwestern University Feinberg School of Medicine, Ann & Robert Lurie Children’s Hospital of Chicago, Chicago, IL, USA.
3Mayo Clinic, Rochester, MN, USA.
4Nationwide Children’s Hospital, Ohio State University, Columbus, OH, USA.
5Children’s Hospital Alabama, Birmingham, AL, USA.
6Golisano Children’s Hospital, Rochester University Medical Center, Rochester, NY, USA.
7University of Virginia, Charlottesville, VA, USA.
8Riley Children’s Hospital, Indiana University School of Medicine, Indianapolis, IN, USA.
9Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
10Mercy Children’s Hospital, University of Missouri-Kansas City School of Medicine, Kansas City, MO, USA.
11Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
12C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI, USA.
13Yale University Medical Center, New Haven, CT, USA.
14St. John’s National Academy of Health Sciences, Bangalore, India.
15Stead Family Children’s Hospital, University of Iowa, Iowa City, IA, USA.
16Seattle Children’s Hospital, University of Washington, Seattle, WA, USA.
17University of Florence, Florence, Italy.
18Medical University of South Carolina, Charleston, SC, USA.
19Lucille Packard Children’s Hospital, Stanford University, Palo Alto, CA, USA.
20Santa Casa dela Belo Horizonte, Belo Horizonte, Brazil.
21Texas Children’s Hospital, Baylor College of Medicine, Houston, TX, USA.
22Hospital for Sick Children, Toronto, Ontario, Canada.
23San Bartolo Hospital, Universiti di Padova, Vicenza, Italy.
24University of California San Diego School of Medicine, La Jolla, CA, USA.
25University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
26Guy’s and St. Thomas’ NHS Foundation Trust, London, UK.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no real or perceived conflicts of interest affecting study design, collection, analysis, and interpretation of data, writing of the report, or the decision to submit for publication. For full disclosure, we provide here an additional list of author commitments and funding sources that are not directly related to this study: D.J.A. is a consultant for Baxter, Nuwellis, Bioporto, and Seastar. His institution receives grant funding for education and research that is not related to this project from the National Institutes of Health, Baxter, Nuwellis, Medtronic, Bioporto, Portero, and Leadiant. He has patents pending on inventions to improve the kidney care of neonates. He is the Founder and Chief Scientific Officer for Zorro-Flow, Inc. J.M. is a consultant for Medtronic.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neumayr, T.M., Bayrakci, B., Chanchlani, R. et al. Programs and processes for advancing pediatric acute kidney support therapy in hospitalized and critically ill children: a report from the 26th Acute Disease Quality Initiative (ADQI) consensus conference. Pediatr Nephrol 39, 993–1004 (2024). https://doi.org/10.1007/s00467-023-06186-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06186-4