Abstract
Texture modification has become one of the most common forms of intervention for dysphagia, and is widely considered important for promoting safe and efficient swallowing. However, to date, there is no single convention with respect to the terminology used to describe levels of liquid thickening or food texture modification for clinical use. As a first step toward building a common taxonomy, a systematic review was undertaken to identify empirical evidence describing the impact of liquid consistency and food texture on swallowing behavior. A multi-engine search yielded 10,147 non-duplicate articles, which were screened for relevance. A team of ten international researchers collaborated to conduct full-text reviews for 488 of these articles, which met the study inclusion criteria. Of these, 36 articles were found to contain specific information comparing oral processing or swallowing behaviors for at least two liquid consistencies or food textures. Qualitative synthesis revealed two key trends with respect to the impact of thickening liquids on swallowing: thicker liquids reduce the risk of penetration–aspiration, but also increase the risk of post-swallow residue in the pharynx. The literature was insufficient to support the delineation of specific viscosity boundaries or other quantifiable material properties related to these clinical outcomes. With respect to food texture, the literature pointed to properties of hardness, cohesiveness, and slipperiness as being relevant both for physiological behaviors and bolus flow patterns. The literature suggests a need to classify food and fluid behavior in the context of the physiological processes involved in oral transport and flow initiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of texture-modified foods and thickened liquids has become a cornerstone of clinical practice to address dysphagia (swallowing impairment) [1, 2]. The principle behind this pervasive practice arises from the assumption that modifying the properties of normal foods and liquids will make them easier and safer to swallow. In the case of liquids, it is widely accepted that thin liquids (such as water) pose safety challenges for people with dysphagia because they flow quickly [3, 4]. The speed of bolus flow from the mouth into the pharynx may be sufficiently fast that it does not provide enough time for the person to engage airway closure before the bolus arrives at the entrance to the larynx and airway. Thickened liquids are frequently recommended with the goal of slowing down the flow of liquids to allow more time for airway closure [4, 5]. Conversely, very thick liquids and solid food materials may require greater strength in terms of the tongue propulsive forces that are used to drive material through the oropharynx. If a person has reduced tongue strength or reduced pharyngeal muscle strength, this is felt to constitute a risk for residues to remain behind in the recesses of the pharynx after a swallow [4, 6–8]. Similarly, solid foods that require chewing may prove challenging for people with dental issues or weakness in the masticatory muscles. Alteration of the properties of solid foods (by dicing, chopping, mincing or pureeing) is a common approach to making these materials easier for oral processing and swallowing.
The widespread use of texture modification as a clinical intervention has created a need to establish clear terminology to describe the target consistencies that are recommended for patients with dysphagia. In the absence of clear terminology and definitions to guide both the production/preparation and the clinical use of modified food textures and liquid consistencies, several countries have developed taxonomies or classification systems, disseminated in the form of clinical guidelines [9–14]. However, different countries have developed different systems of classification [15]. Recognition of the need to agree on terminology both within and across geographic jurisdictions has led to the establishment of the International Dysphagia Diet Standardisation Initiative (www.iddsi.org). The IDDSI task force has set a goal of developing global standardized terminology and definitions for texture-modified foods and thickened liquids for individuals with dysphagia of all ages, in all care settings, and all cultures.
The majority of existing guidelines for texture terminology have been developed based on input derived from expert opinion, focus groups, and interviews with clinicians [10, 12–14]. In addition to best practice and expert or consensus opinion, some guidelines have drawn on evidence from the literature to support their nomenclature [11]. However, it has been seven years since the last review of evidence from the literature [11]. In addition to consensus opinion, the IDDSI project has a goal to consider current empirical evidence when determining the number and characteristics of the terms that should be used in a recommended taxonomy of thickened liquids and texture-modified foods for clinical use. This article describes a systematic review of the literature that has been conducted to identify high quality scientific evidence regarding the influence of bolus consistency on swallowing function and/or physiology, either in healthy or impaired participants. For the purposes of this review, the term swallowing function is used to refer either to swallowing safety (i.e., swallowing without material being aspirated into the airway) and/or swallowing efficiency (i.e., swallowing material in a reasonable timeframe without leaving residual behind in the mouth or pharynx). The term swallowing physiology is used to refer to the biomechanical components of swallowing behavior, such as hyoid and laryngeal movement, tongue function or upper esophageal sphincter opening, which ultimately contribute to functional swallowing outcomes. With respect to labeling levels or categories of texture-modified liquids in this article, we will use the labels “thin”, “nectar-thick”, “honey-thick”, “pudding-thick/puree/paste”, “soft solids” and “hard solids” because these were the terms encountered most frequently in the research literature. It is acknowledged that terms like these are not culturally neutral or transparent, and are open to different interpretations. A previous publication by the IDDSI task force provides tables comparing terms across different guidelines and geographical jurisdictions [15]. For the purposes of this review, the term “nectar-thick” should be interpreted to refer to an initial degree of thickening (i.e., slightly thicker than thin or unthickened liquids), while the terms “honey-thick” and “pudding-thick” refer to progressively greater degrees of thickening, respectively.
The purpose of this review was to identify and review articles describing eating and swallowing in humans of any age, in which at least two different consistencies of food and/or liquid had been tested, and in which objective measures of swallowing function or physiology were reported for different bolus consistencies. The review also included articles describing the rheological or material characteristics of food or liquid stimuli after oral processing (i.e., at the point of swallowing). Articles describing the measures of interest either in healthy people, and/or in people with oropharyngeal dysphagia, without any restrictions related to diagnostic etiology were included. There were no restrictions imposed on the diagnostic or instrumental methods used, provided that some form of objective measurement was performed to capture the parameters of interest. Articles in all languages were accepted, based on the fact that the IDDSI working committee had the necessary expertise to provide or access translation for many non-English languages. Once identified, the intent was to evaluate the selected articles to determine evidence-informed answers to the following research questions:
-
1.
Is there evidence to support or refute a functional or behavioral change resulting from the thickening of liquids and/or texture modification of foods? If yes, how many and which levels of thickening or texture modification are supported by evidence, and what is the quality of evidence)?
-
2.
Does the literature provide trustworthy objective measures (e.g., viscosity, density, yield stress, texture analysis, or other physical measures) to guide the definition of different levels of thickened liquids and texture modified foods?
-
3.
Does the available evidence have application across the lifespan, or is it specific to particular subpopulations, defined either by age or diagnosis?
-
4.
What are the gaps in the literature regarding thickening of liquids and/or texture modification of food as a strategy to manage dysphagia?
Methods
A comprehensive literature search for literature published between 1985 and January, 2013 was conducted using multiple search engines, including Ovid MEDLINE(R), Ovid MEDLINE(R) In-process and other non-indexed citations, AMED (Allied and Complementary Medicine), EMBASE, Health and Psychosocial Instruments, and PsycINFO. The search was also conducted in Scopus using the following subject area limits: medicine, agricultural and biological sciences, pharmacology etc., chemistry, nursing, neuroscience, chemical engineering, engineering, health professions, psychology, materials science, multidisciplinary, dentistry. Search terms were broadly specified with the goal of finding as much relevant literature as possible, and included MeSH Headings of “Swallowing” or “Deglutition” or “Dysphagia”. Inclusion of one or more key-word (Scopus) or text terms (all other search engines) was also specified with the goal of focusing the results on the topic of interest. These terms were: “Visco*”; “Bolus”; “rheo*”; “dens*”; “yield*”; “fluid*”; “mechani*”; “elastic*”; “Newton*”; “carbohydrate”; “colloid*”; “starch”; “gum”; “alginate”; “cohes*”; “thick*”; “consisten*”; “nectar”; “honey”; “puree*”; “pudding”; “thin”; “spoon”; “liqui*”; “textur*”; “smooth*”; “mince*”; “soft”; “dice*”; “chop*”; “fibr*”; “fibe*”; “bread” or “solid*”. The asterisk used at the end of each search term stem allows for different word endings; for example, the stem “textur*” searches for the words “texture”, “textured” or “textural”. Terms were nominated by the authors based on their professional experience and following consultation with peers. The final set of search terms was intended to capture concepts and terms known to be used in the food oral processing and dysphagia research communities to describe food or liquid properties. It should be noted that terms related to choking, airway obstruction, or asphyxiation were not included in the search strategy for this review.
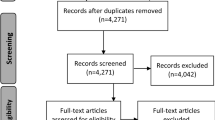
As a step in measuring construct validity, and to confirm that the search was succeeding in finding important articles from the dysphagia and food processing research literature, members of the IDDSI working committee generated a list of known articles that they expected should have been found in the course of the search. A cross-check of these nominated articles with the search results revealed gaps in the search results with respect to articles describing swallowing in children or arising from the food oral processing literature. Consequently, two additional searches were conducted using the same search engines. The first of these sought articles under the MeSH search term “eating and feeding disorders of childhood” while the second search specified the additional MeSH term of “food texture” in combination with the original search terms. Figure 1 summarizes the yield of this literature search strategy according to the criteria laid out in the 2009 PRISMA guideline for systematic reviews [16].
This set of 10,147 non-duplicate articles was subjected to an initial screening review to identify a sub-set of articles for full-text review. A team of three research assistants (LG, CL and HW) screened the titles and abstracts of the complete search yield of 10,147 non-duplicate articles to determine relevance, defined as an article describing a measurement of human swallowing using more than one consistency of food or liquid. This initial screening was conducted blindly in duplicate. Articles were included if they were identified as relevant by at least one reviewer. This led to a set of 488 articles selected for more detailed full-text review. These 488 articles were assigned to an international team of 10 raters who each reviewed between 40 and 70 articles for relevance and quality using a questionnaire administered using SurveyMonkey® (see Table 1). Training in completion of the relevance and quality ratings was provided via teleconference with subsequent email support from the lead author (CMS). The questions addressed during the full-text review are listed in Table 1 and led to a final subset of 36 articles selected for qualitative synthesis. The qualitative synthesis included extraction of trends from the data both within and across specific participant subgroups (e.g., healthy, stroke patients) and critical appraisal of the risk of bias at both the study level and the outcome level based on the food and liquid consistencies and the measurement instruments used in each study. Due to the wide variety of instrumental methods used to measure swallowing behaviors, and the wide variety of foods and liquids used in the selected studies, it was not possible to undertake a quantitative analysis of results across studies. As the final step in this systematic review, the interpretations arising from the qualitative synthesis were shared with members of the IDDSI international working committee for reaction and discussion.
Results
Participant Characteristics
Demographic information regarding the participants of the 36 studies selected for qualitative synthesis is shown in Table 2. Notably, only three of these studies described swallowing or oral processing in children; one of these was a study of swallowing in premature infants [17], while a second [18] explored differences in chewing behaviors in infants aged 6 months to 2 years of age. The third study involving children explored oral processing behaviors in two groups of typically developing girls aged 5 and 8 years old, as well as a control group of healthy adult women [19]. Of the 29 studies describing swallowing or oral processing in adults, 27 reported data for healthy adult participants [19–45], with two studies restricting their focus to denture wearers [46, 47]. A total of 10 studies reported data for adults with dysphagia [20–24, 48–52]. Four of these studies described swallowing in stroke patients in comparison to healthy controls [21–24] and a 5th paper described a group of patients with dysphagia secondary to Chagas’ disease, again with comparison to a group of healthy controls [20]. Two papers reported data for individuals with dysphagia related to head and neck cancer, in one case following surgical resection of the soft palate [48] and the second exploring post-radiation dysphagia in patients treated for nasopharyngeal carcinoma [49]. The remaining papers described swallowing in patients with Parkinson’s disease [50], in unspecified neurogenic dysphagia [51], or in unspecified dysphagia [52]. Sample sizes ranged from 3 [25] to 205 [24] participants.
Stimulus Characteristics
The various food and liquid stimuli used in the studies selected for inclusion in the qualitative synthesis are summarized in Tables 3 (radio-opaque liquid stimuli), 4 (non-opaque liquid stimuli), and 5 (solid stimuli). Of the 36 studies selected for detailed review, seven reported comparative data for swallows of thin liquid (either barium, water or juice) and an extremely thick liquid (i.e., pureed or spoon-thick consistency) [19, 26, 33, 35, 48, 51, 53]. A total of 13 articles described swallowing measures for a narrower contrast, i.e., thin liquid compared to either a mildly thick liquid (also known as nectar-thick) [17, 19, 23, 26, 28–30, 32, 35, 48, 51, 53] and/or a moderately thick liquid (also known as honey-thick) [28–30, 32, 33, 35, 53], with six of these articles including both mildly thick and moderately thick liquids [28–30, 32, 35, 53]. In terms of solid stimuli, which were explored in a total of 18 studies (Table 5) [18, 19, 21, 24, 27, 31, 39–48, 51, 52], there were effectively no stimuli that were the same in any two or more studies. Solid foods ranged from items that were described by authors as being softer (i.e., banana with barium paste [31]; cooked rice mixed with barium [52]; corned beef [45]; gummy bears [19]; konjac jelly [27], or gelatin cubes [18]) to items at the harder end of the continuum (e.g., fresh raw carrots [27]; biscuits or cookies [24, 31, 48, 51] or peanuts [31]). The description of certain items as “softer” in these studies illustrates the subjectivity with which texture descriptors may be applied. For example, “crisp, peeled apple” were described as being “softer” [46] in comparison to raw carrot [46], despite the fact that a crisp apple would not generally be regarded as a soft texture. Three studies, all originating from Asia, explored the combination of solid and liquid consistencies using either corned beef in a liquid barium [45], a thick rice gruel (the consistency of which was not further described) [21] or 12 g of cooked rice added to 100 ml of liquid barium [52].
Given the available data, it appears reasonable to synthesize observations regarding differences in swallowing physiology and function across the spectrum of liquid consistencies, from the thin to the extremely thick end of the continuum. However, caution is warranted with respect to delineating quantitative values to capture levels or categories of liquids along this continuum, based on incomplete reporting and the variety of methods and measures used to characterize liquid flow in the studies reviewed. This variety challenges the idea that the stimulus labels used in the literature (e.g., thin, nectar-thick, honey-thick) map to defined ranges of flow. For example, a wide variety of different studies reported using thin liquid barium, but where recipes were reported, these used different concentrations of barium and different dilutions with water or other thin liquids. Insufficient information was provided in the majority of these studies to support recipe replication, or to calculate the weight to volume concentrations of the resulting barium suspensions. Furthermore, given that commercial barium preparations frequently involve additional components to reduce foaming or aid suspension, including gums and starches, viscosity cannot be presumed without additional information.
Very few studies provided objective measures of stimulus characteristics such as viscosity, yield stress, or density (see Tables 3, 4). In several cases, the authors used metaphors to describe the apparent viscosities of stimuli, such as “with a viscosity similar to water”, but failed to provide adequate evidence to support these descriptions. Indeed, several of the metaphors used were scientifically implausible; for example, 120 % w/v E-Z-HD barium is described as being similar in viscosity to water in one study [51], although barium solutions typically have non-Newtonian flow characteristics and viscosities well above those of water [54, 55]. The fact that both starch- and xanthan-gum thickeners are acknowledged to produce liquids with non-Newtonian flow [54, 56–62] presents a challenge when comparing the stimuli used across these studies; the measured value of viscosity (i.e., “apparent viscosity”) is very sensitive to the shear rate at which the measurement is taken. In cases where viscosity measures were reported, the literature lacked any apparent convention with respect to reporting values at specific shear rates. From the data reported, it can be noted that the non-opaque stimuli labeled as “thin” had viscosities ranging up to 12 mPa s @ 45/s [28], while the radio-opaque “thin” liquid stimuli spanned a larger viscosity range, reaching reported values as high as 351 mPa s at 25/s [29]. Non-opaque liquids described as mildly thick or nectar-thick had viscosities as high as 466 mPa s at 25/s [29] or 325 mPa s at 45/s [28], while the radio-opaque liquids in this category had viscosities up to 863 mPa s at 25/s [29]. Similarly, the stimuli labeled as moderately thick or honey-thick had viscosities reaching 1,541 mPa s at 25/s [29] for radio-opaque liquids or 785 mPa s at 45/s [28] for non-opaque stimuli. It is interesting that even among manuscripts arising from the same lab [28, 29] there is no clear convention regarding the shear rates at which viscosities are reported. Shear rate is the term used to describe the rate of deformation of non-Newtonian stimuli as the fluid layers slide over each other when the bolus is placed under stress or force. During swallowing, shear rate for a bolus may be altered by the speed of biomechanical events including tongue movement and pharyngeal shortening or constriction. Perceptual experiments suggest that a range of shear rates is likely to be operational in the mouth during oral processing and swallowing [59, 63, 64]. However, there is no clear guidance from the literature regarding the shear rates that should be used as references when reporting the apparent viscosities of food and fluid stimuli that are being studied. Such variation in reporting makes for confusion and limits generalizability across studies.
Risk of Bias
The evaluation for risk of bias was performed according to the guidelines suggested by the Cochrane Bias Methods Group [65]. Specifically, the methods of each study were reviewed to determine whether there was potential bias in terms of participant selection, the performance of the particular study tasks by the participants, the detection or measurement of behaviors of interest, attrition or missing data, and reporting of results. As shown in Table 6, for the 36 studies reviewed, there were identified risks with respect to bias for every single study. By far, the most common risk of bias lay in the failure to report whether or not raters were blind to bolus consistency during analysis. In some cases, blinding to participant identity was reported, but given the nature of our interest in determining whether there are objective differences in swallowing or oral processing behaviors across boluses with different textures, blinding to stimulus consistency is an important consideration. It may well be that in some cases, such as videofluoroscopy, blinding to bolus consistency is less practical or feasible; however, the literature reviewed lacked acknowledgment of this issue entirely. This may reflect the fact that the primary question in many of these studies was something other than measuring differences in swallowing as a function of bolus consistency; nevertheless, in future studies where this is the purpose, blinding to bolus type would be desirable to limit bias during data analysis. Similarly, in the majority of cases, the reported data appeared to arise from analysis by a single rater with no reporting of inter- or intra-rater reliability. In some cases, measures appeared to be taken online and involved some degree of subjectivity, such that measurement validity and reliability are concerns for many of the studies reviewed. Finally, a subtle but important risk of bias must be mentioned regarding this literature to the extent that investigators selected particular stimuli to study and the reasons guiding these choices were not always reported. As described in the previous section, the stimuli covered by this literature represent a wide variety of discrete points along any sort of viscosity or material characteristic continuum. As such, caution is warranted in drawing conclusions that may be generalized to other ranges on these continua.
Observed Trends and Levels of Evidence
Notwithstanding the caveats mentioned in the previous three sections, the identified studies do provide sufficient preliminary information to support a trend analysis regarding differences in swallowing physiology and function related to differences in stimulus consistency. Table 7 summarizes the main findings from each of the 36 reviewed studies, which are grouped according to the type of instrumentation used to measure swallowing or oral processing behavior. Videofluoroscopy and surface electromyography were used in 12 and 10 studies, respectively, thereby accounting for the bulk of the observed trends, but in total, 12 different types of instrumentation were used.
The level of evidence for each main finding is shown in the far right column of Table 6, according to the scheme used by the National Health and Medical Research Council of Australia [66]. It can be noted that the selected studies fall into one of two types with respect to level of evidence. In total, 28 studies [17, 25–33, 36–53] were classified as reporting level IV evidence, that is, evidence arising from case series, post-test or pre-test and post-test studies without any comparison to controls. The remaining 8 studies [18–24, 35] were classified as level III-2 studies, reporting evidence from non-randomized cohort, case–control or interrupted time-series studies involving comparison to a control group.
Comparing results across studies, it is possible to identify patterns associated with thickened liquids or food texture modification. With respect to liquids, thicker liquids were reported to increase the duration of swallowing events compared to thin liquids in accelerometry [32], electromagnetic articulography [29], ultrasound [53] and surface electromyography signals [19, 26, 30], and also on videofluoroscopy for pharyngeal transit time measures [17, 24]. In patients with stroke-related dysphagia, longer upper esophageal sphincter opening durations [22] were also reported for paste consistency stimuli than with thin liquids, while longer oral transit times were observed with the paste consistency in patients with Parkinson’s disease [50] and those who had received radiation therapy for nasopharyngeal carcinoma [49]. Electromyographic measures of oral processing duration were longer for agar gels compared to water data [43]. Findings regarding the influence of liquid consistency on pharyngeal delay times in stroke patients were equivocal, with one study reporting longer delays with a pudding-thick consistency [24] and a second study reporting the opposite trend [22]. Two further reports found results that conflicted with the generally observed trend of longer duration events being seen with increasing viscosity. One study reported that the sounds associated with swallowing water were longer than those seen with either yogurt or konjac jelly [27]. Hyoid movement durations were also described to be shorter with paste consistency compared to thin barium following radiation treatment for nasopharyngeal carcinoma [49]. Two studies describe measures that did not change as a function of liquid consistency: swallow apnea duration was reported to remain unaffected by bolus consistency in healthy adults [33] while measures of swallow response time (also known as stage transition duration) and laryngeal vestibule closure duration did not differ for a thin to nectar-thick liquid barium comparison in stroke patients [23].
In addition to observations regarding physiological timing measures, other reported measures support the impression that thicker and harder items require greater effort in oral processing and swallowing. Measures that contribute to this observation include more prominent and well-defined accelerometry signal peaks [32], greater variability in tongue movement patterns [28, 29], higher surface electromyography amplitudes [19, 36, 41, 47], higher velocities of jaw movement [39], greater variability in surface electromyography patterns [26], and increased amplitudes of tongue-palate pressure [35]. Several studies concur that boluses with increased hardness elicit timing differences in chewing, involving faster rates, longer cycle durations, and a greater number of cycles [18, 41–44, 46, 47]. Findings were mixed with respect to the influence of bolus consistency on the magnitude of hyoid and laryngeal movements. One large study reported larger hyoid and laryngeal excursion for paste consistency and bread boluses compared to thin liquids [24], while a smaller study in healthy adults failed to find differences across different solid boluses [31].
With respect to functional swallowing measures, an important question is to determine whether penetration–aspiration of material into the airway is effectively reduced by altering bolus consistency? Several of the videofluoroscopic studies concur on this question, as illustrated in Fig. 2a, b. Bingjie and colleagues reported that the frequency of penetration–aspiration in stroke patients decreased as liquid viscosity increased [24]. This trend was also seen in the studies by Chen et al. [51], Barata et al. [48], Troche et al. [50] and by Lee et al. [52], who further described that aspiration was worst for thin liquids, better with a mixed consistency involving rice in liquid barium, and best for rice served without combining it with liquid. However, a cautionary note is also warranted on the basis of the selected studies, in that greater vallecular residue was observed with paste consistency barium than with thin liquid barium [48, 49] and with a plain rice bolus compared to a rice and barium mixed consistency [52]. Troche and colleagues [50] also observed that patients with Parkinson’s disease used a greater number of tongue pumps to successfully swallow a pudding-thick consistency, than for a thin liquid bolus, suggesting that clearance was worse with the thicker consistency. A recent report by Hind and colleagues [67], also reports a trend toward greater pharyngeal residues for barium stimuli with increasing viscosity.
a Prevalence of penetration–aspiration by liquid bolus consistency, as reported in a study of stroke patients by Bingjie et al. [24]. Penetration–aspiration scale scores of 1 and 2 are considered normal; scores of 3–5 indicate penetration of the laryngeal vestibule, while scores of 6–8 indicate aspiration of material below the true vocal folds. b Differences in the severity of penetration–aspiration as a function of liquid bolus consistency, as reported in a study of patients with Parkinson’s disease by Troche et al. [50]
An interesting study exploring swallowing with liquid barium, mixed consistency and a solid food (corned beef) demonstrated that for mixed consistencies and the solid food, the location of the bolus at swallow onset was lower in the hypopharynx than with liquids [45]. However, in a clever twist in their experimental design, these authors also asked participants to engage in chewing with the liquid barium stimulus and showed that this led to accumulation of the liquid bolus in the vallecular space, as seen with the mixed and solid consistencies.
With respect to solid foods, the literature search identified several studies in which rheological or texture profile analysis methods were used to measure the characteristics of the food bolus were at the end of oral processing, when the bolus was considered to be ready for swallowing [21, 40, 42–44]. These articles suggest that the property of cohesiveness remains stable during chewing and oral processing while other mechanical properties change [40] and are influenced by dry matter content [42], the degree to which salivary enzymes are absorbed by the bolus and contribute to starch hydrolysis [42], and the composition of the bolus with respect to the use of single gelling agents or complex gel combinations [43, 44]. In the food oral processing literature, the construct of cohesiveness is defined as a mechanical textural attribute relating to the degree to which a substance can be deformed before it breaks. The standard method for measuring cohesiveness during sensory panel testing involves placing a sample between the molar teeth, compressing the sample, and evaluating the degree of deformation before rupture [68–70]. Adjectives that are listed as descriptors of cohesiveness include: fracturable, crumbly, crunchy, brittle, crispy, crusty, chewy, tender, tough, short, mealy, pasty, and gummy. The texture reference scale developed by Munoz [71] is recommended in ISO guidelines for sensory ratings of cohesiveness, but it is acknowledged that no suitable set of reference products has been developed for this attribute.
Discussion
Evidence Supporting or Refuting Thickening of Liquids
Collectively, the selected studies clearly show a reduction in the risk of penetration–aspiration with liquids, as they progress from the thin to the very thick end of the viscosity continuum. This finding is limited, by definition, to studies in which objective measures of penetration–aspiration were available, which for the purposes of the present review meant studies involving barium swallowed under videofluoroscopy. Evidence regarding penetration–aspiration was also limited to studies involving adult participants with dysphagia.
However, an important cautionary note arises from this review given the convergence of evidence across several studies, showing a heightened risk of post-swallow residue in the pharynx for liquids with higher viscosities. This points to an important clinical challenge in terms of identifying suitable and safe consistencies for patients with dysphagia; namely, that of identifying liquids that are thick enough to be swallowed safely (without penetration–aspiration) while avoiding the pitfall of post-swallow residue.
As a post-script on this particular question, an additional source of data was brought to the attention of the authors after completion of the qualitative synthesis of the selected articles. This as-yet unpublished doctoral dissertation [72] involved rigorous videofluoroscopic exploration of swallowing with thin and nectar-thick Varibar™ barium by infants aged 3 weeks to 3 months, referred for evaluation of swallowing secondary to respiratory compromise. This dissertation is particularly noteworthy given the relative dearth of information regarding pediatric feeding and swallowing uncovered in our search process [17–19]. The Gosa study [72] reports several findings that concur with the observations gleaned from our qualitative synthesis, including prolongations of oral transit time and a reduction in penetration–aspiration with the nectar-thick stimulus compared to the thin liquid barium. Additionally, residue was reported to be present for 80 % of the nectar-thick swallows compared to only 44 % of thin liquid swallows. An additional gap to highlight with respect to the lack of identified studies in either the healthy or dysphagic infant population is the challenge of matching assessment stimuli to the rheological properties of breast milk or infant formula. This is a question of emerging interest in the dysphagia literature [9, 73] and definitely an area where additional research is needed.
Evidence Regarding the Number and Definitions of Levels of Liquid Thickening
Although this systematic review finds a convergence of evidence showing that thicker liquids are less likely to be aspirated, and more likely to cause post-swallow residue, the available data are insufficient to suggest particular viscosity values or other quantitative measures of material properties along the continuum from thin to extremely thick liquids that represent boundaries of clinical importance [74]. Historically, clinical guidelines regarding the use of thickened liquids have proposed quantitative boundaries that arise either from clinical consensus, or represent an educated guess. For example, the National Dysphagia Diet in the United States proposes 4 levels of liquid viscosity, labeled “thin”, “nectar-thick”, “honey-thick” and “spoon-thick” and corresponding to apparent viscosity ranges of 1–50, 51–350, 351–1,750, and ≥1,751 mPa s, measured at a shear rate of 50/s [10]. The Japanese guideline provides a larger number of categories with viscosity ranges of 1–50, 51–150, 151–300, 301–500, and >500 mPa s, again measured at a shear rate of 50/s [14, 15]. In the current review, we found no specific evidence to support or refute the specific numeric categorical viscosity boundaries suggested in these or other guidelines. We found no evidence to suggest that there are transitions of clinical relevance occurring at the boundaries between categories in these guidelines. Furthermore, the available evidence to date does not help us to ascertain how large a viscosity difference needs to be, in order to have a beneficial and measurable effect with respect to reducing penetration–aspiration, nor at what point the risk of residue accumulation becomes a real concern. Similarly, the available evidence does not provide clear evidence to suggest how many incremental levels of increasing viscosity might be meaningful in the clinical context. The available data also lack evidence regarding the important possibility that properties of a liquid bolus other than apparent viscosity, such as density, yield stress, cohesiveness, or slipperiness (to list a few) might influence swallowing physiology and function. On this latter point, we are aware of a recent publication describing differences in the rates of occurrence of penetration–aspiration for liquids, depending on the type of thickener used (starch vs xanthan-gum), albeit thickened to different degrees [75]. A recent conference abstract also reports differences in residue accumulation for liquids thickened with corn-starch versus xanthan-gum thickeners, and attributes these differences to subjectively judged cohesive properties of the bolus [76]. Similarly, several articles reviewed in this study revealed that different thickening agents produced products that had different rheological or material property profiles, as suggested in prior studies [60], and were shown to require different degrees of oral processing and suggested to have different rates of flow [43, 44]. Thus, it is naïve and not appropriate to assume that liquids thickened to similar viscosities using different agents will behave similarly in the oropharynx. The possibility that properties other than viscosity may have clinical relevance is both intriguing and important, and poses a challenge to the scientific community to develop rigorous studies that characterize such properties according to validated methods, in order to explore such phenomena.
Given the recognition that particular numeric viscosity boundaries for levels of liquid thickening are neither empirically supported nor empirically refuted, we conclude that the most appropriate clinical course of action with respect to identifying the optimal consistency of liquids for a patient who aspirates thin liquids is to increase viscosity in relatively small increments until swallowing safety is demonstrated. How large these increments might need to be can perhaps be informed by evidence from the sensory literature, based on the assumption that changes in behavior result from perceived differences in bolus consistency. The literature shows that the scaling of oral viscosity perception does not grow in a linear manner, but rather in an exponential fashion [77, 78]. Human ability to discriminate viscosity is proportional to the viscosity of the sample itself, as described by Weber’s law [79]. Recently, Withers and colleagues [80] manipulated the cream/fat content, and viscosity of skimmed milk using 0.1 % w/v increments of a starch-based thickener to explored the thresholds of just-noticeable differences (JND) in perceived thickness by healthy adults. They found no age differences in viscosity discrimination for liquids with apparent viscosities between 45 and 135 mPa s at 44/s, and reported that the average JND was between 0.26 and 0.32 % w/v in terms of thickener concentration, which equated to approximately a 1.8-fold increase in apparent viscosity (i.e., from 45 to 83 mPa s). Another recently published study explored just-noticeable differences using 0.1 % w/v increments in the concentration of a xanthan-gum thickener for sweetened cordial stimuli between 190 and 380 mPa s at 50/s [56]. In this case, the JNDs were found to be narrower, namely 0.38-fold for thickener concentration, equating to a 0.67-fold increase in viscosity [56]. Differences in the nature of the liquids studied (i.e., dairy products vs cordials), the viscosity ranges probed (i.e., 45–135 vs. 190–390 mPa s) and the testing methodologies used (i.e., two-stimulus forced choice comparisons versus three-stimulus triangle test paradigms) may have contributed to the observed differences in the resolution of perceivably different viscosities across these two studies. The authors of the second study [56] extrapolated from their findings to suggest that an array of liquids with apparent viscosities of 5, 8, 13, 22, 36, 60, 100, 170, 280, 470, 790, 1320, and 2200 mPa s at 50/s might provide a starting point for evaluating the influence of perceivably different viscosities on swallowing. However, it should also be noted that all of the viscosity discrimination studies cited [56, 77, 78, 80] were conducted using healthy volunteers with intact sensory systems. Alterations to oral or pharyngeal sensation, such as may be seen in individuals with dysphagia secondary to stroke, may alter perceptual viscosity discrimination abilities compared to healthy individuals. It should also be noted that the perceptual thresholds for noticeable viscosity differences arising from both of these studies are smaller than the categorical boundaries suggested by current clinical guidelines. Certainly, the results of our qualitative synthesis point to a significant gap both in literature and knowledge regarding the impact of small increments of viscosity on swallowing, and illustrate the need for new studies, which explore both the physiological and functional consequences of thickening in both narrow and larger increments.
As a final comment in this section on thickened liquids, some attention is required on the issue of viscosity measurement and its dependence on shear rate, which is a quantified measure of the speed of flow. The addition of either starch or xanthan-gum thickeners to liquids will result in non-Newtonian characteristics [56–62], meaning that the apparent (measured) viscosity is strongly dependent on shear rate. This is also true of most, but not all contrast media used for swallowing evaluation [54, 55]. Thus, if a measure of viscosity is reported, the shear rate used in the measurement is critical to understanding the measurement and to enabling comparison across studies. Although it has been common to report apparent viscosity at a shear rate of 50 reciprocal seconds (i.e., 50/s) [10, 14, 56], none of the studies identified for inclusion in this systematic review followed this convention. Notably, available information regarding the viscosity of Varibar™ (a line of commercially available barium products for swallowing evaluation used frequently in the United States) is quoted at a shear rate of 30/s. The actual shear rates involved in oral processing and swallowing depend on the rate and degree of pressures applied as well as the material properties of the fluids, and as such, can vary widely. The oral preparatory phase probably involves low shear rates (particularly for thicker fluids) and the perception of thickness has been shown to be best-related to objective measurements of viscosity taken at 10/s [64]. The pharyngeal and esophageal stages of swallowing are thought to involve much more rapid flow, with computer simulations suggesting shear rates in the order of 400/s for water [81].
Until such time as new research is available to describe the shear rates that are actually operating during swallowing [82], both in healthy and impaired contexts, it is paramount that apparent viscosities of thickened liquids intended both for assessment and therapeutic clinical purposes be reported across a range of shear rates. As a starting point, we recommend that shear rates of 1, 10 [64], 30, 50, and 100 reciprocal seconds would provide a reasonable basis for comparison. We particularly encourage consideration of liquid flow behaviors at low shear rates due to the likelihood that motoric deficits in dysphagia may impact a person’s ability to generate the shear forces and physiological behaviors that are typical of healthy swallowing.
Evidence Supporting or Refuting Texture Modification of Foods
If the literature on thickened liquids is sparse, this is even more apparent when reviewing the literature regarding texture-modified foods and swallowing. As illustrated in Table 5, the identified literature discussed only a small number and variety of solid foods. With the exception of longer duration and higher amplitude masseter surface electromyography signals when ingesting solid foods with increasing thickness or hardness [19, 30, 36–38, 43, 44, 46], the findings of the identified studies do not clearly point to measurable differences in either oral processing or swallowing parameters across the particular solid foods tested. We did not, for example, find literature that specifically explored the particle size of solid foods after a specific timeframe of chewing by people with partial or missing dentition or with reduced chewing strength. Penman and Thomson suggest that particles of 1.5 cm2 constitute a choking hazard for people with dysphagia [83], but the studies that we found describing the characteristics of solid foods after oral processing focused more on textural profiling than on particle size. Although this information may exist in the dental or food oral processing literature, it was not found given the specified search strategy, and, as previously acknowledged, the term “chok*” was not included in our search. Data regarding solid food particle size after oral processing under both normal and abnormal dental conditions would be interesting to consider alongside autopsy results suggesting that individuals with partial or missing dentition are more prone to choking on food [84]. A recent report by the Japanese Food Safety Commission [85], concludes that food texture (surface smoothness, elasticity, hardness), size, and shape are all relevant with respect to choking risk. In their investigations, sticky rice cakes were found to be the leading cause of choking accidents, but jelly cups were also mentioned as a not infrequent cause of choking. The report highlights that the risk of choking on a particular food item needs to be understood both in terms of the textural properties of the bolus and of the physiological behaviors commonly used during ingestion of that item. Thus, the jelly cups, for which they describe a common behavior of tilting the head backwards to suck the jelly out of the cup, are not without risk.
The review revealed common use in the food oral processing literature of accepted terminology to describe the textural attributes of solid foods as laid out in ISO guidelines [68, 69]. The construct of cohesiveness, mentioned earlier, is one example of such terminology. One term, which was encountered in the food oral processing literature, but which remains poorly understood, is “ease of swallowing” [86]. This appears to be an attribute that is commonly captured in sensory profiling of food textures; however, whether and how this attribute maps to objective, quantifiable measures of bolus flow or physiology remains unclear.
An interesting question arising from this review is whether adhesive paste consistency stimuli such as cheese spread or peanut butter should be considered to be semi-solid foods or extremely thick liquids? These items can be compressed and spread across the palate with the tongue, and do not fracture; as such, in a physiological sense, they behave quite differently and involve different oral processing behaviors from foods that require mastication [87]. On the other hand, they do not flow either under gravity, or under the typical pressures applied by the tongue, and require handling by the tongue for transport through the oral cavity. In this respect, they are quite different from liquids. It is recommended that future investigations with respect to differences in oral processing and swallowing of solid foods and thickened liquids make a clear distinction based on the physiological processes that are required for transport (i.e., mastication, oral containment, tongue-sweeping, and propulsion or simple tongue compression), rather than using texture descriptors derived based on physical properties alone [88]. Furthermore, it may be important to note that a given stimulus may behave more like a liquid for one person and a semi-solid for another person, based on the person’s ability to generate forces or movement with their tongue. As such, physiological definitions of texture may have different boundaries for different consumer groups. Considerations of temperature inside the mouth and the slipperiness of the oral surfaces given differences in the levels of saliva across the duration of oral processing are undoubtedly also relevant to developing a texture classification system for the dysphagia population, which is founded on a physiological framework.
The paucity of studies captured in our search describing oral processing or swallowing of texture-modified foods comes as both a disappointment and a surprise. On reflection, we believe that the rules of our search strategy may have overly limited the search results, given mandatory inclusion of the MeSH terms “Swallowing” or “Deglutition” or “Dysphagia”, even when the supplementary search for articles was performed with the additional MeSH heading term of “food texture”. We are aware that there is an entire field of scholarship known as “food oral processing”, with its own journals and conferences. Although our search strategy employed search engines intended to tap the engineering and non-medical domains, it may well be that key words related to swallowing and dysphagia are not commonly used in publications within this subspecialty, leading our search to capture only a limited number of articles from this domain. Certainly, a direction for future research will be to explore this literature in greater detail for relevant evidence regarding differences in oral processing behaviors for foods with different textural characteristics.
From a clinical perspective, the lack of guidance regarding the classification, labeling, and preparation of texture-modified foods for people with dysphagia is a concern. It is not uncommon for coroner’s inquiries into fatal choking episodes in people at risk for dysphagia to conclude that food of an inappropriate consistency was ingested [84, 89–91]. On the basis of the current review, we are obliged to point out that the best available evidence regarding the selection of an optimal food consistency for a person with dysphagia comes from the careful exploration of tolerance for different foods in a comprehensive clinical swallowing assessment. This systematic review found a lack of research evidence providing support for the selection or avoidance of specific consistencies. Our review points to an urgent need to generate empirical evidence to describe different classes of chewable food, so that the corresponding expected differences in oral processing and swallowing behavior can be defined. Additionally, the development of valid methods for observing, describing, and measuring oral stage behaviors during assessment tasks that probe a variety of different solid foods would be a valuable addition to current subjective clinical methods. Collaboration with the research field food oral processing is strongly advised as a first step in developing such methods.
Conclusions
At the outset of this review, we identified several main questions for our investigations regarding the impact of liquid consistency and food texture on swallowing. Our first question was to determine whether evidence supports or refutes the practices of thickening liquids and modifying food textures in the context of the clinical management of dysphagia. We conclude that evidence shows a benefit associated with thickening liquids in terms of reducing penetration and aspiration, but that this benefit brings with it a risk of post-swallow residue in the pharynx with thicker consistencies. We were unable to find evidence to delineate particular boundaries in measured viscosity that may predict these clinical outcomes. We found very little evidence to guide practice with respect to different degrees of modifying solid foods for patients with dysphagia. The literature strongly suggests that there are several relevant properties of food texture for swallowing, including cohesiveness, hardness, and slipperiness.
With respect to objective measures that might be used to guide the classification of thickened liquids and texture-modified foods, our review identified an absence of convention, particularly in terms of the shear rates that are used for reporting apparent viscosity. Exceptionally limited information was available for objective measurement of texture-modified foods. Collaboration with experts in the sensory aspects of food oral processing emerges as an important direction for future research in this respect. The adoption of sensory terms and scaling methods that have become standard in the food oral processing world to capture the characteristics of foods used in dysphagia management would be a very worthwhile pursuit both for research and clinical food production.
This systematic review has identified some major gaps in our understanding of the impact of liquid consistency and food texture on swallowing physiology, both in healthy and disordered populations. Looking to the future, we conclude that classifications of these properties should take into consideration the physiological behaviors that are observed when ingesting different stimuli. Potential delineations with clinical utility include differentiating liquids into those that flow easily in the context of minimally applied tongue pressures in the mouth versus those that require more active tongue movement to initiate flow. The behavior of a bolus in the context of bolus containment, active tongue movement, or chewing (i.e., spreading vs flow vs fracture) may be another useful way of capturing clinically relevant properties of food texture for swallowing, and also appears to be relevant in terms of choking risk. These speculations raise the intriguing possibility that different boundaries of bolus texture and flow may be needed for different subpopulations within the larger clinical consumer group of people with dysphagia, depending on their physiological capabilities. Finally, this manuscript reminds us that the dysphagia field is still in relative infancy. Given the prevalent use of texture-modified foods and thickened liquids in the treatment of dysphagia, it is timely that gaps in these areas are identified and provide strong grounds for clinically relevant research to guide best practice.
References
Robbins J, Nicosia MA, Hind JA, Gill GD, Blanco R, Logemann JA: Defining physical properties of fluids for dysphagia evaluation and treatment.: Perspectives on Swallowing and Swallowing Disorders (Dysphagia) American Speech-Language Hearing Association Special Interest Division 13 Newsletter 2002, pp. 16–19.
Garcia JM, Chambers ET, Molander M. Thickened liquids: practice patterns of speech-language pathologists. Am J Speech Lang Pathol. 2005;14:4–13.
Logemann JA. Swallowing physiology and pathophysiology. Otolaryngol Clin N Am. 1988;21:613–23.
Clave P, de Kraa M, Arreola V, Girvent M, Farre R, Palomera E, Serra-Prat M. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24:1385–94.
Logemann JA. Noninvasive approaches to deglutitive aspiration. Dysphagia. 1993;8:331–3.
Steele CM, Huckabee ML. The influence of oro-lingual pressure on the timing of pharyngeal pressure events. Dysphagia. 2007;22:30–6.
Huckabee ML, Steele CM. An analysis of lingual contribution to submental sEMG measures and pharyngeal biomechanics during effortful swallow. Arch Phys Med Rehabil. 2006;87:1067–72.
Clave P, Rofes L, Carrion S, Ortega O, Cabre M, Serra-Prat M, Arreola V. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Series. 2012;72:57–66.
September C, Nicholson TM, Cichero JA. Implications of changing the amount of thickener in thickened infant formula for infants with dysphagia. Dysphagia. 2014;29:432–7.
National Dysphagia Diet Task Force, National Dysphagia Diet: Standardization for Optimal Care/American Dietetic Association. 2002.
Cichero JAY, Atherton M, Bellis-Smith N, Suter M. Texture-modified foods and thickened fluids as used for individuals with dysphagia: Australian standardised labels and definitions. Nutr Diet. 2007;64:S53–76.
United Kingdom National Patient Safety Agency, Dysphagia Diet Food Texture Descriptors. 2011.
Irish Association of Speech-Language Therapists and Irish Nutrition and Dietetic Institute, Irish consistency descriptors for modified fluids and food, 2009.
Fujitani J, Uyama R, Okoshi H, Kayashita J, Koshiro A, Takahashi K, Maeda H, Fujishima I, Ueda K. Japanese Society of Dysphagia Rehabilitation: classification of dysphagia modified food. Jpn J Dysphagia Rehabil. 2013;17:255–67.
Cichero JAY, Steele CM, Duivestein J, Clave P, Chen J, Kayashita J, Dantas R, Lecko C, Speyer R, Lam P. The need for international terminology and definitions for texture modified foods and thickened liquids used in dysphagia management: foundations of a global initiative. Curr Phys Med Rehabil Rep. 2013;1:280–91.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12.
Goldfield EC, Smith V, Buonomo C, Perez J, Larson K. Preterm infant swallowing of thin and nectar-thick liquids: changes in lingual-palatal coordination and relation to bolus transit. Dysphagia. 2013;28:234–44.
Gisel EG. Effect of food texture on the development of chewing of children between six months and two years of age. Dev Med Child Neurol. 1991;33:69–79.
Ruark JL, McCullough GH, Peters RL, Moore CA. Bolus consistency and swallowing in children and adults. Dysphagia. 2002;17:24–33.
Dos Santos CM, Cassiani RA, Dantas RO. Videofluoroscopic evaluation of swallowing in Chagas’ disease. Dysphagia. 2011;26:361–5.
Kim IS, Han TR. Influence of mastication and salivation on swallowing in stroke patients. Arch Phys Med Rehabil. 2005;86:1986–90.
Bisch EM, Logemann JA, Rademaker AW, Kahrilas PJ, Lazarus CL. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J Speech HearRes. 1994;37:1041–59.
Oommen ER, Kim Y, McCullough G. Stage transition and laryngeal closure in poststroke patients with dysphagia. Dysphagia. 2011;26:318–23.
Bingjie L, Tong Z, Xinting S, Jianmin X, Guijun J. Quantitative videofluoroscopic analysis of penetration–aspiration in post-stroke patients. Neurol India. 2010;58:42–7.
Linden P, Tippett D, Johnston J, Siebens A, French J. Bolus position at swallow onset in normal adults: preliminary observations. Dysphagia. 1989;4:146–50.
Reimers-Neils L, Logemann J, Larson C. Viscosity effects on EMG activity in normal swallow. Dysphagia. 1994;9:101–6.
Taniwaki M, Gao Z, Nishinari K, Kohyama K. Acoustic analysis of the swallowing sounds of food with different physical properties using the cervical auscultation method. J Texture Stud. 2013;44:169–75.
Steele CM, Van Lieshout PH. Influence of bolus consistency on lingual behaviors in sequential swallowing. Dysphagia. 2004;19:192–206.
Steele CM, van Lieshout PH. Does barium influence tongue behaviors during swallowing? Am J Speech Lang Pathol. 2005;14:27–39.
Igarashi A, Kawasaki M, Nomura S, Sakai Y, Ueno M, Ashida I, Miyaoka Y. Sensory and motor responses of normal young adults during swallowing of foods with different properties and volumes. Dysphagia. 2010;25:198–206.
Ishida R, Palmer JB, Hiiemae KM. Hyoid motion during swallowing: factors affecting forward and upward displacement. Dysphagia. 2002;17:262–72.
Lee J, Sejdic E, Steele CM, Chau T. Effects of liquid stimuli on dual-axis swallowing accelerometry signals in a healthy population. Biomed Eng Online. 2010;9:7.
Butler SG, Postma GN, Fischer E. Effects of viscosity, taste, and bolus volume on swallowing apnea duration of normal adults. Otolaryngol Head Neck Surg. 2004;131:860–3.
Chi-Fishman G, Sonies BC. Effects of systematic bolus viscosity and volume changes on hyoid movement kinematics. Dysphagia. 2002;17:278–87.
Youmans SR, Youmans GL, Stierwalt JA. Differences in tongue strength across age and gender: is there a diminished strength reserve? Dysphagia. 2009;24:57–65.
Inagaki D, Miyaoka Y, Ashida I, Yamada Y. Influence of food properties and body posture on durations of swallowing-related muscle activities. J Oral Rehabil. 2008;35:656–63.
Inagaki D, Miyaoka Y, Ashida I, Yamada Y. Activity pattern of swallowing-related muscles, food properties and body position in normal humans. J Oral Rehabil. 2009;36:703–9.
Inagaki D, Miyaoka Y, Ashida I, Yamada Y. Influence of food properties and body position on swallowing-related muscle activity amplitude. J Oral Rehabil. 2009;36:176–83.
Anderson K, Throckmorton GS, Buschang PH, Hayasaki H. The effects of bolus hardness on masticatory kinematics. J Oral Rehabil. 2002;29:689–96.
Yamaya M, Nishimura H, Hatachi Y, Yoshida M, Fujiwara H, Asada M, Nakayama K, Yasuda H, Deng X, Sasaki T, Kubo H, Nagatomi R. Procaterol inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Eur J Pharmacol. 2011;650:431–44.
Karkazis HC, Kossioni AE. Re-examination of the surface EMG activity of the masseter muscle in young adults during chewing of two test foods. J Oral Rehabil. 1997;24:216–23.
Hoebler C, Karinthi A, Devaux MF, Guillon F, Gallant DJG, Bouchet B, Melegari C, Barry JL. Physical and chemical transformations of cereal food during oral digestion in human subjects. Br J Nutr. 1998;80:429–36.
Funami T, Ishihara S, Nakauma M, Kohyama K, Nishinari K. Texture design for products using food hydrocolloids. Food Hydrocolloids. 2012;26:412–20.
Ashida I, Iwamori H, Kawakami SY, Miyaoka Y, Murayama A. Analysis of physiological parameters of masseter muscle activity during chewing of agars in healthy young males. J Texture Stud. 2007;38:87–99.
Saitoh E, Shibata S, Matsuo K, Baba M, Fujii W, Palmer JB. Chewing and food consistency: effects on bolus transport and swallow initiation. Dysphagia. 2007;22:100–7.
Karkazis HC. EMG activity of the masseter muscle in implant supported overdenture wearers during chewing of hard and soft food. J Oral Rehabil. 2002;29:986–91.
Karkazis HC, Kossioni AE. Surface EMG activity of the masseter muscle in denture wearers during chewing of hard and soft food. J Oral Rehabil. 1998;25:8–14.
Barata LF, De Carvalho GB, Carrara-De Angelis E, De Faria JCM, Kowalski LP. Swallowing, speech and quality of life in patients undergoing resection of soft palate. Eur Arch Oto-Rhino-Laryngol. 2013;270:305–12.
Lin P, Hsiao T, Chang Y, Ting L, Chen W, Chen S, Wang T. Effects of functional electrical stimulation on dysphagia caused by radiation therapy in patients with nasopharyngeal carcinoma. Support Care Cancer. 2011;19:91–9.
Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson’s disease. Dysphagia. 2008;23:26–32.
Chen MYM, Peele VN, Donati D, Ott DJ, Donofrio PD, Gelfand DW. Clinical and videofluoroscopic evaluation of swallowing in 41 patients with neurologic disease. Gastrointest Radiol. 1992;17:95–8.
Lee KL, Kim WH, Kim EJ, Lee JK. Is swallowing of all mixed consistencies dangerous for penetration–aspiration? Am J Phys Med Rehabil. 2012;91:187–92.
Chi-Fishman G, Sonies BC. Effects of systematic bolus viscosity and volume changes on hyoid movement kinematics. Dysphagia. 2002;17:278–87.
Popa Nita S, Murith M, Chisholm H, Engmann J. Matching the rheological properties of videofluoroscopic contrast agents and thickened liquid prescriptions. Dysphagia. 2013;28:245–52.
Steele CM, Molfenter SM, Peladeau-Pigeon M, Stokely S. Challenges in preparing contrast media for videofluoroscopy. Dysphagia. 2013;28(3):464–7.
Steele CM, James DF, Hori S, Polacco RC, Yee C. Oral perceptual discrimination of viscosity differences for non-Newtonian liquids in the nectar- and honey-thick ranges. Dysphagia. 2014;. doi:10.1007/s00455-014-9518-9.
Hanson B, Cox B, Kaliviotis E, Smith CH. Effects of saliva on starch-thickened drinks with acidic and neutral pH. Dysphagia. 2012;27:427–35.
O’Leary M, Hanson B, Smith C. Viscosity and non-Newtonian features of thickened fluids used for dysphagia therapy. J Food Sci. 2010;75:E330–8.
O’Leary M, Hanson B, Smith CH. Variation of the apparent viscosity of thickened drinks. Int J Lang Commun Disord. 2011;46:17–29.
Garcia JM, Chambers ET, Matta Z, Clark M. Viscosity measurements of nectar- and honey-thick liquids: product, liquid, and time comparisons. Dysphagia. 2005;20:325–35.
Garcia JM, Chambers ET, Matta Z, Clark M. Serving temperature viscosity measurements of nectar- and honey-thick liquids. Dysphagia. 2008;23:65–75.
Dewar RJ, Joyce MJ. Time-dependent rheology of starch thickeners and the clinical implications for dysphagia therapy. Dysphagia. 2006;21:264–9.
Shama F, Sherman P. Identification of stimuli controlling the sensory evaluation of viscosity. J Texture Stud. 1973;4:111–8.
Cutler AN, Morris ER, Taylor LJ. Oral perception of viscosity in fluid foods and model systems. J Texture Stud. 1983;14:377–95.
Lundh A, Gotzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008;8:22.
National Health and Medical Research Council of Australia. How to use the evidence: assessment and application of scientific evidence. Canberra: Biotext; 2000.
Hind J, Divyak E, Zielinski J, Taylor A, Hartman M, Gangnon R, Robbins J. Comparison of standardized bariums with varying rheological parameters on swallowing kinematics in males. J Rehabil Res Dev. 2012;49:1399–404.
International Organization for Standardization: ISO 11036: Sensory analysis—methodology—texture profile, 1994.
International Organization for Standardization: ISO 11035: Sensory analysis—identification and selection of descriptors for establishing a sensory profile by a multidimensional approach, 1994.
Szczesniak AS. Classification of textural characteristics. J Food Sci. 1963;28:385–9.
Munoz AM. Development and application of texture reference scale. J Sens Stud. 1986;1:55–83.
Gosa MM. Videofluoroscopic analysis to determine the effects of thickened liquids on orophayrngeal swallowing function in infants with respiratory compromise Communication Sciences and Disorders. Memphis: University of Memphis; 2012.
Cichero J, Nicholson T, Dodrill P. Liquid barium is not representative of infant formula: characterisation of rheological and material properties. Dysphagia. 2010;26(3):264–71.
Steele CM, Cichero JA. A question of rheological control. Dysphagia. 2008;23:199–201.
Leonard RJ, White C, McKenzie S, Belafsky PC. Effects of bolus rheology on aspiration in patients with dysphagia. J Acad Nutr Diet. 2014;114:590–4.
Vilardell N, Rofes L, Arreola V, Speyer R, Clave P. A comparative study between modified starch and xanthan gum thickeners in post-stroke oropharyngeal dysphagia. 22nd Dysphagia Research Society. Nashville: Springer; 2014.
Smith C. Oral and oropharyngeal perception of fluid viscosity accross the age span. Dysphagia. 1999;21(4):209–17.
Smith CH, Logemann JA, Burghardt WR, Carrell TD, Zecker SG. Oral sensory discrimination of fluid viscosity. Dysphagia. 1997;12:68–73.
Chen J. Food oral processing: some important underpinning principles of eating and sensory processing. Food Structure. 2014;1:91–105.
Withers C, Gosney MA, Methven L. Perception of thickness, mouth coating and mouth drying of dairy beverages by younger and older volunteers. J Sens Stud. 2013;28:230–7.
Meng Y, Rao MA, Datta AK. Computer simulation of the pharyngeal bolus transport of Newtonian and non-Newtonian fluids. Trans Inst Chem Eng C. 2005;83:297–305.
Nicosia MA. Theoretical estimation of shear rate during the oral phase of swallowing: effect of partial slip. J Texture Stud. 2013;44:132–9.
Penman JP, Thomson M. A review of the textured diets developed for the management of dysphagia. J Hum Nutr Diet. 1998;11:51–60.
Berzlanovich AM, Fazeny-Dorner B, Waldhoer T, Fasching P, Keil W. Foreign body asphyxia: a preventable cause of death in the elderly. Am J Prev Med. 2005;28:65–9.
Japanese Food Safety Commission, Risk Assessment Report: choking accidents caused by foods, 2010.
Alsanei WA, Chen J. Studies of the oral capabilities in relation to bolus manipulations and the ease of initiation bolus flow. J Texture Stud. 2014;45:1–12.
Hiiemae KM, Palmer JB. Food transport and bolus formation during complete feeding sequences on foods of different initial consistency. Dysphagia. 1999;14:31–42.
Pascua Y, Koc H, Foegeding EA. Food structure: roles of mechanical properties and oral processing in determining sensory texture of soft materials. Curr Opin Colloid Interface Sci. 2013;18:324–33.
Samuels R, Chadwick DD. Predictors of asphyxiation risk in adults with intellectual disabilities and dysphagia. J Intellect Disabil Res. 2006;50:362–70.
Morley RE, Ludemann JP, Moxha JP, Kozak FK, Riding KH. Foreign body aspiration in infants and toddlers: recent trends in British Columbia. J Otolaryngol. 2004;33:37–41.
Wick R, Gilbert JD, Byard RW. Cafe coronary syndrome-fatal choking on food: an autopsy approach. J Clin Forensic Med. 2006;13:135–8.
Acknowledgments
The authors gratefully acknowledge Dr. Wendy Dahl for her advice and review of an earlier version of this manuscript. Additionally, Dr. Jun Kayashita is acknowledged for his assistance in obtaining information regarding the Japanese Dysphagia Diet guidelines. Funding for the initial screening stage of the literature review described in this manuscript was provided by the International Dysphagia Diet Standardisation Initiative (IDDSI). IDDSI is an independent, not-for-profit entity, which receives sponsorship funding from several industry partners. None of these industry sponsors were involved with the design, development, writing or approval of this manuscript.
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Steele, C.M., Alsanei, W.A., Ayanikalath, S. et al. The Influence of Food Texture and Liquid Consistency Modification on Swallowing Physiology and Function: A Systematic Review. Dysphagia 30, 2–26 (2015). https://doi.org/10.1007/s00455-014-9578-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-014-9578-x