Abstract
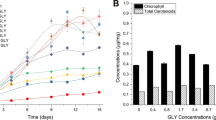
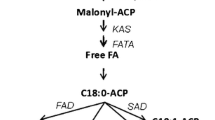
Compounds from microalgae such as ω3-fatty acids or carotenoid are commercially exploited within the pharmacology, nutraceutical, or cosmetic sectors. The co-stimulation of several compounds of interest may improve the cost-effectiveness of microalgal biorefinery pipelines. This study focussed on Phaeodactylum tricornutum to investigate the effects on lipogenesis and carotenogenesis of combined stressors, here cold temperature and addition of NaCl salt or the phytohormone abscisic acid, using a two-stage cultivation strategy. Cold stress with NaCl or phytohormone addition increased the neutral lipid content of the biomass (20 to 35%). These treatments also enhanced the proportions of EPA (22% greater than control) in the fatty acid profile. Also, these treatments had a stimulatory effect on carotenogenesis, especially the combination of cold stress with NaCl addition, which returned the highest production of fucoxanthin (33% increase). The gene expression of diacylglycerol acyltransferase (DGAT) and the ω-3 desaturase precursor (PTD15) were enhanced 4- and 16-fold relative to the control, respectively. In addition, zeaxanthin epoxidase 3 (ZEP3), was downregulated at low temperature when combined with abscisic acid. These results highlight the benefits of applying a combination of low temperature and salinity stress, to simultaneously enhance the yields of the valuable metabolites EPA and fucoxanthin in Phaeodactylum tricornutum.




Similar content being viewed by others
References
Rizwan M, Mujtaba G, Memon SA et al (2018) Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sustain Energy Rev 92:394–404. https://doi.org/10.1016/j.rser.2018.04.034
Yang R, Wei D, Xie J (2020) Diatoms as cell factories for high-value products: chrysolaminarin, eicosapentaenoic acid, and fucoxanthin. Crit Rev Biotechnol 40:993–1009. https://doi.org/10.1080/07388551.2020.1805402
Maeda Y, Nojima D, Yoshino T, Tanaka T (2017) Structure and properties of oil bodies in diatoms. Phil Trans R Soc B Biol Sci 372:20160408. https://doi.org/10.1098/rstb.2016.0408
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Dolch L-J, Maréchal E (2015) Inventory of fatty acid desaturases in the pennate diatom Phaeodactylum tricornutum. Mar Drugs 13:1317–1339. https://doi.org/10.3390/md13031317
Pérez-López P, González-García S, Allewaert C et al (2014) Environmental evaluation of eicosapentaenoic acid production by Phaeodactylum tricornutum. Sci Total Environ 466–467:991–1002. https://doi.org/10.1016/j.scitotenv.2013.07.105
Ryckebosch E, Bruneel C, Muylaert K, Foubert I (2012) Microalgae as an alternative source of omega-3 long chain polyunsaturated fatty acids. Lipid Technol 24:128–130. https://doi.org/10.1002/lite.201200197
Lafourcade M, Larrieu T, Mato S et al (2011) Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci 14:345–350. https://doi.org/10.1038/nn.2736
Narayan B, Miyashita K, Hosakawa M (2006) Physiological effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—a Review. Food Rev Int 22:291–307. https://doi.org/10.1080/87559120600694622
Shin SY, Bajpai VK, Kim HR, Kang SC (2007) Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int J Food Microbiol 113:233–236. https://doi.org/10.1016/j.ijfoodmicro.2006.05.020
Becker EW (2013) Microalgae for aquaculture: nutritional aspects. In: Richmond A, Hu Q (eds) Handbook of microalgal culture. John Wiley and Sons, Ltd., Oxford, pp 671–691
Hemaiswarya S, Raja R, Ravi Kumar R et al (2011) Microalgae: a sustainable feed source for aquaculture. World J Microbiol Biotechnol 27:1737–1746. https://doi.org/10.1007/s11274-010-0632-z
Latowski D, Kuczyńska P, Strzałka K (2011) Xanthophyll cycle—a mechanism protecting plants against oxidative stress. Redox Rep 16:78–90. https://doi.org/10.1179/174329211X13020951739938
Niyogi KK, Björkman O, Grossman AR (1997) The roles of specific xanthophylls in photoprotection. Proc Natl Acad Sci 94:14162–14167. https://doi.org/10.1073/pnas.94.25.14162
Rmiki N-E, Brunet C, Cabioch J, Lemoine Y (1996) Xanthophyll-cycle and photosynthetic adaptation to environment in macro- and microalgae. In: Lindstrom SC, Chapman DJ (eds) Fifteenth international seaweed symposium. Springer, Dordrecht, pp 407–413
Peng J, Yuan J-P, Wu C-F, Wang J-H (2011) Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar Drugs 9:1806–1828. https://doi.org/10.3390/md9101806
Wang H, Zhang Y, Chen L et al (2018) Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess Biosyst Eng 41:1061–1071. https://doi.org/10.1007/s00449-018-1935-y
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553. https://doi.org/10.3390/en5051532
Zhao Y, Wang H-P, Han B, Yu X (2019) Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: a review. Bioresour Technol 274:549–556. https://doi.org/10.1016/j.biortech.2018.12.030
Nagappan S, Devendran S, Tsai P-C et al (2019) Potential of two-stage cultivation in microalgae biofuel production. Fuel 252:339–349. https://doi.org/10.1016/j.fuel.2019.04.138
Boelen P, van Dijk R, Sinninghe Damsté JS et al (2013) On the potential application of polar and temperate marine microalgae for EPA and DHA production. AMB Express 3:26. https://doi.org/10.1186/2191-0855-3-26
Mimouni V, Couzinet-Mossion A, Ulmann L, Wielgosz-Collin G (2018) Chapter 5–Lipids From microalgae. In: Levine IA, Fleurence J (eds) Microalgae in health and disease prevention. Academic Press, Cambridge, pp 109–131
Sayanova O, Mimouni V, Ulmann L et al (2017) Modulation of lipid biosynthesis by stress in diatoms. Phil Trans R Soc B Biol Sci 372:20160407. https://doi.org/10.1098/rstb.2016.0407
Yoshida K, Igarashi E, Mukai M et al (2003) Induction of tolerance to oxidative stress in the green alga, Chlamydomonas reinhardtii, by abscisic acid. Plant Cell Environ 26:451–457. https://doi.org/10.1046/j.1365-3040.2003.00976.x
Zhang H, Yin W, Ma D et al (2021) Phytohormone supplementation significantly increases fatty acid content of Phaeodactylum tricornutum in two-phase culture. J Appl Phycol 33:13–23. https://doi.org/10.1007/s10811-020-02074-8
Zulu NN, Zienkiewicz K, Vollheyde K, Feussner I (2018) Current trends to comprehend lipid metabolism in diatoms. Prog Lipid Res 70:1–16. https://doi.org/10.1016/j.plipres.2018.03.001
Bertrand M (2010) Carotenoid biosynthesis in diatoms. Photosynth Res 106:89–102. https://doi.org/10.1007/s11120-010-9589-x
Kuczynska P, Jemiola-Rzeminska M, Strzalka K (2015) Photosynthetic pigments in diatoms. Mar Drugs 13:5847–5881. https://doi.org/10.3390/md13095847
Domergue F, Lerchl J, Zähringer U, Heinz E (2002) Cloning and functional characterization of Phaeodactylum tricornutum front-end desaturases involved in eicosapentaenoic acid biosynthesis. Eur J Biochem 269:4105–4113. https://doi.org/10.1046/j.1432-1033.2002.03104.x
Eilers U, Dietzel L, Breitenbach J et al (2016) Identification of genes coding for functional zeaxanthin epoxidases in the diatom Phaeodactylum tricornutum. J Plant Physiol 192:64–70. https://doi.org/10.1016/j.jplph.2016.01.006
Coesel S, Oborník M, Varela J et al (2008) Evolutionary origins and functions of the carotenoid biosynthetic pathway in marine diatoms. PLoS ONE 3:e2896. https://doi.org/10.1371/journal.pone.0002896
Conceição D, Lopes RG, Derner RB et al (2020) The effect of light intensity on the production and accumulation of pigments and fatty acids in Phaeodactylum tricornutum. J Appl Phycol 32:1017–1025. https://doi.org/10.1007/s10811-019-02001-6
Kuczynska P, Jemiola-Rzeminska M, Nowicka B et al (2020) The xanthophyll cycle in diatom Phaeodactylum tricornutum in response to light stress. Plant Physiol Biochem 152:125–137. https://doi.org/10.1016/j.plaphy.2020.04.043
Lopes RG, Cella H, Mattos JJ et al (2019) Effect of phosphorus and growth phases on the transcription levels of EPA biosynthesis genes in the diatom Phaeodactylum tricornutum. Braz J Bot 42:13–22. https://doi.org/10.1007/s40415-019-00515-4
Siaut M, Heijde M, Mangogna M et al (2007) Molecular toolbox for studying diatom biology in Phaeodactylum tricornutum. Gene 406:23–35. https://doi.org/10.1016/j.gene.2007.05.022
Kwak HS, Kim JYH, Woo HM et al (2016) Synergistic effect of multiple stress conditions for improving microalgal lipid production. Algal Res 19:215–224. https://doi.org/10.1016/j.algal.2016.09.003
Aziz MMA, Kassim KA, Shokravi Z et al (2020) Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: a review. Renew Sustain Energy Rev 119:109621. https://doi.org/10.1016/j.rser.2019.109621
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport. Springer, Boston, pp 29–60
Qiao H, Cong C, Sun C et al (2016) Effect of culture conditions on growth, fatty acid composition and DHA/EPA ratio of Phaeodactylum tricornutum. Aquaculture 452:311–317. https://doi.org/10.1016/j.aquaculture.2015.11.011
Mc Gee D, Archer L, Paskuliakova A et al (2018) Rapid chemotaxonomic profiling for the identification of high-value carotenoids in microalgae. J Appl Phycol 30:385–399. https://doi.org/10.1007/s10811-017-1247-7
Egeland ES, Garrido JL, Clementson L et al (2011) Data sheets aiding identification of phytoplankton carotenoids and chlorophylls. In: Llewellyn CA, Egeland ES, Johnsen G, Roy S (eds) Phytoplankton pigments: characterization, chemotaxonomy and applications in oceanography. Cambridge University Press, Cambridge, pp 665–674
Johnson ZI, Bidigare RR, Blinebry SK et al (2017) Screening for lipids from marine microalgae using Nile red. In: Lee SY (ed) Consequences of microbial interactions with hydrocarbons, oils, and lipids: production of fuels and chemicals. Springer, Berlin, pp 87–108
Barone ME, Parkes R, Herbert H et al (2021) Comparative response of marine microalgae to H2O2-induced oxidative stress. Appl Biochem Biotechnol 193:4052–4067. https://doi.org/10.1007/s12010-021-03690-x
Archer L, McGee D, Parkes R et al (2021) Antioxidant bioprospecting in microalgae: characterisation of the potential of two marine heterokonts from irish waters. Appl Biochem Biotechnol 193:981–997. https://doi.org/10.1007/s12010-020-03467-8
Bowler C, Allen AE, Badger JH et al (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456:239–244. https://doi.org/10.1038/nature07410
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45. https://doi.org/10.1093/nar/29.9.e45
Curran-Everett D (2018) Explorations in statistics: the log transformation. Adv Physiol Educ 42:343–347. https://doi.org/10.1152/advan.00018.2018
Glazier DS (2013) Log-transformation is useful for examining proportional relationships in allometric scaling. J Theor Biol 334:200–203. https://doi.org/10.1016/j.jtbi.2013.06.017
Adarme-Vega TC, Thomas-Hall SR, Schenk PM (2014) Towards sustainable sources for omega-3 fatty acids production. Curr Opin Biotechnol 26:14–18. https://doi.org/10.1016/j.copbio.2013.08.003
Fu W, Wichuk K, Brynjólfsson S (2015) Developing diatoms for value-added products: challenges and opportunities. New Biotechnol 32:547–551. https://doi.org/10.1016/j.nbt.2015.03.016
Liang M-H, Wang L, Wang Q et al (2019) High-value bioproducts from microalgae: strategies and progress. Crit Rev Food Sci Nutr 59:2423–2441. https://doi.org/10.1080/10408398.2018.1455030
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542. https://doi.org/10.1016/j.biotechadv.2013.07.011
Huerlimann R, de Nys R, Heimann K (2010) Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol Bioeng 107:245–257. https://doi.org/10.1002/bit.22809
Jung J-H, Sirisuk P, Ra CH et al (2019) Effects of green LED light and three stresses on biomass and lipid accumulation with two-phase culture of microalgae. Process Biochem 77:93–99. https://doi.org/10.1016/j.procbio.2018.11.014
Sun X-M, Ren L-J, Zhao Q-Y et al (2018) Microalgae for the production of lipid and carotenoids: a review with focus on stress regulation and adaptation. Biotechnol Biofuels 11:272. https://doi.org/10.1186/s13068-018-1275-9
Li H-Y, Lu Y, Zheng J-W et al (2014) Biochemical and genetic engineering of diatoms for polyunsaturated fatty acid biosynthesis. Mar Drugs 12:153–166. https://doi.org/10.3390/md12010153
Lavens P, Sorgeloos P (1996) Manual on the production and use of live food for aquaculture. FAO, Rome
Kudo I, Miyamoto M, Noiri Y, Maita Y (2000) Combined effects of temperature and iron on the growth and physiology of the marine diatom Phaeodactylum Tricornutum (bacillariophyceae). J Phycol 36:1096–1102. https://doi.org/10.1046/j.1529-8817.2000.99042.x
Chapman KD, Ohlrogge JB (2012) Compartmentation of triacylglycerol accumulation in plants *. J Biol Chem 287:2288–2294. https://doi.org/10.1074/jbc.R111.290072
Chen JE, Smith AG (2012) A look at diacylglycerol acyltransferases (DGATs) in algae. J Biotechnol 162:28–39. https://doi.org/10.1016/j.jbiotec.2012.05.009
Harwood JL (1988) Fatty acid metabolism. Annu Rev Plant Physiol Plant Mol Biol 39:101–138. https://doi.org/10.1146/annurev.pp.39.060188.000533
Barrero-Sicilia C, Silvestre S, Haslam RP, Michaelson LV (2017) Lipid remodelling: unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci 263:194–200. https://doi.org/10.1016/j.plantsci.2017.07.017
Jiang H, Gao K (2004) Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum Tricornutum (bacillariophyceae)1. J Phycol 40:651–654. https://doi.org/10.1111/j.1529-8817.2004.03112.x
Erdoğan A, Karataş AB, Demirel Z, Dalay MC (2022) Purification of fucoxanthin from the diatom Amphora capitellata by preparative chromatography after its enhanced productivity via oxidative stress. J Appl Phycol 34:301–309. https://doi.org/10.1007/s10811-021-02625-7
Ishika T, Moheimani NR, Bahri PA et al (2017) Halo-adapted microalgae for fucoxanthin production: effect of incremental increase in salinity. Algal Res 28:66–73. https://doi.org/10.1016/j.algal.2017.10.002
Ishika T, Laird DW, Bahri PA, Moheimani NR (2019) Co-cultivation and stepwise cultivation of Chaetoceros muelleri and Amphora sp. for fucoxanthin production under gradual salinity increase. J Appl Phycol 31:1535–1544. https://doi.org/10.1007/s10811-018-1718-5
Parihar P, Singh S, Singh R et al (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075. https://doi.org/10.1007/s11356-014-3739-1
Rijstenbil JW (2003) Effects of UVB radiation and salt stress on growth, pigments and antioxidative defence of the marine diatom Cylindrotheca closterium. Mar Ecol Prog Ser 254:37–48. https://doi.org/10.3354/meps254037
Sommella E, Conte GM, Salviati E et al (2018) Fast profiling of natural pigments in different Spirulina (Arthrospira platensis) dietary supplements by DI-FT-ICR and evaluation of their antioxidant potential by pre-column DPPH-UHPLC assay. Molecules 23:1152. https://doi.org/10.3390/molecules23051152
Lepetit B, Volke D, Gilbert M et al (2010) Evidence for the existence of one antenna-associated, lipid-dissolved and two protein-bound pools of diadinoxanthin cycle pigments in diatoms. Plant Physiol 154:1905–1920. https://doi.org/10.1104/pp.110.166454
Lepetit B, Goss R, Jakob T, Wilhelm C (2012) Molecular dynamics of the diatom thylakoid membrane under different light conditions. Photosynth Res 111:245–257. https://doi.org/10.1007/s11120-011-9633-5
Blommaert L, Chafai L, Bailleul B (2021) The fine-tuning of NPQ in diatoms relies on the regulation of both xanthophyll cycle enzymes. Sci Rep 11:12750. https://doi.org/10.1038/s41598-021-91483-x
Cui Y, Thomas-Hall SR, Chua ET, Schenk PM (2021) Development of High-level omega-3 eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum. J Phycol 57:258–268. https://doi.org/10.1111/jpy.13082
Wang Z, Mou J, Qin Z et al (2022) An auxin-like supermolecule to simultaneously enhance growth and cumulative eicosapentaenoic acid production in Phaeodactylum tricornutum. Bioresour Technol 345:126564. https://doi.org/10.1016/j.biortech.2021.126564
Wu H, Li T, Wang G et al (2016) A comparative analysis of fatty acid composition and fucoxanthin content in six Phaeodactylum tricornutum strains from different origins. Chin J Oceanol Limnol 34:391–398. https://doi.org/10.1007/s00343-015-4325-1
Yang Y-H, Du L, Hosokawa M et al (2017) Fatty acid and lipid class composition of the microalga Phaeodactylum tricornutum. J Oleo Sci 66:363–368. https://doi.org/10.5650/jos.ess16205
Hamilton ML, Warwick J, Terry A et al (2015) Towards the industrial production of omega-3 long chain polyunsaturated fatty acids from a genetically modified diatom Phaeodactylum tricornutum. PLoS ONE 10:e0144054. https://doi.org/10.1371/journal.pone.0144054
Cui Y, Thomas-Hall S, Schenk P (2020) Isolation and cultivation of a Phaeodactylum tricornutum strain from the east coast of Australia for EPA production. Geol Earth Mar Sci. 2:1–7. https://doi.org/10.31038/GEMS.2020222
Ruenwai R, Neiss A, Laoteng K et al (2011) Heterologous production of polyunsaturated fatty acids in Saccharomyces cerevisiae causes a global transcriptional response resulting in reduced proteasomal activity and increased oxidative stress. Biotechnol J 6:343–356. https://doi.org/10.1002/biot.201000316
Sun X-M, Geng L-J, Ren L-J et al (2018) Influence of oxygen on the biosynthesis of polyunsaturated fatty acids in microalgae. Bioresour Technol 250:868–876. https://doi.org/10.1016/j.biortech.2017.11.005
Bautista-Chamizo E, Sendra M, Cid Á et al (2018) Will temperature and salinity changes exacerbate the effects of seawater acidification on the marine microalga Phaeodactylum tricornutum? Sci Total Environ 634:87–94. https://doi.org/10.1016/j.scitotenv.2018.03.314
Santos MMD, Moreno-Garrido I, Gonçalves F et al (2002) An in situ bioassay for estuarine environments using the microalga Phaeodactylum tricornutum. Environ Toxicol Chem 21:567–574. https://doi.org/10.1002/etc.5620210315
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16. https://doi.org/10.1016/j.plantsci.2003.10.024
Chawla S, Jain S, Jain V (2013) Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J Plant Biochem Biotechnol 22:27–34. https://doi.org/10.1007/s13562-012-0107-4
Pancha I, Chokshi K, Maurya R et al (2015) Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 189:341–348. https://doi.org/10.1016/j.biortech.2015.04.017
AbdElgawad H, Zinta G, Hegab MM et al (2016) High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front Plant Sci 7:276
Azachi M, Sadka A, Fisher M et al (2002) Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina. Plant Physiol 129:1320–1329. https://doi.org/10.1104/pp.001909
Chen H, Jiang J-G (2009) Osmotic responses of Dunaliella to the changes of salinity. J Cell Physiol 219:251–258. https://doi.org/10.1002/jcp.21715
Lu Y, Xu J (2015) Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci 20:273–282. https://doi.org/10.1016/j.tplants.2015.01.006
Tuteja N (2007) Abscisic acid and abiotic stress signaling. Plant Signal Behav 2:135–138. https://doi.org/10.4161/psb.2.3.4156
Han X, Zeng H, Bartocci P et al (2018) Phytohormones and effects on growth and metabolites of microalgae: a review. Fermentation 4:25. https://doi.org/10.3390/fermentation4020025
Sivaramakrishnan R, Incharoensakdi A (2020) Plant hormone induced enrichment of Chlorella sp. omega-3 fatty acids. Biotechnol Biofuels 13:7. https://doi.org/10.1186/s13068-019-1647-9
Cho D, Shin D, Jeon BW, Kwak JM (2009) ROS-mediated ABA signaling. J Plant Biol 52:102–113. https://doi.org/10.1007/s12374-009-9019-9
Postiglione AE, Muday GK (2020) The role of ROS homeostasis in ABA-induced guard cell signaling. Front Plant Sci 11:968
Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran L-SP (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol 202:35–49. https://doi.org/10.1111/nph.12613
Sairam RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–421
Hirsch R, Hartung W, Gimmler H (1989) Abscisic acid content of algae under stress*. Bot Acta 102:326–334. https://doi.org/10.1111/j.1438-8677.1989.tb00113.x
Kempa S, Krasensky J, Santo SD et al (2008) A central role of abscisic acid in stress-regulated carbohydrate metabolism. PLoS ONE 3:e3935. https://doi.org/10.1371/journal.pone.0003935
Qiao T, Zhao Y, Zhong D, Yu X (2021) Hydrogen peroxide and salinity stress act synergistically to enhance lipids production in microalga by regulating reactive oxygen species and calcium. Algal Res 53:102017. https://doi.org/10.1016/j.algal.2020.102017
Acknowledgements
The authors acknowledge financial support from the VES4US project funded by the European Union's Horizon 2020 research and innovation program under grant Agreement No 801338.
Author information
Authors and Affiliations
Contributions
DF, MEB and NT developed the experimental design. DF carried out the cell cultivation. DF and MEB performed the HPLC and GC–MS analyses. MEB carried out the Nile Red and TBARS analyses. DF and VG conducted the molecular biology analyses. DF and MEB led the drafting of the manuscript. All authors contributed to editing and finalising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no conflict of interest and no affiliations with or involvement in any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fierli, D., Barone, M.E., Graceffa, V. et al. Cold stress combined with salt or abscisic acid supplementation enhances lipogenesis and carotenogenesis in Phaeodactylum tricornutum (Bacillariophyceae). Bioprocess Biosyst Eng 45, 1967–1977 (2022). https://doi.org/10.1007/s00449-022-02800-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02800-1