Abstract
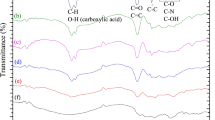
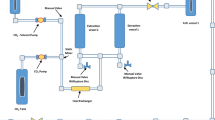
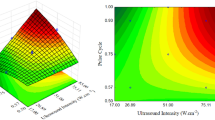
Endophytic fungi have been highlight in the production of secondary metabolites with different bioactive properties, such as in the production of the antioxidant compounds. Therefore, the objective of this work was the extraction of the antioxidant compounds from the biomass of Diaporthe schini using supercritical carbon dioxide (CO2) without and with ethanol as cosolvent. The biomass was produced by submerged fermentation and the parameters evaluated in the extraction process were: pressure (150–250 bar), temperature (40–60 ºC) and cosolvent [biomass: cosolvent ratio, 1:0, 1:0.75 and 1:1.5 (w/v)]. Extraction yield, antioxidant activity and chemical composition of the extracts were determined. The highest extraction yield (3.24 wt.%) and the best antioxidant activity against the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical (96.62%) were obtained at 40 ºC, 250 bar and biomass:cosolvent ratio of 1:1.5 (w/v). The chemical compounds 1,4-diaza-2,5-dioxo-3-isobutyl bicyclo[4.3.0]nonane and benzeneethanol identified in GC/MS could be responsible for the antioxidant activity found in this study.
Graphic abstract




Similar content being viewed by others
References
Lupatini M, Jacques RJS, Antoniolli ZI et al (2013) Land-use change and soil type are drivers of fungal and archaeal communities in the Pampa biome. World J Microbiol Biotechnol 29:223–233. https://doi.org/10.1007/s11274-012-1174-3
de Souza ARC, Baldoni DB, Lima J et al (2017) Selection, isolation, and identification of fungi for bioherbicide production. Braz J Microbiol 48:101–108. https://doi.org/10.1016/j.bjm.2016.09.004
Bilal S, Ali L, Khan AL et al (2018) Endophytic fungus Paecilomyces formosus LHL10 produces sester-terpenoid YW3548 and cyclic peptide that inhibit urease and α-glucosidase enzyme activities. Arch Microbiol 200:1493–1502. https://doi.org/10.1007/s00203-018-1562-7
Zhang G, Zhang Y, Qin J et al (2013) Antifungal metabolites produced by Chaetomium globosum No.04, an endophytic fungus isolated from Ginkgo biloba. Indian J Microbiol 53:175–180. https://doi.org/10.1007/s12088-013-0362-7
Daniel JJ, Zabot GL, Tres MV et al (2018) Fusarium fujikuroi: A novel source of metabolites with herbicidal activity. Biocatal Agric Biotechnol 14:314–320. https://doi.org/10.1016/j.bcab.2018.04.001
de Souza ARC, Baldoni DB, Lima J et al (2015) Bioherbicide production by Diaporthe sp. isolated from the Brazilian Pampa biome. Biocatal Agric Biotechnol 4:575–578. https://doi.org/10.1016/j.bcab.2015.09.005
dos Reis CM, da Rosa BV, da Rosa GP et al (2019) Antifungal and antibacterial activity of extracts produced from Diaporthe schini. J Biotechnol 294:30–37. https://doi.org/10.1016/j.jbiotec.2019.01.022
Valente IL, Confortin TC, Luft L et al (2018) Extraction of bioactive compounds from Botryosphaeria dothidea using supercritical carbon dioxide and compressed liquefied petroleum gas. J Supercrit Fluids 136:52–59. https://doi.org/10.1016/j.supflu.2018.02.013
Carvalho CR, Gonçalves VN, Pereira CB et al (2012) The diversity, antimicrobial and anticancer activity of endophytic fungi associated with the medicinal plant Stryphnodendron adstringens (Mart.) Coville (Fabaceae) from the Brazilian savannah. Symbiosis 57:95–107. https://doi.org/10.1007/s13199-012-0182-2
Sharma V, Singamaneni V, Sharma N et al (2018) Valproic acid induces three novel cytotoxic secondary metabolites in Diaporthe sp., an endophytic fungus from Datura inoxia Mill. Bioorg Med Chem Lett 28:2217–2221. https://doi.org/10.1016/j.bmcl.2018.04.018
El-Gendy MMAA, Yahya SMM, Hamed AR et al (2018) Phylogenetic analysis and biological evaluation of marine endophytic fungi derived from red sea sponge Hyrtios erectus. Appl Biochem Biotechnol 185:755–777. https://doi.org/10.1007/s12010-017-2679-x
Tanney JB, McMullin DR, Green BD et al (2016) Production of antifungal and antiinsectan metabolites by the Picea endophyte Diaporthe maritima sp. nov. Fungal Biol 120:1448–1457. https://doi.org/10.1016/j.funbio.2016.05.007
Li G, Kusari S, Kusari P et al (2015) Endophytic Diaporthe sp. LG23 produces a potent antibacterial tetracyclic triterpenoid. J Nat Prod 78:2128–2132. https://doi.org/10.1021/acs.jnatprod.5b00170
Sebastianes FLS, Cabedo N, El Aouad N et al (2012) 3-Hydroxypropionic acid as an antibacterial agent from endophytic fungi Diaporthe phaseolorum. Curr Microbiol 65:622–632. https://doi.org/10.1007/s00284-012-0206-4
Tanapichatsakul C, Monggoot S, Gentekaki E, Pripdeevech P (2018) Antibacterial and antioxidant metabolites of Diaporthe spp. isolated from flowers of Melodorum fruticosum. Curr Microbiol 75:476–483. https://doi.org/10.1007/s00284-017-1405-9
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042. https://doi.org/10.1021/np9904509
Demirci MA, Ipek Y, Gul F et al (2018) Extraction, isolation of heat-resistance phenolic compounds, antioxidant properties, characterization and purification of 5-hydroxymaltol from Turkish apple pulps. Food Chem 269:111–117. https://doi.org/10.1016/j.foodchem.2018.06.147
Kungel PTAN, Correa VG, Corrêa RCG et al (2018) Antioxidant and antimicrobial activities of a purified polysaccharide from yerba mate (Ilex paraguariensis). Int J Biol Macromol 114:1161–1167. https://doi.org/10.1016/j.ijbiomac.2018.04.020
Lin T, Liu Y, Lai C et al (2018) The effect of ultrasound assisted extraction on structural composition, antioxidant activity and immunoregulation of polysaccharides from Ziziphus jujuba Mill var. spinosa seeds. Ind Crops Prod 125:150–159. https://doi.org/10.1016/j.indcrop.2018.08.078
Trentini CP, Cuco RP, Cardozo-Filho L, da Silva C (2019) Extraction of macauba kernel oil using supercritical carbon dioxide and compressed propane. Can J Chem Eng 97:785–792. https://doi.org/10.1002/cjce.23236
Şahin S, Bilgin M, Dramur MU (2011) Investigation of oleuropein content in olive leaf extract obtained by supercritical fluid extraction and soxhlet methods. Sep Sci Technol 46:1829–1837. https://doi.org/10.1080/01496395.2011.573519
Scapin G, Abaide ER, Nunes LF et al (2017) Effect of pressure and temperature on the quality of chia oil extracted using pressurized fluids. J Supercrit Fluids 127:90–96. https://doi.org/10.1016/j.supflu.2017.03.030
de Melo MMR, Silvestre AJD, Silva CM (2014) Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J Supercrit Fluids 92:115–176. https://doi.org/10.1016/j.supflu.2014.04.007
Kitzberger CSG, Smânia A Jr, Pedrosa RC, Ferreira SRS (2007) Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J Food Eng 80:631–638. https://doi.org/10.1016/j.jfoodeng.2006.06.013
Mazzutti S, Ferreira SRS, Riehl CAS et al (2012) Supercritical fluid extraction of Agaricus brasiliensis: Antioxidant and antimicrobial activities. J Supercrit Fluids 70:48–56. https://doi.org/10.1016/j.supflu.2012.06.010
Confortin TC, Todero I, Soares JF et al (2017) Extraction and composition of extracts obtained from Lupinus albescens using supercritical carbon dioxide and compressed liquefied petroleum gas. J Supercrit Fluids 128:395–403. https://doi.org/10.1016/j.supflu.2017.06.006
de Souza ARC, Guedes AR, Rodriguez JMF et al (2018) Extraction of Arctium Lappa leaves using supercritical CO2 + ethanol: kinetics, chemical composition, and bioactivity assessments. J Supercrit Fluids 140:137–146. https://doi.org/10.1016/j.supflu.2018.06.011
Sallet D, Abaide E, Marcuz C et al (2017) Obtaining fatty acids from Mortierella isabellina using supercritical carbon dioxide and compressed liquefied petroleum gas. J Supercrit Fluids 122:79–87. https://doi.org/10.1016/j.supflu.2016.12.005
NIST–Chemistry WebBook (2017) https://webbook.nist.gov/chemistry/. Accessed 11 Mar 2019. https://doi.org/10.18434/T4D303
Meireles MAA (2008) Extraction of bioactive compounds from Latin American plants, in: J. Martinez (Ed.), Supercritical fluid extraction of nutraceuticals and bioactive compounds, CRC Press – Taylor & Francis Group, Boca Raton.
Dal Prá V, Dolwitsch CB, Lima FO, et al (2015) Ultrasound-assisted extraction and biological activities of extracts of Brassica oleracea var. capitata. Food Technol Biotechnol 53:102–109. https://doi.org/10.17113/ftb.53.01.15.3533
Dal Prá V, Soares JF, Monego DL et al (2016) Extraction of bioactive compounds from palm (Elaeis guineensis) pressed fiber using different compressed fluids. J Supercrit Fluids 112:51–56. https://doi.org/10.1016/j.supflu.2016.02.011
Goyeneche R, Fanovich A, Rodrigues CR et al (2018) Supercritical CO2 extraction of bioactive compounds from radish leaves: yield, antioxidant capacity and cytotoxicity. J Supercrit Fluids 135:78–83. https://doi.org/10.1016/j.supflu.2018.01.004
Elgndi MA, Filip S, Pavlić B et al (2017) Antioxidative and cytotoxic activity of essential oils and extracts of Satureja montana L., Coriandrum sativum L. and Ocimum basilicum L. obtained by supercritical fluid extraction. J Supercrit Fluids 128:128–137. https://doi.org/10.1016/j.supflu.2017.05.025
Zabot GL, Moraes MN, Petenate AJ, Meireles MAA (2014) Influence of the bed geometry on the kinetics of the extraction of clove bud oil with supercritical CO2. J Supercrit Fluids 93:56–66. https://doi.org/10.1016/j.supflu.2013.10.001
Zhao H, Dong J, Lu J et al (2006) Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.). J Agric Food Chem 54:7277–7286. https://doi.org/10.1021/jf061087w
Pereira P, Bernardo-Gil MG, Cebola MJ et al (2013) Supercritical fluid extracts with antioxidant and antimicrobial activities from myrtle (Myrtus communis L.) leaves. Response surface optimization. J Supercrit Fluids 83:57–64. https://doi.org/10.1016/j.supflu.2013.08.010
Takaya Y, Furukawa T, Miura S et al (2007) Antioxidant constituents in distillation residue of Awamori spirits. J Agric Food Chem 55:75–79. https://doi.org/10.1021/jf062029d
Tan LTH, Chan KG, Chan CK et al (2018) Antioxidative potential of a Streptomyces sp. MUM292 isolated from mangrove soil. Biomed Res Int 2018:1–13. https://doi.org/10.1155/2018/4823126
Kim MK, Nam P-W, Lee S-J, Lee K-G (2014) Antioxidant activities of volatile and non-volatile fractions of selected traditionally brewed Korean rice wines. J Inst Brew 120:537–542. https://doi.org/10.1002/jib.180
Xia M, Liu L, Qiu R et al (2018) Anti-inflammatory and anxiolytic activities of Euphorbia hirta extract in neonatal asthmatic rats. AMB Express 8:179–189. https://doi.org/10.1186/s13568-018-0707-z
Tyagi T, Agarwal M (2017) Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J Pharmacogn Phytochem 6:195–206
Wei LS, Wee W, Siong JYF, Syamsumir DF (2011) Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Med Iran 49:670–674
Patil L, Kaliwal BB (2019) Microalga Scenedesmus bajacalifornicus BBKLP-07, a new source of bioactive compounds with in vitro pharmacological applications. Bioprocess Biosyst Eng 42:979–994. https://doi.org/10.1007/s00449-019-02099-5
Hou R, Liu X, Xiang K et al (2019) Characterization of the physicochemical properties and extraction optimization of natural melanin from Inonotus hispidus mushroom. Food Chem 277:533–542. https://doi.org/10.1016/j.foodchem.2018.11.002
Li S, Tang D, Wei R et al (2019) Polysaccharides production from soybean curd residue via Morchella esculenta. J Food Biochem 43:1–12. https://doi.org/10.1111/jfbc.12791
Wang Y, Ma H, Ding Z et al (2019) Three-phase partitioning for the direct extraction and separation of bioactive exopolysaccharides from the cultured broth of Phellinus baumii. Int J Biol Macromol 123:201–209. https://doi.org/10.1016/j.ijbiomac.2018.11.065
Vasundhara M, Baranwal M, Sivaramaiah N, Kumar A (2017) Isolation and characterization of trichalasin-producing endophytic fungus from Taxus baccata. Ann Microbiol 67:255–261. https://doi.org/10.1007/s13213-017-1256-4
Acknowledgements
The authors thank Coordination for the Improvement of Higher Education Personnel (CAPES) for scholarships, as well as National Council for Scientific and Technological Development (CNPq) and Research Support Foundation of the State of Rio Grande do Sul (FAPERGS) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there are no conflicts of interest regarding this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Rosa, B.V., Kuhn, K.R., Ugalde, G.A. et al. Antioxidant compounds extracted from Diaporthe schini using supercritical CO2 plus cosolvent. Bioprocess Biosyst Eng 43, 133–141 (2020). https://doi.org/10.1007/s00449-019-02211-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02211-9