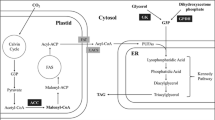
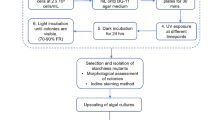
Abstract
In this study, the microalga Chlorella saccharophila was subjected to ultraviolet (UV) mutagenesis, and mutant screening was conducted based on acidity tolerance to generate mutants with increased triacylglycerol (TAG) and polyunsaturated fatty acid (PUFA) contents. Two improved mutant strains (M1 and M5) were generated. M1 and M5 accumulated 27.2% and 27.4% more TAG, respectively, and showed stronger fluorescence intensity than the wild-type (WT) strain when the cells of these mutants were stained with the lipophilic Nile Red stain. In the M1 mutant, 50.5% of the fatty acid methyl esters (FAMEs) were saturated (C16:0 and C18:0) and 25.27% were monounsaturated (C18:1) fatty acids which are suitable for biofuels production. In the M5 mutant, 65.19% of the total FAMEs were nutritional PUFAs (C16:2, C18:2, and C18:3), while these FAMEs were not detected in the WT. These results demonstrated that UV mutagenesis coupled to an acid pH screening strategy represents a valuable and fast platform to generate mutants of C. saccharophila with improved TAG and PUFA contents for biofuels and nutraceutical applications, respectively.






Similar content being viewed by others
References
Sarayloo E, Simsek S, Unlu YS et al (2018) Enhancement of the lipid productivity and fatty acid methyl ester profile of Chlorella vulgaris by two rounds of mutagenesis. Bioresour Technol 250:764–769. https://doi.org/10.1016/j.biortech.2017.11.105
Klok AJ, Lamers PP, Martens DE et al (2014) Edible oils from microalgae: insights in TAG accumulation. Trends Biotechnol 32:521–528. https://doi.org/10.1016/j.tibtech.2014.07.004
Paliwal C, Mitra M, Bhayani K et al (2017) Abiotic stresses as tools for metabolites in microalgae. Bioresour Technol 244:1216–1226. https://doi.org/10.1016/j.biortech.2017.05.058
Jagadevan S, Banerjee A, Banerjee C et al (2018) Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol Biofuels 11:185. https://doi.org/10.1186/s13068-018-1181-1
Contreras-Pool PY, Peraza-Echeverria S, Ku-González AF, Herrera-Valencia VA (2016) The phytohormone abscisic acid increases triacylglycerol content in the green microalga Chlorella saccharophila (Chlorophyta). Algae 31:267–276
Remmers IM, Wijffels RH, Barbosa MJ, Lamers PP (2018) Can we approach theoretical lipid yields in microalgae? Trends Biotechnol 36:265–276. https://doi.org/10.1016/j.tibtech.2017.10.020
Steensels J, Snoek T, Meersman E et al (2014) Improving industrial yeast strains: exploiting natural and artificial diversity. FEMS Microbiol Rev 38:947–995. https://doi.org/10.1111/1574-6976.12073
Mehtani J, Arora N, Patel A et al (2017) Augmented lipid accumulation in ethyl methyl sulphonate mutants of oleaginous microalga for biodiesel production. Bioresour Technol 242:121–127. https://doi.org/10.1016/j.biortech.2017.03.108
Sivaramakrishnan R, Incharoensakdi A (2017) Enhancement of lipid production in Scenedesmus sp. by UV mutagenesis and hydrogen peroxide treatment. Bioresour Technol 235:366–370. https://doi.org/10.1016/j.biortech.2017.03.102
de Jaeger L, Verbeek RE, Draaisma RB et al (2014) Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus: (I) mutant generation and characterization. Biotechnol Biofuels 7:69. https://doi.org/10.1186/1754-6834-7-69
Sirikhachornkit A, Vuttipongchaikij S, Suttangkakul A et al (2016) Increasing the triacylglycerol content in Dunaliella tertiolecta through isolation of starch-deficient mutants. J Microbiol Biotechnol 26:854–866. https://doi.org/10.4014/jmb.1510.10022
Lim DKY, Schuhmann H, Sharma K, Schenk PM (2015) Isolation of high-lipid tetraselmis suecica strains following repeated UV-C mutagenesis, FACS, and high-throughput growth selection. Bioenergy Res 8:750–759. https://doi.org/10.1007/s12155-014-9553-2
Sarayloo E, Tardu M, Unlu YS et al (2017) Understanding lipid metabolism in high-lipid-producing Chlorella vulgaris mutants at the genome-wide level. Algal Res 28:244–252. https://doi.org/10.1016/j.algal.2017.11.009
Tatsuzawa H, Takizawa E, Wada M, Yamamoto Y (1996) Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. J Phycol 32:598–601
Poerschmann J, Spijkerman E, Langer U (2004) Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb Ecol 48:78–89. https://doi.org/10.1007/s00248-003-0144-6
Eibl JK, Corcoran JD, Senhorinho GN et al (2014) Bioprospecting for acidophilic lipid-rich green microalgae isolated from abandoned mine site water bodies. AMB Express 4:7. https://doi.org/10.1186/2191-0855-4-7
Fuggi A, Pinto G, Pollio A (1988) Effects of NaCI, Na2SO4, H2SO4, and glucose on growth, photosynthesis, and respiration in the acidophilic alga Dunaliella acidophila (Volvocales, Chlorophyta). Phycologia 27:334–339
Fuggi A, Pinto G, Pollio A, Taddei R (1988) The role of glycerol in osmoregulation of the acidophilic alga. Phycologia 27:439–446
Herrera-Valencia VA, Contreras-Pool PY, López-Adrián SJ et al (2011) The green microalga Chlorella saccharophila as a suitable source of oil for biodiesel production. Curr Microbiol 63:151–157. https://doi.org/10.1007/s00284-011-9956-7
Harris EH (1989) The Chlamydomonas sourcebook: a comprehensive guide to biology and laboratory use. Academic Press, San Diego
Wimpenny JW, Waters P (1984) Growth of micro-organisms in gel-stabilized two-dimensional diffusion gradient systems. J Gen Microbiol 130:2921–2926
Cakmak T, Angun P, Demiray YE, Ozkan AD (2012) Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol Bioeng 109:1947–1957. https://doi.org/10.1002/bit.24474
Herrera-Valencia VA, Us-Vázquez RA, Larqué-Saavedra FA, Barahona-Pérez LF (2012) Naturally occurring fatty acid methyl esters and ethyl esters in the green microalga Chlamydomonas reinhardtii. Ann Microbiol 62:865–870. https://doi.org/10.1007/s13213-011-0361-z
Pérez L, Bugja R, Lorenschat J et al (2011) Aquatic ecosystems of the Yucatán Peninsula (Mexico), Belize, and Guatemala. Hydrobiologia 661:407–433. https://doi.org/10.1007/s10750-010-0552-9
Drago L, Nicola L, Mattina R, De Vecchi E (2010) In vitro selection of resistance in Escherichia coli and Klebsiella spp. at in vivo fluoroquinolone concentrations. BMC Microbiol 10:119. https://doi.org/10.1186/1471-2180-10-119
Hong ME, Lee KS, Yu BJ et al (2010) Identification of gene targets eliciting improved alcohol tolerance in Saccharomyces cerevisiae through inverse metabolic engineering. J Biotechnol 149:52–59. https://doi.org/10.1016/j.jbiotec.2010.06.006
Luo L, Wen Q, Ren J et al (2014) Dynamic encoding of perception, memory, and movement in a C. elegans chemotaxis circuit. Neuron 82:1115–1128. https://doi.org/10.1016/j.neuron.2014.05.010
Patnaik R, Louie S, Gavrilovic V et al (2002) Genome shuffling of Lactobacillus for improved acid tolerance. Nat Biotechnol 20:707–712. https://doi.org/10.1038/nbt0702-707
Guihneuf F, Fouqueray M, Mimouni V et al (2010) Effect of UV stress on the fatty acid and lipid class composition in two marine microalgae Pavlova lutheri (Pavlovophyceae) and Odontella aurita (Bacillariophyceae). J Appl Phycol 22:629–638. https://doi.org/10.1007/s10811-010-9503-0
Beacham TA, Macia VM, Rooks P et al (2015) Altered lipid accumulation in Nannochloropsis salina CCAP849/3 following EMS and UV induced mutagenesis. Biotechnol Rep 7:87–94. https://doi.org/10.1016/j.btre.2015.05.007
Hu Q, Sommerfeld M, Jarvis E et al (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639. https://doi.org/10.1111/j.1365-313X.2008.03492.x
Anthony J, Rangamaran VR, Gopal D et al (2014) Ultraviolet and 5′ fluorodeoxyuridine Induced random mutagenesis in Chlorella vulgaris and its impact on fatty acid profile: a new insight on lipid-metabolizing genes and structural characterization of related proteins. Mar Biotechnol 17:66–80. https://doi.org/10.1007/s10126-014-9597-5
Doan TTY, Obbard JP (2011) Improved Nile Red staining of Nannochloropsis sp. J Appl Phycol 23:895–901. https://doi.org/10.1007/s10811-010-9608-5
Sharma KK, Li Y, Schenk PM (2015) Rapid lipid induction in Chlorella sp. by UV-C radiation. Bioenergy Res 8:1824–1830. https://doi.org/10.1007/s12155-015-9633-y
Norashikin MN, Loh SH, Aziz A, Cha TS (2018) Metabolic engineering of fatty acid biosynthesis in Chlorella vulgaris using an endogenous omega-3 fatty acid desaturase gene with its promoter. Algal Res 31:262–275. https://doi.org/10.1016/j.algal.2018.02.020
Acknowledgements
This study was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT México) Project no. 169217. Jalsen Iván Teco Bravo received support from CONACYT (scholarship no. 404430). The authors thank Ileana C. Borges Argáez, Tanit Toledano Thompson and Fray Martin Baas Espinola for technical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have not conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig.
1 Comparison of the tolerance to acidity between the WT and mutant strains on a pH gradient plate. A) Control plate; only the WT strain was inoculated. B-F) The WT and mutant strains were streaked on the same pH gradient plate. Approximately 1x105 WT and mutagenized cells were streaked on the gradient in the direction from the acid to alkaline region (TIFF 5889 kb)
Supplementary Fig.
2 Growth patterns observed with the WT and mutant strains of C. saccharophila on pH gradient agar plates. Approximately 4x107 cells were spread over the surface (TIFF 8373 kb)
Supplementary Fig.
3 Lipid content of the WT and mutant strains of C. saccharophila at pH 6 and 7. A) Lipid extract in mg L-1 culture; B) Lipid content as a percentage of dry biomass weight (% DBW). Cells were incubated in 50 mL of TAP medium adjusted to the indicated pH for 10 days. Values are the mean ± standard deviation (n = 3). Means with a common letter are not significantly different (Fisher´s least significant difference, p ˂ 0.05) (TIFF 3264 kb)
Supplementary Fig.
4 Productivity of the WT and mutant strains of C. saccharophila cultured at pH 6 and 7. A) Biomass productivity; B) Lipid productivity; C) TAG productivity. Cells were incubated in 50 mL of TAP medium adjusted to the pH indicated for 10 days. Values are the mean ± standard deviation (n = 3). Means with a common letter are not significantly different (Fisher´s least significant difference, p ˂ 0.05) (TIFF 2595 kb)
Supplementary Table
1 Fatty acid methyl ester (FAME) composition of the WT and mutant strains (M1 and M5) of C. saccharophila (percentage of total FAMEs) (TIFF 795 kb)
Rights and permissions
About this article
Cite this article
Teco-Bravo, J.I., Barahona-Pérez, L.F., Reyes-Sosa, C.F. et al. Enhanced production of triacylglycerols and polyunsaturated fatty acids in novel acid-tolerant mutants of the green microalga Chlorella saccharophila. Bioprocess Biosyst Eng 42, 1561–1571 (2019). https://doi.org/10.1007/s00449-019-02153-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02153-2