Abstract
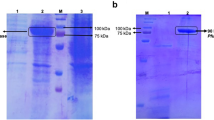
An efficient induction strategy that consisted of multiple additions of small doses of isopropyl-β-D-thiogalactopyranoside (IPTG) in the early cell growth phase was developed for enhancing Pfu DNA polymerase production in Escherichia coli. In comparison to the most commonly used method of a single induction of 1 mM IPTG, the promising induction strategy resulted in an increase in the Pfu activity of 13.5 % in shake flasks, while simultaneously decreasing the dose of IPTG by nearly half. An analysis of the intracellular IPTG concentrations indicated that the cells need to maintain an optimum intracellular IPTG concentration after 6 h for efficient Pfu DNA polymerase production. A significant increase in the Pfu DNA polymerase activity of 31.5 % under the controlled dissolved oxygen concentration of 30 % in a 5 L fermentor was achieved using the multiple IPTG induction strategy in comparison with the single IPTG induction. The induction strategy using multiple inputs of IPTG also avoided over accumulation of IPTG and reduced the cost of Pfu DNA polymerase production.





Similar content being viewed by others
References
Hansen CJ, Wu L, Fox JD, Arezi B, Hogrefe HH (2011) Engineered split in Pfu DNA polymerase fingers domain improves incorporation of nucleotide gamma-phosphate derivative. Nucleic Acids Res 39:1801–1810
Melissis S, Labrou NE, Clonis YD (2006) Nucleotide-mimetic synthetic ligands for DNA-recognizing enzymes—One-step purification of Pfu DNA polymerase. J Chromatogr A 1122:63–75
Norholm MH (2010) A mutant Pfu DNA polymerase designed for advanced uracil-excision DNA engineering. BMC Biotech 10:21
Fiala G, Stetter KO (1986) Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100 °C. Arch Microbiol 145:56–61
Lu CL, Erickson HP (1997) Expression in Escherichia coli of the thermostable DNA polymerase from Pyrococcus furiosus. Protein Expres Purif 11:179–184
Uemori T, Ishino Y, Toh H, Asada K, Kato I (1993) Organization and nucleotide-sequence of the DNA-polymerase gene from the archaeon Pyrococcus furiosus. Nucleic Acids Res 21:259–265
Dabrowski S, Kur J (1998) Cloning and expression in Escherichia coli of the recombinant his-tagged DNA polymerases from Pyrococcus furiosus and Pyrococcus woesei. Protein Expr Purif 14:131–138
Sun Z, Cai J (2006) Purification of recombinant Pfu DNA polymerase using a new JK110 resin. Korean J Chem Eng 23:607–609
Fernandez A, Ruiz J, Caminal G, Lopez-Santin J (2010) Development and validation of a liquid chromatography-mass spectrometry assay for the quantitation of IPTG in E. Coli fed-batch cultures. Anal Chem 82:5728–5734
Donovan RS, Robinson CW, Glick BR (1996) Review: optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter. J Ind Microbiol 16:145–154
Einsfeldt K, Severo Junior JB, Correa Argondizzo AP, Medeiros MA, Moitinho Alves TL, Almeida RV, Larentis AL (2011) Cloning and expression of protease ClpP from Streptococcus pneumoniae in Escherichia coli: study of the influence of kanamycin and IPTG concentration on cell growth, recombinant protein production and plasmid stability. Vaccine 29:7136–7143
Malakar P, Venkatesh KV (2012) Effect of substrate and IPTG concentrations on the burden to growth of Escherichia coli on glycerol due to the expression of Lac proteins. Appl Microbiol Biotechnol 93:2543–2549
Pinsach J, de MasC Lopez-Santin J (2008) Induction strategies in fed-batch cultures for recombinant protein in Escherichia coli: application to rhamnulose 1-phosphate aldolase. Biochem Eng J 41:181–187
Striedner G, Cserjan-Puschmann M, Potschacher F, Bayer K (2003) Tuning the transcription rate of recombinant protein in strong Escherichia coli expression systems through repressor titration. Biotechnol Progr 19:1427–1432
Lecina M, Sarro E, Casablancas A, Godia F, Cairo JJ (2013) IPTG limitation avoids metabolic burden and acetic acid accumulation in induced fed-batch cultures of Escherichia coli M15 under glucose limiting conditions. Biochem Eng J 70:78–83
Durany O, Caminal G, de Mas C, Lopez-Santin J (2004) Studies on the expression of recombinant fuculose-1-phosphate aldolase in E. coli. Process Biochem 39:1677–1684
Lim HK, Jung KH, Park DH, Chung SI (2000) Production characteristics of interferon-alpha using an L-arabinose promoter system in a high-cell-density culture. Appl Microbiol Biotechnol 53:201–208
Olaofe OA, Burton SG, Cowan DA, Harrison STL (2010) Improving the production of a thermostable amidase through optimising IPTG induction in a highly dense culture of recombinant Escherichia coli. Biochem Eng J 52:19–24
Bentley WE, Davis RH, Kompala DS (1991) Dynamics of induced cat expression in Escherichia coli. Biotechnol Bioeng 38:749–760
Duan KJ, Lin MT, Hung YC, Lin CT, Chen CW, Sheu DC (2000) Production of GST-SOD fusion protein by recombinant E coli XL1 Blue. J Chem Technol Biotechnol 75:722–728
Miao F, Kompala DS (1992) Overexpression of cloned genes using recombinant Escherichia coli regulated by a T7-promoter: I. batch cultures and kinetic modeling. Biotechnol Bioeng 40:787–796
Kim M, Elvin C, Brownlee A, Lyons R (2007) High yield expression of recombinant pro-resilin: lactose-induced fermentation in E coli and facile purification. Protein Expres Purif 52:230–236
Bentley WE, Mirjalili N, Andersen DC, Davis RH, Kompala DS (1989) Plasmid-encoded protein: the principal factor in the “Metabolic Burden” associated with recombinant bacteria. Biotechnol Bioeng 102:1284–1297
Chen SJ, Chang MC, Cheng CY (1997) Effect of induction conditions on production and excretion of Aeromonas hydrophila chitinase by recombinant Escherichia coli. J Ferment Bioeng 84:610–613
Ren Q, Henes B, Fairhead M, Thony-Meyer L (2013) High level production of tyrosinase in recombinant Escherichia coli. BMC Biotech 13:18
Srivastava P, Mukherjee KJ (2005) Kinetic studies of recombinant human interferon-alpha (rhIFN-alpha) expression in transient state continuous cultures. Biochem Eng J 26:50–58
Fernandez-Castane A, Vine CE, Caminal G, Lopez-Santin J (2012) Evidencing the role of lactose permease in IPTG uptake by Escherichia coli in fed-batch high cell density cultures. J Biotechnol 157:391–398
Herzenberg LA (1961) Isolation and identification of derivates formed in the course of intracellular accumulation of thiogalactosides by Escherichia coli. Arch Biochem Biophys 93:314–315
Roderick SL (2005) The lac operon galactoside acetyltransferase. C R Biol 328:568–575
Wang H, Wang F, Wei D (2009) Impact of oxygen supply on rtPA expression in Escherichia coli BL21 (DE3): ammonia effects. Appl Microbiol Biotechnol 82:249–259
Losen M, Frolich B, Pohl M, Buchs J (2004) Effect of oxygen limitation and medium composition on Escherichia coli fermentation in shake-flask cultures. Biotechnol Prog 20:1062–1068
Qoronfleh MW (1999) Dissolved oxygen concentration affects the accumulation of HIV-1 recombinant proteins in Escherichia coli. Appl Biochem Biotechnol 80:107–120
Konz JO, King J, Cooney CL (1998) Effects of oxygen on recombinant protein expression. Biotechnol Prog 14:393–409
Acknowledgments
This work was financially supported by the National Key Technology Research and Development Program of China (Nos. 2012BAK25B01), and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSZD-EW-Z-015). We acknowledge Dr. Amanda Stiles (the Chinese Academy of Sciences Fellowships for Young International Scientists, No. 2011Y1GA01) for the help in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, JH., Wang, F. & Liu, CZ. Development of an efficient process intensification strategy for enhancing Pfu DNA polymerase production in recombinant Escherichia coli . Bioprocess Biosyst Eng 38, 651–659 (2015). https://doi.org/10.1007/s00449-014-1304-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1304-4