Abstract
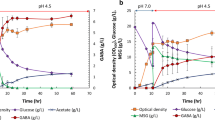
In shake flasks, good oxygen supply tended to decrease rtPA expression in media containing only yeast extract and tryptone, while oxygen limitation would increase rtPA synthesis in the same medium. Our data showed that though the drop of rtPA expression in the 20-ml cultures of LBG or 2YTG was accompanied with a severe acetate accumulation, it was actually caused by low ammonia. The rtPA expression level could be significantly improved by increasing culture ammonium ion up to 500 mM. The effects of exogenous high ammonia on cell growth and rtPA expression were further examined in shake flasks and a 4-l fermentor. The high ammonia had no significant impact on cell growth and oxygen respiratory activity but significantly depressed the activities of glutamine synthetase/glutamate synthase and glutamate dehydrogenase, suggesting that ammonium ion as a nitrogen source improved the protein expression by mediating ammonia-assimilating enzymes. We thus propose in our work that E. coli cells, which were grown to a certain density to produce rtPA, would undergo nitrogen starvation under the low ammonia conditions even when the organic nitrogen sources remained abundant. The scale-up of rtPA production from shake flasks to fermentors could be readily achieved in the media containing rich ammonium ion.








Similar content being viewed by others
References
Atkinson MR, Ninfa AJ (1998) Role of the glnK gene in nitrogen-regulation of gene transcription in Escherichia coli. Mol Microbiol 29:431–447
Atkinson MR, Blauwkamp TA, Bondarenko V, Studitsky V, Ninfa AJ (2002) Activation of the glnA, glnK, and nac promoters as Escherichia coli undergoes the transition from nitrogen excess growth to nitrogen starvation. J Bacteriol 184:5358–5363
Axe DD, Bailey JE (1995) Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnol Bioeng 47:8–19
Bockman AT, Reeve CA, Matin A (1986) Stabilization of glucose-starved Escherichia coli K-12 and Salmonella typhimurium LT2 by peptidase-deficient mutants. J Gen Microbiol 132:231–235
Boogerd FC, Bos P, Kuenen JG, Heijnen JJ, van der Lans RG (1990) Oxygen and carbon dioxide mass transfer and the aerobic, autotrophic cultivation of moderate and extreme thermophiles: A case study related to the microbial desulfurization of coal. Biotechnol Bioeng 35:1111–1119
Ernsting BR, Denninger JW, Blumenthal RM, Matthews RG (1993) Regulation of the gltBDF operon of Escherichia coli: how is a leucine-insensitive operon regulated by the leucine-responsive regulatory protein? J Bacteriol 175:7160–7169
Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28:2077–2080
Grabherr R, Nilsson E, Striedner G, Bayer K (2002) Stabilizing plasmid copy number to improve recombinant protein production. Biotechnol Bioeng 77:142–147
Groat RG, Schultz JE, Zychlinsky E, Bockman A, Matin A (1986) Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol 168:486–493
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418
Javelle A, Severi E, Thornton J, Merrick M (2004) Ammonium sensing in Escherichia coli: Role of the ammonium transporter AmtB and AmtB-GlnK complex formation. J Biol Chem 279:8530–8538
Jeanette RP, Dale LO (1968) Amino acid transport systems in Escherichia coli K12. J Biol Chem 243:5914–5920
Jensen EB, Carlsen S (1990) Production of recombinant human growth hormone in Escherichia coli: expression of different precursors and physiological effects of glucose, acetate, and salts. Biotechnol Bioeng 36:1–11
Kleiner D (1985) Bacterial ammonium transport. FEMS Microbiol Rev 32:87–100
Kleman GL, Strohl WR (1994) Acetate metabolism by Escherichia coli in high-cell-density fermentation. Appl Environ Microbiol 60:3952–3958
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lasko DR, Zamboni N, Sauer U (2000) Bacterial response to acetate challenge: a comparion of tolerance among species. Appl Microbiol Biotechnol 54:243–247
Lee SY (1996) High cell-density culture of Escherichia coli. Trends Biotechol 14:98–105
Leigh JA, Dodsworth JA (2007) Nitrogen regulation in bacteria and archaea. Annu Rev Microbiol 61:349–77
Losen M, Frölich B, Pohl M, Büchs J (2004) Effect of oxygen limitation and medium composition on Escherichia coli fermentation in shake-flask cultures. Biotechnol Prog 20:1062–1068
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lu C, Bentley WE, Rao G (2003) Comparisons of oxidative stress response genes in aerobic Escherichia coli fermentations. Biotechnol Bioeng 83:864–870
Makrides SC (1996) Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev 60:512–538
Margareta W, Erik H, Anneli A, Christina K, Bjorn L, Benny R, Lennart H, Gunnar P (1991) Synthesis and secretion of a fibrinolytically active tissue-type plasminogen activator variant in Escherichia coli. Gene 99:243–248
Maurizi MR, Rasulova F (2002) Degradation of L-glutamate dehydrogenase from Escherichia coli: allosteric regulation of enzyme stability. Arch Biochem Biophys 397:206–216
McFall E, Newman EB (1996) Amino acids as carbon sources. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasani k B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella: cellular and molecular biology, 2nd edn. ASM Press, Washington, DC, pp 358–379
Merrick MJ, Edwards RA (1995) Nitrogen control in bacteria. Microbiol Rev 59:604–622
Miller RE, Stadtman ER (1972) Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem 247:7407–7419
Naider F, Becker JM (1975) Multiplicity of oligopeptide transport systems in Escherichia coli. J Bacteriol 122:1208–1215
Neubauer P, Lin HY, Mathiszik B (2003) Metabolic load of recombinant protein production: Inhibition of cellular capacities for glucose uptake and respiration after induction of a heterologous gene in Escherichia coli. Biotechnol Bioeng 83:53–64
Ninfa AJ, Atkinson MR (2000) PII signal transduction proteins. Trends Microbiol 8:172–179
Payne JW, Bell G (1979) Direct determination of the properties of peptide transport systems in Escherichia coli, using a fluorescent-labeling procedure. J Bacteriol 137:447–455
Qoronfleh MW (1999) Dissolved oxygen concentration affects the accumulation of HIV-1 recombinant proteins in Escherichia coli. Appl Biochem Biotechnol 80:107–120
Reitzer L (2003) Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol 57:155–176
Reitzer L, Schneider BL (2001) Metabolic context and possible physiological themes of σ54-dependent genes in Escherichia coli. Microbiol Mol Biol Rev 65:422–444
Ryan W, Parulekar SJ, Stark BC (1989) Expression of β-lactamase by recombinant Escherichia coli strains containing plasmids of different sizes - effects of pH, phosphate, and dissolved oxygen. Biotechnol Bioeng 34:309–319
Saito Y, Ishii Y, Sasaki H, Hayashi M, Fujimura T, Imai Y, Nakamura S, Suzuki S, Notani J, Asada T, Horiai H, Masakazu K, Mineo N (1994) Production and characterization of a novel tissue-type plasminogen activator derivative in Escherichia coli. Biotechnol Prog 10:472–479
Senior PJ (1975) Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: studies with the continuous-culture technique. J Bacteriol 123:407–418
Tribe LA, Briens CL, Margaritis A (1995) Determination of the volumetric mass transfer coefficient (k La) using the dynamic “gas out-gas in” method: analysis of errors caused by dissoved oxygen probes. Biotechnol Bioeng 46:388–392
Verheijen JH, Mullaart E, Chang GT, Kluft C, Wijngaards G (1982) A simple, sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurements in plasma. Thromb Haemost 48:266–269
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 39:971–974
Woolfolk CA, Shapiro B, Stadtman ER (1966) Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys 116:177–192
Zhao YC, Wang G, Kong Y, Zhang CK (2003) Cloning, expression and renaturation studies of retaplase. J Microbiol Biotechnol 13:989–992
Zheng L, Kostrewa D, Bernèche S, Winkler FK, Li XD (2004) The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci U S A 101:17090–17095
Zimmer DP, Soupene E, Lee HL, Wendisch VF, Khodursky AB, Peter BJ, Bender RA, Kustu S (2000) Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc Natl Acad Sci U S A 97:14674–14679
Acknowledgments
We thank Shandong Yanglong Biotech. Co., Ltd (Ji’nan, China) for kindly supplying the original rtPA expressing strain, E. coli BL21 (DE3)/pET22b(+)-rtPA. This work was supported by a grant (No. 2009ZX09306) from National Key Technologies R&D Programs of the Ministry of Science and Technology of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Wang, F. & Wei, D. Impact of oxygen supply on rtPA expression in Escherichia coli BL21 (DE3): ammonia effects. Appl Microbiol Biotechnol 82, 249–259 (2009). https://doi.org/10.1007/s00253-008-1756-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1756-z