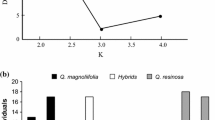
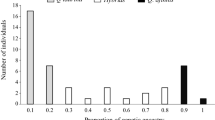
Abstract
In a previous study, we showed that the geographic proximity of hybrid plants to the allopatric areas of parental species increases their morphological and genetic similarity with them. In the present work, we explored whether the endophagous fauna of hybrid plants show the same pattern. We studied the canopy species richness, diversity and composition of leaf-mining moths (Lepidoptera: Tischeridae, Citheraniidae) and gall-forming wasps (Hymenoptera: Cynipidae) associated with two species of red oaks (Quercus crassifolia and Quercus crassipes) and their interspecific hybrid (Quercus×dysophylla Benth pro sp.) in seven hybrid zones in central Mexico, during four seasons in 2 years. The study was conducted on 194 oak trees with known genetic status [identified by leaf morphology and molecular markers (random amplified polymorphic DNAs)], and the results indicate a bidirectional pattern of gene flow. Hybrid plants supported intermediate levels of infestation of gall-forming and leaf-mining insects compared to their putative parental species. The infestation level of leaf-mining insects varied significantly following the pattern: Q. crassifolia>hybrids>Q. crassipes, whereas the gall-forming insects showed an inverse pattern. A negative and significant relationship was found between these two types of insect guilds in each host taxa, when the infestation percentage was evaluated. It was found that 31.5% (n=11) of the endophagous insects were specific to Q. crassipes, 22.9% (n=8) to Q. crassifolia, and 8.6% (n=3) to hybrid individuals. The hybrid bridge hypothesis was supported in the case of 25.7% (n=9) of insects, which suggests that the presence of a hybrid intermediary plant may favor a host herbivore shift from one plant species to another. Greater genetic diversity in a hybrid zone is associated with greater diversity in the endophagous community. The geographic proximity of hybrid plants to the allopatric site of a parental species increases their similarity in terms of endophagous insects and the Eje Neovolcánico acts as a corridor favoring this pattern.






Similar content being viewed by others
References
Aguilar JM, Boecklen WJ (1992) Patterns of herbivory in the Quercus grisea×Quercus gambelii species complex. Oikos 64:498–504
Anderson E (1949) Introgressive hybridization. Wiley, New York
Ananthakrisham NT (1984) The biology of gall insects. Arnold, London
Bacilieri R, Ducousso A, Kremer A (1995) Genetic, morphological, ecological and phenological differentiation between Quercus petrea (Matt.) Liebl. and Quercus robur L. in a hybrid zone of the northwest. Silvae Genet 44:1–1
Boecklen WJ, Spellenberg R (1990) Structure of herbivore communities in two oak (Quercus spp.) hybrid zones. Oecologia 85:92–100
Boecklen WJ, Larson KC (1994) Gall-forming wasp (Hymenoptera: Cynipidae) in an oak hybrid zone: testing hypotheses about hybrid susceptibility to herbivores. In: Price PW, Mattson WJ, Baranchikov YN (eds) The ecology and evolution of gall-forming insects. North Central Forest Experimental Station, Forest Service, USDA, St. Paul, Min., pp 110–120
Bruschi P, Vendramin GG, Bussotti F, Grossoni P (2000) Morphological and molecular differentiation between Quercus petrea (Matt.) Liebl. and Quercus pubescens Willd. (Fagaceae) in northern and central Italy. Ann Bot 85:325–333
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Connor EF, Faeth SH, Simberloff D (1983) Leaf-miners on oaks: the role of immigration in situ reproductive recruitment. Ecology 64:191–204
Cornell HV (1986) Oak species attributes and host size influence cynipine wasp species richness. Ecology 67:1582–1592
Cornell HV, Washburn JO (1979) Evolution of the richness-area correlation for cynipid gall wasp on oak tree: a comparison of two geographic areas. Evolution 33:257–274
Dumolin-Lapeguè S, Demesure B, Fineschi S, LeCorre V, Petit RJ (1997) Phylogeographic structure of white oaks throughout the European Continent. Genetics 146:1475–1487
Dungey HS, Potts BM, Whitham TG, Li HF (2000) Plant genetics effects arthropod community richness and composition: evidence from a synthetic eucalypt hybrid population. Evolution 54:1938–1946
Faith DP, Minchin PR, Belbin L (1987) Compositional dissimilarity as a robust measure of ecological distance. Vegetation 69:57–68
Ferrusquía-Villafranca I (1993) Geology of Mexico: a synopsis. In: Ramamoorthy TP, Bye R, Lot A, Fa J (eds) Biological diversity of Mexico: origins and distribution. Oxford University Press, New York, pp 3–107
Floate KD, Whitham TG (1993) The “hybrid bridge” hypothesis: host shifting via plant hybrid swarms. Am Nat 141:651–662
Fritz RS, Simms EL (1992) Plant resistance to herbivores and phatogens. University of Chicago Press, Chicago, Ill.
Fritz RS, Nichols-Orians CM, Brunsfeld SJ (1994) Interspecific hybridization of plants and resistance to herbivores: hypothesis, genetics, and variable responses in a diverse herbivore community. Oecologia 97:106–117
Fritz RS, Roche BM, Brunsfeld SJ, Orians CM (1996) Interspecific and temporal variation in herbivores responses to hybrid willows. Oecologia 108:121–129
Fritz RS, Roche BM, Brunsfeld SJ (1998) Genetic variation in resistance of hybrid willows to herbivores. Oikos 83:117–128
Fritz RS, Moulia C, Newcombe G (1999) Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Annu Rev Ecol Syst 30:565–591
Grant V (1981) Plant speciation. 2nd edn. Columbia University Press, New York
Guttman SI, Weight LA (1989) Electrophoretic evidence of relationships among Quercus (oaks) of eastern North America. Can J Bot 67:339–351
Hall RW, Towsend AM (1987) Suitability of Ulmus wilsoniana, the “urban” elm, and their hybrids for the elm leaf beetle, Xanthogaleruca luteola (Müller) (Coleoptera: Chrysomelidae). Environ Entomol 16:1042–1044
Hanhimäki S, Senn J, Haukioja E (1994) Permanence of insect herbivores on hybridizing trees: the case of the subarctic birches. J Anim Ecol 63:163–175
Hardin JW (1975) Hybridization and introgression in Quercus alba. J Arnold Arbor 56:336–363
Jensen RJ, Eshbaugh WH (1976) Numerical taxonomic studies of hybridization in Quercus. Populations of restricted aerial distribution and low taxonomic diversity. Syst Bot 1:1–10
Knops JMH, Tilman D, Haddad NM, Naeem S, Mitchell CE, Haarstad J (1999) Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundance and diversity. Ecol Lett 2:286–293
Lawton JH (1978) Host-plant influences on insect diversity: the effects of space and time. Symp R Entomol Soc Lond 9:105–125
Messina FJ, Richards JH, McArthur ED (1996) Variable responses of insects to hybrid versus parental sagebrush in common gardens. Oecologia 107:513–521
Moorehead JR, Taper ML, Case TJ (1993) Utilization of hybrid oak hosts by a monophaghous gall wasp: how little host character is sufficient? Oecologia 95:385–392
Morrow PA, Whitham TG, Potts BM, Ladiges P, Ashton DH, Williams J (1994) Gall-forming insects concentrate on hybrid phenotypes of Eucalyptus host. In: Price PW, Mattson WJ Jr, Baranchikov YN (eds) The ecology and evolution of gall-forming insects. North Central Forest Experimental Station, Forest Service, USDA, St. Paul, Min.
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nixon KC (1993) The genus Quercus in Mexico. In: Nixon KC (ed) Biological diversity of Mexico: origins and distributions, Oxford University Press, New York, pp 447–458
Orians CM, Fritz RS (1995) Secundary chemistry of hybrid and parental willows: phenolic glycosides and condensed tannins in Salix sericea, S. eriocephala, and their hybrids. J Chem Ecol 21:1245–1253
Orians CM (2000) The effects of hybridization in plants on secondary chemistry: implications for the ecology and evolution of plant-herbivore interactions. Am J Bot 87:1749–1756
Osier TL, Lindroth RL (2001) Effects of genotype, nutrient availability, and defoliation on aspen phytochemistry and insect performance. J Chem Ecol 27:1289–1313
Price PW, Abrahamson WG, Hunter MD, Melika G (2004) Using gall wasp on oaks to test broad ecological concepts. Conserv Biol 18:1405–1416
Rieseberg LH, Brunsfeld SJ (1992) Molecular evidence and plant introgression. In: Soltis PS, Soltis DE, Doyle JD (eds) Molecular systematic of plants. Chapman and Hall, New York, pp 151–176
Rieseberg LH, Elltrand NC (1993) What can molecular and morphological markers tell us about plant hybridization? Crit Rev Plant Sci 12:213–241
Rieseberg LH, Wendel JF (1993) Introgression and its consequences in plants. In: Harrison RG (ed) hybrid zones and the evolutionary process, Oxford University Press, Oxford, pp 70–109
Romero RS (1993) El género Quercus (Fagaceae) en el Estado de México. M.Sc. thesis, Universidad Nacional Autónoma de México
Rowell-Rahier M (1984) The food plant preferentes of Phratora vitellinae (Coleoptera: Chrysomelinae). Oecologia 64:375–380
Shoonhoven LM, Jermy T, Van Loon JJA (1998) Insect–plant biology: from physiology to evolution. Chapman and Hall, London
Simberloff D, Stiling P (1987) Larval dispersion and survivorship in a leaf-mining moth. Ecology 68:1647–1657
Simms EL, Rausher MD (1993) Patterns of selection on phytophage resistance in Ipomoea purpurea. Evolution 47:970–976
Slansky F, Scriber JM (1985) Food composition and utilization. In: Kerkurt G, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology. Pergamon, Oxford, pp 87–163
Soetens P, Rowell-Rahier M, Pasteels JM (1991) Influence of phenolglucosides and trichome density on the distribution of insects herbivores on willows. Entomol Exp Appl 59:175–187
Stace CA (1987) Hybridization and the plant species. In: Urbanska KM (ed) Differentiation patterns in higher plants. Academic Press, New York, pp 115–127
Stone GN, Schönrogge K, Atkinson RJ, Pujade-Villar J (2002) The population biology of gall wasp (Hymenoptera: Cynipidae). Annu Rev Entomol 47:633–668
Strauss SY (1994) Levels of herbivory and parasitism in host hybrid zones. Trends Ecol Evol 9:209–214
Strong DR, Lawton JH, Southwood TRE (1984) Insects on plants: community patterns and mechanisms. Harvard University Press, Cambridge, Mass.
Tovar-Sánchez E, Oyama K (2004) Natural hybridization and hybrid zones between Quercus crassifolia and Q. crassipes in Mexico. Morphological and molecular evidence. Am J Bot 91:1352–1363
Warwick RM, Clarke KR, Suharsono A (1990) A statistical analysis of coral community responses to the 1982–1983 El Niño in the Thousand Island, Indonesia. Coral Reefs 8:171–179
Wendel LH (1960) Cynipid Galls of the Southwest. Privately printed, Ann Arbor, Mich.
Wendel JF, Steward JMcD, Rettig JH (1991) Molecular evidence for homoploid reticulate evolution among Australian species of Gossypium. Evolution 45:694–711
Whitham TG, Morrow PA, Potts BM (1994) Plant hybrid zones as center of biodiversity: the herbivore community of two endemic Tasmanian eucalypts. Oecologia 97:481–490
Whitham TG, Martinsen GD, Floate KD, Dungey H, Potts BM, Keim P (1999) Plant hybrid zones affect biodiversity: tools for a genetic-based understanding of community structure. Ecology 80:416–428
Whittemore AT, Schaal BA (1991) Interspecific gene flow in sympatric oaks. Proc Natl Acad Sci USA 88:2540–2544
Wimp GM, Young WP, Woolbright SA, Martinsen GD, Keim P, Whitham TG (2004) Conserving plant genetic diversity for dependent animal communities. Ecol Lett 7:776–780
Wimp GM, Gregory D, Martinsen GD, Floate KD, Bangert RK, Whitham TG (2005) Plant genetic determinants of arthropod community structure and diversity. Evolution 59:61–69
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Englewood Cliffs, N.J.
Acknowledgements
We thank Jim Cronin and two anonymous reviewers for their comments that improved the original manuscript considerably. The authors thank Patricia Mussali, Susana Valencia, Rocio Esteban, Marco Romero, Mauricio Mora, and Maribel Paniagua for technical assistance. This research was supported by a PAEP–UNAM grant and a CONACYT scholarship to E. T. S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jim Cronin
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tovar-Sánchez, E., Oyama, K. Effect of hybridization of the Quercus crassifolia×Quercus crassipes complex on the community structure of endophagous insects. Oecologia 147, 702–713 (2006). https://doi.org/10.1007/s00442-005-0328-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-005-0328-5