Abstract
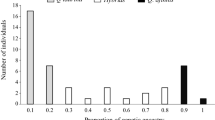
We found the hybrid zone between Eucalyptus amygdalina and Eucalyptus risdonii to be a center of insect and fungal species richness and abundance. Of 40 taxa examined, 73% were significantly more abundant in the hybrid zone than in pure zones, 25% showed on significant differences, and 2% were most abundant on a pure host species. The average hybrid tree supported 53% more insect and fungal species, and relative abundances were, on average, 4 times greater on hybrids than on either eucalypt species growing in pure stands. Hybrids may act as refugia for rare species: 5 of 40 species were largely restricted to the hybrid zone. Also, 50% of the species coexisted only in the hybrid zone, making for mique species assemblages. Although hybrids support more species and greater abundances, all hybrids are not equal: 68% of the 40 taxa examined were significantly more abundant on one hybrid phenotype than another. While herbivore concentrations on F1 type intermediates were rare, concentrations were common on phenotypes resembling backcrosses either to E. amygdalina or E. risdonii. For specialist herbivores, the hybrid phenotype most heavily utilized appears to be determined by its phenotypic affinity to its host species. Generalists exhibit an overall greater abundance on hybrids, but are less likely to utilize one hybrid phenotype over another. Mechanistic explanations for these distributions are numerous and probably species specific, but are likely to include: increased genetic susceptibility of hybrids due to hybrid breakdown; increased stress in the hybrid zone resulting in greater plant susceptibility; and a greater diversity of resources in the hybrid zone which could support more species. Seed capsule production by hybrids and their parental species is negatively correlated with herbivory. However, it is difficult to determine whether herbivores cause this pattern as hybrids may have inherently lower sexual reproduction. Laws enacted to protect rare and endangered species do not include hybrids. We argue that a re-examination of our current “hybrid policy” is warranted. Plant hybrid zones are centers of plant evolution and speciation, sources of economically important plants and potential biocontrol agents, and, as our study suggests, also provide essential habitats for phytophagous communities.
Similar content being viewed by others
References
Aguilar JM, Boecklen WJ (1991) Patterns of herbivory in the Quercus grisea × Q. gambelii species complex. Oikos 64:498–504
Barker JF (1990) Sunflower trichome defenses avoided by a sunflower stem weevil, Cylindrocopturus adspersus LeConte (Coleoptera: Curculionidae). J Kans Entomol Soc 63:638–641
Boecklen WJ, Spellenberg R (1990) Structure of herbivore communities in two oak (Quercus spp.) hybrid zones. Oecologia 85:92–100
Brussard PF (1984) Geographic patterns and environmental gradients: the central-marginal model in Drosophilia revisited. Annu Rev Ecol Syst 15:25–64
Bunce JA, Chabot BF, Miller LN (1979) Role of annual leaf carbon balance in the distribution of plant species along an elevational gradient. Bot Gaz 140:288–294
Dowling TE, DeMarais BD (1993) Evolutionary significance of introgressive hybridization in cyprinid fishes. Nature 362:444–446
Dowling TE, DeMarais BD, Minckley WL, Douglas ME (1992) Use of genetic characters in conservation biology. Conserv Biol 6:7–8
Drake DW (1981) Reproductive success of two Eucalyptus hybrid populations. I. Generalized seed output model and comparison of fruit parameters. Aust J Bot 29:25–35
Duncan F (1989) Systematic affinities, hybridization and clinal variation within Tasmanian eucalypts. Tas For 1:13–25
Dupont F, Criyelli AJ (1988) Do parasites confer a disadvantage to hybrids? Oecologia 75:587–592
Ericson L, Burdon JJ, Wennstrom A (1993) Inter-specific host hybrids and phalacrid beetles implicated in the local survival of smut pathogens. Oikos 68:393–400
Floate K, Kearsley MJC, Whitham TG (1993) Elevated herbivory in plant hybrid zones: Chrysomela confluens, Populus and phenological sinks. Ecology 74:2056–2065
Floate KD, Whitham TG (1993) The “hybrid bridge” hypothesis: host shifting via plant hybrid swarms. Am Nat 141:651–662
Floate KD, Whitham TG (1994) Aphid-ant interaction reduces chrysomilid herbivory in a cottonwood hybrid zone. Oecologia (in press)
Fox LR, Morrow PA (1983) Estimates of grazing damage to Eucalyptus trees. Aust J Ecol 8:139–144
Fox LR, Morrow PA (1986) On comparing herbivore damage in Australian and North American systems. Aust J Ecol 11:387–393
Frankel R (1983) Heterosis. Springer, Berlin Heidelberg New York
Fritz RS, Simms EL (1992) Plant resistance to herbivores and pathogens. University of Chicago Press, New York
Fritz RS, Nichols-Orians CM, Brunsfeld SJ (1994) Interspecific hybridization of plants and resistance to herbivores: hypotheses, genetics, and variable responses in a diverse herbivore community. Oecologia (in press)
Grant V (1971) Plant speciation. Columbia University Press, New York
Greenslade P, New TR (1990) Australia: conservation of continental insect fauna. In: Collins NM, Thomas JA (eds) The conservation of insects and their habitats. Academic Press, London, pp 33–70
Griffin AR, Burgess IP, wolf L (1988) Patterns of natural and manipulated hybridisation in the genus Eucalyptus L'Herit —a review Aust J Bot 36:41–66
Head MJ, Lacey CJ (1988) Radiocarbon age determinations from lignotubers. Aust J Bot 36:93–100
Heaney LR, Timm RM (1985) Morphology, genetics, and ecology of pocket gophers (genus Geomys) in a narrow hybrid zone. Biol J Linn Soc 25:301–317
Kat FW (1985) Historical evidence for fluctuation in levels of hybridization. Evolution 39:1164–1169
Keim P, Paige KN, Whitham TG, Lark KG (1989) Genetic analysis of an interspecific hybrid swarm of Populus: occurrence of unidirectional introgression. Genetics 123:557–565
Levin DA (1983) Polyploidy and novelty in flowering plants. Am Nat 122:1–25
Lewis WH (1979) Polyploidy. Plenum, New York
Lewis WH (1980) Polyploidy: biological relevance. Plenum, New York
Lewontin RC, Birch LC (1966) Hybridization as a source of variation for adaptation to new environments. Evolution 20:315–336
Manley AM, Fowler DP (1969) Spruce budworm defoliation in relation to introgression in red and black spruce. For Sci 15:365–366
Martinsen GD, Whitham TG (1994) More nests in hybrid cottonwoods. Wilson Bulletin (in press)
Maxwell FG, Jennings PR (1980) Breeding plants resistant to insects. Wiley, New York
McClure MS (1985) Patterns of abundance, survivorship, and fecundity of Nuculaspis tsugae (Homoptera: Diaspididae) on Tsuga species in Japan in relation to elevation. Environ Entomol 14:413–415
Mitton JB, Grant MC (1984) Relationships among protein heterozygosity, growth rate, and developmental stability. Annu Rev Ecol Syst 15:479–499
Moran NA (1991) Phenotype fixation and genotypic diversity in the complex life cycle of the aphid Pemphigus betae. Evolution 45:957–970
Moran NA, Whitham TG (1988) Evolutionary reduction of complex lifecycles: loss of host-alternation in Pemphigus (Homoptera: Aphididae). Evolution 42:717–728
Morris MG, Collins NM, Vane-Wright RI, Waage J (1990) The utilization and value of nondomesticated insects. In: Collins NM, Thomas JA (eds) The conservation of insects and their habitats. Academic Press, London, pp 319–347
Morrow PA, Fox LR (1989) Estimates of pre-settlement insect damage in Australian and North American forests. Ecology 70:1055–1060
Morrow PA, LaMarche VC (1978) Tree ring evidence for chromic insect suppression of productivity in subalpine Eucalyptus. Science 201:1244–1245
Morrow PA, Whitham TG, Potts BM, Ladiges P, Ashton DH, Williams J (1994) Gall-forming insects concentrate on hybrid phenotypes of Eucalyptus hosts. In: Baranchikov YN, Price PW, Mattson WJ Jr (eds) Gall-forming insects: ecology, physiology and evolution. USDA For Serv Northeast For Exp St Gen Tech Rep (in press)
O'Brien SJ, Mayr E (1991) Bureaucratic mischief: recognizing endangered species and subspecies. Science 251:1187–1188
Paige KN, Capman WC (1993) the effects of host-plant genotype, hybridization, and environment on gall-aphid attack and survival in cottonwood: the importance of genetic studies and the utility of RFLPS. Evolution 47:36–45
Potts BM (1986) Population dynamics and regeneration of a hybrid zone between Eucalyptus risdonii Hook.f. and E. amygdalina. Aust J Bot 34:305–329
Potts BM, Reid JB (1985) Analysis of a hybrid swarm between Eucalyptus risdonii Hook.f. and E. amygdalina Labill. Aust J Bot 33:543–562
Potts BM, Reid JB (1988) Hybridization as a dispersal mechanism. Evolution 42:1245–1255
Prvor LD (1976) The biology of the Eucalyptus. Camelot Press, Southampton
Pryor LD (1981) Australian endangered species: eucalypts. Aust Nat Parks Wildl Serv Spec Publ No. 5
Pryor LD, Johnson LAS (1971) A classification of the eucalypts. Australian National University Press, Canberra
Rattenbury JA (1962) Cyclic hybridization as a survival mechanism in the New Zealand forest flora. Evolution 16:348–363
Rieseberg LH (1991) Homoploid reticulate evolution in Helianthus (Asteraceae): evidence from ribosomal genes. Am J Bot 78:1218–1237
Rojas M (1992) The species problem and conservation: what are we protecting? Conserv Biol 6:170–178
Sage RD, Heyneman D, Lim K, Wilson AC (1986) Wormy mice in a hybrid zone. Nature 324:60–63
SAS (1988) SAS/STAT user's guide for personal computers, release 6.03 edition. SAS Institute Inc. Cary, NC, USA
Stace CA (1987) Hybridization and the plant species. In: Urbanska KM (ed) Differentiation patterns in higher plants Academic Press, New York, pp 115–127
Stebbins GL (1950) Variation and evolution in plants. Columbia University Press, New York
Stebbins GL (1971) Chromosomal evolution in higher plants. Arnold, London
Uyeno T, Smith GR (1972) Tetraploid origin of the karyotype of Catostomid fishes. Science 175:644–646
Waring GL, cobb NS (1992) The impact of plant stress on herbivore population dynamics In: Bernays EA (ed) Insect-plant interactions. CRC Press, Boca Raton, pp 167–226
Wendel JF, Stewart JM, Rettig JH (1991) Molecular evidence for homoploid reticulate evolution among Australian species of Gossypium. Evolution 45:694–711
White TCR (1969) An index to measure weather-induced stress of trees associated with outbreaks of psyllids in Australia. Ecology 50:905–909
White TCR (1976) Weather, food and plagues of locusts. Oecologia 22:119–134
White TCR (1984) The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63:90–105
Whitham TG (1989) Plant hybrid zones as sinks for pests. Science 244:1490–1493
Whitham TG, Morrow PA, Potts BM (1991) Conservation of hybrid plants. Science 254:779–780
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Whitham, T.G., Morrow, P.A. & Potts, B.M. Plant hybrid zones as centers of biodiversity: the herbivore community of two endemic Tasmanian eucalypts. Oecologia 97, 481–490 (1994). https://doi.org/10.1007/BF00325886
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00325886