Abstract
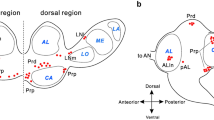
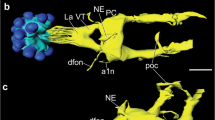
Input regions of pars intercerebralis (PI) neurons are examined by confocal and electron microscopies with special reference to their connections with neurons immunoreactive for pigment-dispersing factor (PDF) in the blow fly, Protophormia terraenovae. PI neurons are a prerequisite for ovarian development under long-day conditions. Backfills from the cardiac recurrent nerve after severance of the posterior lateral tracts labeled thin fibers derived from the PI neurons in the superior medial protocerebrum. These PI fibers were mainly synapsin-negative and postsynaptic to unknown varicose profiles containing dense-core vesicles. Backfilled fibers in the periesophageal neuropils, derived from the PI neurons or neurons with somata in the subesophageal zone, were varicose and some were synapsin-positive. Electron microscopy revealed the presence of both presynaptic and postsynaptic sites in backfilled fibers in the periesophageal neuropils. Many PDF-immunoreactive varicosities were found in the superior medial and lateral protocerebrum and double-labeling showed that 60–88 % of PDF-immunoreactive varicosities were also synapsin-immunoreactive. Double-labeling with the backfills and PDF immunocytochemistry showed that the PI fibers and PDF-immunoreactive varicosities were located close to each other in the superior medial protocerebrum. Results of triple-labeling of PI neurons, PDF-immunoreactive neurons and synapsin-immunoreactive terminals demonstrated that the synapsin-positive PDF-immunoreactive varicosities contacted the PI fibers. These data suggest that PI neurons receive synaptic contacts from PDF-immunoreactive fibers, which are derived from circadian clock neurons, of small ventral lateral neurons (previously called OL2) or posterior dorsal (PD) neurons with somata in the pars lateralis.





Similar content being viewed by others
References
Agnati LF, Fuxe K, Zoli M, Ozoni I, Toffano G, Ferraguti F (1986) A correlation analysis of the regional distribution of central enkephalin and b-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand 128:201–207
Beck SD (1980) Insect photoperiodism, 2nd edn. Academic Press, New York
Birse RT, Söderberg JAE, Luo J, Winther ÅME, Nässel DR (2011) Regulation of insulin-producing cells in the adult Drosophila brain via the tachykinin peptide receptor DTKR. J Exp Biol 214:4201–4208
Broughton SJ, Piper MDW, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, Partridge L (2005) Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A 102:3105–3110
Cavanaugh DJ, Geratowski JD, Wooltorton JRA, Spaethling JM, Hector CE, Zheng X, Johnson EC, Eberwine JH, Sehgal A (2014) Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell 157:689–701
Crocker A, Shahidullah M, Levitan IB, Sehgal A (2010) Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65:670–681
Davis NT (1985) Serotonin-immunoreactive visceral nerves and neurohemal system in the cockroach Periplaneta americana (L.). Cell Tissue Res 240:593–600
de Velasco B, Erclik T, Shy D, Sclafani J, Lipshitz H, Mclnnes R, Hartenstein V (2007) Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Dev Biol 302:309–323
Hamanaka Y, Numata H, Shiga S (2004) Morphology and electrophysiological properties of neurons projecting to the retrocerebral complex in the blow fly, Protophormia terraenovae. Cell Tissue Res 318:403–418
Hamanaka Y, Yasuyama K, Numata H, Shiga S (2005) Synaptic connections between pigment-dispersing factor-immunoreactive neurons and neurons in the pars lateralis of the blow fly Protophormia terraenovae. J Comp Neurol 491:390–399
Hamanaka Y, Tanaka S, Numata H, Shiga S (2007) Peptide immunocytochemistry of neurons projecting to the retrocerebral complex in the blow fly, Protophormia terraenovae. Cell Tissue Res 329:581–593
Helfrich-Förster C, Homberg U (1993) Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol 337:177–190
Hermann-Luibl C, Helfrich-Förster C (2015) Clock network in Drosophila. Curr Opin Insect Sci 7:1–6
Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, Keshishian H, Restifo LL, Rössler W, Simpson JH, Strausfeld NJ, Strauss R, Vosshall LB, Insect Brain Name Working Group (2014) A systematic nomenclature for the insect brain. Neuron 81:755–765
Kaneko M, Hall JC (2000) Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 422:66–94
Kono Y (1975) Daily changes of neurosecretory type-II cell structure of Pieris larvae entrained by short and long days. J Insect Physiol 21:249–264
Lear BC, MerrillCE LJ-M, SchroederA ZL, Allada R (2005) A G protein-coupled receptor, groom-of PDF, is required for PDF neuron action in circadian behavior. Neuron 48:221–227
Leitinger G, Pabst MS, Kral K (1999) Serotonin-immunoreactive neurones in the visual system of the praying mantis: an immunohistochemical, confocal laser scanning and electron microscopic study. Brain Res 823:11–23
Luo J, Lushchak OV, Goergen P, Williams MJ, Nässel DR (2014) Drosophila insulin-producing cells are differentially modulate by serotonin and octopamine receptors and affect social behavior. Plos One 9:e99732
Meinertzhagen IA, O’Neil SD (1991) Synaptic organization of columnar elements in the lamina of the wild-type in Drosophila melanogaster. J Comp Neurol 305:232–263
Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH (2005) PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 48:213–219
Nässel DR, Shiga S, Mohrherr CJ, Rao KR (1993) Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: immunocytochemistry and partial characterization. J Comp Neurol 331:183–198
Numata H, Shiga S (1995) Induction of adult diapause by photoperiod and temperature in Protophormia terraenovae (Diptera: Calliphoridae) in Japan. Environ Entomol 24:1633–1636
Raabe M (1989) Recent developments in insect neurohormones. Plenum, New York
Renn SCP, Park JH, Rosbash M, Hall JC, Taghertet PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99:791–802
Rulifson EJ, Kim SK, Nussel R (2002) Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296:1118–1120
Sakai T, Watanabe K, Ohashi H, Sato S, Inami S, Shimada N, Kitamoto T (2014) Insulin-producing cells regulate the sexual receptivity through the painless TRP channel in Drosophila virgin females. Plos One 9:e88175
Saunders DS (1981) Insect photoperiodism—the clock and the counter: a review. Physiol Entomol 6:99–116
Saunders DS (2002) Insect clocks, 3rd edn. Elsevier, Amsterdam
Shafer OT, Yao Z (2014) Pigment-dispersing factor signaling and circadian rhythms in insect locomotor activity. Curr Opin Insect Sci 1:73–80
Shiga S, Numata H (2000) The role of neurosecretory neurons in the pars intercerebralis and pars lateralis in reproductive diapause of the blowfly, Protophormia terraenovae. Naturwissenschaften 87:125–128
Shiga S, Numata H (2009) Roles of PER immunoreactive neurons in circadian rhythms and photoperiodism in the blow fly, Protophormia terraenovae. J Exp Biol 212:867–877
Shiga S, Toyoda I, Numata H (2000) Neurons projecting to the retrocerebral complex of the adult blow fly, Protophormia terraenovae. Cell Tissue Res 299:427–439
Yasuyama K, Okada Y, Hamanaka Y, Shiga S (2006) Synaptic connections between eyelet photoreceptors and pigment dispersing factor-immunoreactive neurons of the blowfly Protophormia terraenovae. J Comp Neurol 494:331–344
Acknowledgments
We thank Kazuko Yamane of the Kawasaki Medical School for expert help with confocal microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a Grant-in Aid for Scientific Research (B) 24370032 by the Japan Society for Promotion of Science to K.Y. and S.S.
Rights and permissions
About this article
Cite this article
Yasuyama, K., Hase, H. & Shiga, S. Neuroanatomy of pars intercerebralis neurons with special reference to their connections with neurons immunoreactive for pigment-dispersing factor in the blow fly Protophormia terraenovae . Cell Tissue Res 362, 33–43 (2015). https://doi.org/10.1007/s00441-015-2192-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2192-x