Abstract
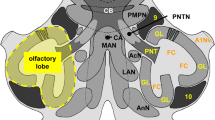
We reveal the neuroanatomy of the optic ganglia and central brain in the water flea Daphnia magna by use of classical neuroanatomical techniques such as semi-thin sectioning and neuronal backfilling, as well as immunohistochemical markers for synapsins, various neuropeptides and the neurotransmitter histamine. We provide structural details of distinct neuropiles, tracts and commissures, many of which were previously undescribed. We analyse morphological details of most neuron types, which allow for unravelling the connectivities between various substructural parts of the optic ganglia and the central brain and of ascending and descending connections with the ventral nerve cord. We identify 5 allatostatin-A-like, 13 FMRFamide-like and 5 tachykinin-like neuropeptidergic neuron types and 6 histamine-immunoreactive neuron types. In addition, novel aspects of several known pigment-dispersing hormone-immunoreactive neurons are re-examined. We analyse primary and putative secondary olfactory pathways and neuronal elements of the water flea central complex, which displays both insect- and decapod crustacean-like features, such as the protocerebral bridge, central body and lateral accessory lobes. Phylogenetic aspects based upon structural comparisons are discussed as well as functional implications envisaging more specific future analyses of ecotoxicological and endocrine disrupting environmental chemicals.












Similar content being viewed by others
References
Andrew DR, Brown SM, Strausfeld NJ (2012) The minute brain of the copepod Tigriopus californicus supports a complex ancestral ground pattern of the tetraconate cerebral nervous systems. J Comp Neurol 520:3446–3470
Aramant R, Elofsson R (1976) Distribution of monoaminergic neurons in the nervous system of non- malacostracan crustaceans. Cell Tissue Res 166:1–24
Binder G (1932) Das Muskelsystem von Daphnia. Int Rev Ges Hydrobiol Hydrograph 26:54–111
Blaustein DN, Derby CD, Simmons RB, Beall AC (1988) Structure of the brain and medulla terminalis of the spiny lobster Panulirus argus and the crayfish Procambarus clarkii, with an emphasis on olfactory centers. J Crustacean Biol 8:493–519
Boyan G, Williams L, Liu Y (2015) Conserved patterns of axogenesis in the panarthropod brain. Arthropod Struct Dev 44:101–112
Callaway JC, Stuart AE (1989) Biochemical and physiological evidence that histamine is the transmitter of barnacle photoreceptors. Vis Neurosci 3:311–325
Claus C (1876) Zur kenntnis der organisation und des feineren baues der daphniden und verwandter cladoceren. Z Wiss Zool 27:362–402
Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, Bauer DJ, Caceres CE, Carmel L, Casola C, Choi JH, Detter JC, Dong Q, Dusheyko S, Eads BD, Fröhlich T, Geiler-Samerotte KA, Gerlach D, Hatcher P, Jogdeo S, Krijgsveld J, Kriventseva EV, Kultz D, Laforsch C, Lindquist E, Lopez J, Manak JR, Muller J, Pangilinan J, Patwardhan RP, Pitluck S, Pritham EJ, Rechtsteiner A, Rho M, Rogozin IB, Sakarya O, Salamov A, Schaack S, Shapiro H, Shiga Y, Skalitzky C, Smith Z, Souvorov A, Sung W, Tang Z, Tsuchiya D, Tu H, Vos H, Wang M, Wolf YI, Yamagata H, Yamada T, Ye Y, Shaw JR, Andrews J, Crease TJ, Tang H, Lucas SM, Robertson HM, Bork P, Koonin EV, Zdobnov EM, Grigoriev IV, Lynch M, Boore JL (2011) The ecoresponsive genome of Daphnia pulex. Science 331:555–561
Consi TR, Macagno ER, Necles N (1987) The oculomotor system of Daphnia magna. the eye muscles and their motor neurons. Cell Tissue Res 247:515–523
Cunnington WA (1903) Studien an einer Daphnide, Simocephalus sima. Beiträge zur Kenntnis des Centralnervensystems und der feineren Anatomie der Daphniden. Jena Z Naturwiss 37:447–520
Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP (1987) The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: Identification by an antiserum against synthetic PDH. Cell Tissue Res 250:377–387
Dircksen H, Neupert S, Predel R, Verleyen P, Huybrechts J, Strauss J, et al. (2011) Genomics, transcriptomics, and peptidomics of Daphnia pulex neuropeptides and protein hormones. J Proteome Res 10:4478–4504
Downing AC (1974) The hydraulic suspension of the Daphnia eye - a new kind of universal joint? Vision Res 14:647–652
Elofsson R (2006) The frontal eyes of crustaceans. Arthropod Struct Dev 35:275–291
Elofsson R, Dahl E (1970) The optic neuropiles and chiasmata of Crustacea. Z Zellforsch 107:343–360
Elofsson R, Nässel D, Myhrberg H (1977) A catecholaminergic neuron connecting the first two optic neuropiles (lamina ganglionaris and medulla externa) of the crayfish Pacifastacus leniusculus. Cell Tissue Res 182:287–297
Eriksson BJ, Ungerer P, Stollewerk A (2013) The function of Notch signalling in segment formation in the crustacean Daphnia magna (Branchiopoda). Dev Biol 383:321–330
Flaster MS, Macagno ER (1984) Cellular interactions and pattern formation in the visual system of the branchiopod crustacean, Daphnia magna. III. the relationship between cell birth dates and cell fates in the optic lamina. J Neurosci 4:1486–1498
Fritsch M, Richter S (2010) The formation of the nervous system during larval development in Triops cancriformis (Bosc) (Crustacea, Branchiopoda): an immunohistochemical survey. J Morphol 271:1457–1481
Fritsch M, Richter S (2012) Nervous system development in Spinicaudata and Cyclestherida (Crustacea, Branchiopoda)--comparing two different modes of indirect development by using an event pairing approach. J Morphol 273:672–695
Fritsch M, Bininda-Emonds O, Richter S (2013) Unraveling the origin of Cladocera by identifying heterochrony in the developmental sequences of Branchiopoda. Front Zool 10:35
Gicklhorn J (1931) Beobachtungen an den lateralen Frontalorganen von Daphnia magna M. nach elektiver Vitalfärbung. Protoplasma 13:725–739
Gupta N, Stopfer M (2012) Functional analysis of a higher olfactory center, the lateral horn. J Neurosci 32:8138–8148
Halcrow K (1969) Sites of presumed neurosecretory activity in Daphnia magna Straus. Can J Zool 47:575–577
Hallberg E, Johansson KU, Elofsson R (1992) The aesthetasc concept: structural variations of putative olfactory receptor cell complexes in Crustacea. Microsc Res Tech 22:325–335
Hanström B (1928) Vergleichende Anatomie des Nervensystems der wirbellosen Tiere. Springer, Berlin
Harzsch S (2002) The phylogenetic significance of crustacean optic neuropils and chiasmata: a re-examination. J Comp Neurol 453:10–21
Harzsch S (2006) Neurophylogeny: architecture of the nervous system and a fresh view on arthropod phyologeny. Integr Comp Biol 46:162–194
Harzsch S, Dawirs RR (1996) Development of neurons exhibiting FMRFamide-related immunoreactivity in the central nervous system of larvae of the spider crab Hyas araneus L. (Decapoda: Majidae). J Crustacean Biol 16:10–19
Harzsch S, Glötzner J (2002) An immunohistochemical study of structure and development of the nervous system in the brine shrimp Artemia salina Linnaeus, 1758 (Branchiopoda, Anostraca) with remarks on the evolution of the arthropod brain. Arthropod Struct Dev 30:251–270
Harzsch S, Hansson BS (2008) Brain architecture in the terrestrial hermit crab Coenobita clypeatus (Anomura, Coenobitidae), a crustacean with a good aerial sense of smell. BMC Neurosci 9:58
Harzsch S, Walossek D (2001) Neurogenesis in the developing visual system of the branchiopod crustacean Triops longicaudatus (LeConte, 1846): corresponding patterns of compound-eye formation in Crustacea and Insecta? Dev Genes Evol 211:37–43
Harzsch S, Dircksen H, Beltz BS (2009) Development of pigment-dispersing hormone-immunoreactive neurons in the American lobster: homology to the insect circadian pacemaker system? Cell Tissue Res 335:417–429
Harzsch S, Sandeman D, Chaigneau J (2012) Morphology and development of the central nervous system. In: Forest J, von Vaupel Klein JC (eds) Treatise on zoology – anatomy, taxonomy, biology. the crustacea, vol 3. Brill, Leiden, pp 9–236
Hausen K (1993) Decoding of retinal image flow in insects. In: Miles FA, Wallman J (eds) Visual motion and its role in the stabilization of gaze. Elsevier, Amsterdam, pp 203–235
Helfrich-Förster C (2009) Neuropeptide PDF plays multiple roles in the circadian clock of Drosophila melanogaster. Sleep Biol Rhythms 7:130–143
Helfrich-Förster C, Homberg U (1993) Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol 337:177–190
Homberg U (2008) Evolution of the central complex in the arthropod brain with respect to the visual system. Arthropod Struct Dev 37:347–362
Homberg U, Hildebrand JG (1991) Histamine-immunoreactive neurons in the midbrain and suboesophageal ganglion of sphinx moth Manduca sexta. J Comp Neurol 307:647–657
Homberg U, Würden S, Dircksen H, Rao KR (1991) Comparative anatomy of pigment-dispersing hormone- immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res 266:343–357
Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR Jr, Luo L (2007) Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell 128:1187–1203
Kenning M, Müller C, Wirkner CS, Harzsch S (2013) The Malacostraca (Crustacea) from a neurophylogenetic perspective: new insights from brain architecture in Nebalia herbstii Leach, 1814 (Leptostraca, Phyllocarida). Zool Anz 252:319–336
Kirsch R, Richter S (2007) The nervous system of Leptodora kindtii (Branchiopoda, Cladocera) surveyed with confocal scanning microscopy (cLSM), including general remarks on the branchiopod neuromorphological ground pattern. Arthropod Struct Dev 36:143–156
Klagges BR, Heimbeck G, Godenschwege TA, Hofbauer A, Pflugfelder GO, Reifegerste R, Reisch D, Schaupp M, Buchner S, Buchner E (1996) Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J Neurosci 16:3154–3165
Krieger J, Sandeman RE, Sandeman DC, Hansson BS, Harzsch S (2010) Brain architecture of the largest living land arthropod, the Giant Robber Crab Birgus latro (Crustacea, Anomura, Coenobitidae): evidence for a prominent central olfactory pathway? Front Zool 7:25
LeBlanc GA (2007) Crustacean endocrine toxicology: a review. Ecotoxicology 16:61–81
Leder H (1915) Untersuchungen über den feineren Bau des Nervensystems der Cladoceren. Arb Zool Inst Univ Wien Triest 20:297–392
Leydig F (1860) Naturgeschichte der Daphniden. Laupp & Siebeck Verlag, Tübingen
Loesel R, Homberg U (1999) Histamine-immunoreactive neurons in the brain of the cockroach Leucophaea maderae. Brain Res 842:408–418
Loesel R, Nässel DR, Strausfeld NJ (2002) Common design in a unique midline neuropil in the brains of arthropods. Arthropod Struct Dev 31:77–91
Loesel R, Wolf H, Kenning M, Harzsch S, Sombke A (2013) Architectural principles and evolution of the arthropod central nervous system. In: Minelli A, Boxshall G, Fusco G (eds) Arthropod biology and evolution: molecules, development, morphology. Springer, Berlin, pp 299–342
Lopresti V, Macagno ER, Levinthal C (1973) Structure and development of neuronal connections in isogenic organisms: cellular interactions in the development of the optic lamina of Daphnia. Proc Natl Acad Sci U S A 70:433–437
Lopresti V, Macagno ER, Levinthal C (1974) Structure and development of neuronal connections in isogenic organisms: transient gap junctions between growing optic axons and lamina neuroblasts. Proc Natl Acad Sci U S A 71:1098–1102
Lundquist CT, Clottens FL, Holman GM, Riehm JP, Bonkale W, Nässel DR (1994) Locustatachykinin immunoreactivity in the blowfly central nervous system and intestine. J Comp Neurol 341:225–240
Macagno ER (1979) Cellular interactions and pattern formation in the development of the visual system of Daphnia magna (Crustacea, Branchiopoda). I. Interactions between embryonic retinular fibers and laminar neurons. Dev Biol 73:206–238
Macagno ER (1981) Cellular interactions and pattern formation in the development of the visual system of Daphnia magna (Crustacea, Branchiopoda). II. Induced retardation of optic axon ingrowth results in a delay in laminar neuron differentiation. J Neurosci 1:945–955
Macagno E (1984) Formation of ordered connections in the visual system of Daphnia magna. Bioscience 34:308–312
Macagno ER, Levinthal C (1973) Synaptic organization of visual complex of Daphnia magna. J Gen Physiol 61:265–265
Macagno ER, Lopresti V, Levinthal C (1973) Structure and development of neuronal connections in isogenic organisms: variations and similarities in the optic system of Daphnia magna. Proc Natl Acad Sci U S A 70:57–61
Mangerich S, Keller R (1988) Localization of pigment-dispersing hormone (PDH) immunoreactivity in the central nervous system of Carcinus maenas and Orconectes limosus (Crustacea), with reference to FMRFamide immunoreactivity in O. limosus. Cell Tissue Res 253:199–208
Mangerich S, Keller R, Dircksen H, Rao KR, Riehm JP (1987) Immunocytochemical localization of pigment-dispersing hormone (PDH) and its coexistence with FMRFamide-immunoreactive material in the eyestalks of the decapod crustaceans Carcinus maenas and Orconectes limosus. Cell Tissue Res 250:365–375
McCoole MD, Baer KN, Christie AE (2011) Histaminergic signaling in the central nervous system of Daphnia and a role for it in the control of phototactic behavior. J Exp Biol 214:1773–1782
Mellon D Jr (2000) Convergence of multimodal sensory input onto higher-level neurons of the crayfish olfactory pathway. J Neurophysiol 84:3043–3055
Mellon D Jr (2007) Combining dissimilar senses: central processing of hydrodynamic and chemosensory inputs in aquatic crustaceans. Biol Bull 213:1–11
Mittmann B, Ungerer P, Klann M, Stollewerk A, Wolff C (2014) Development and staging of the water flea Daphnia magna (Straus, 1820; Cladocera, Daphniidae) based on morphological landmarks. EvoDevo 5:12
Müller M, Homberg U, Kühn A (1997) Neuroarchitecture of the lower division of the central body in the brain of the locust (Schistocerca gregaria). Cell Tissue Res 288:159–176
Nässel DR (1999) Histamine in the brain of insects: a review. Microsc Res Tech 44:121–136
Nässel DR, Elekes K (1992) Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res 267:147–167
Nässel DR, Elofsson R, Odselius R (1978) Neural connectivity patterns in the compound eyes of Artemia salina and Daphnia magna. Cell Tissue Res 190:435–457
Nässel DR, Shiga S, Mohrherr CJ, Rao KR (1993) Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: immunocytochemistry and partial characterization. J Comp Neurol 331:183–198
Nilsson DE, Osorio D (1998) Homology and parallelism in arthropod sensory processing. In: Fortey RA, Thomas RH (eds) Arthropod relationships. The Systematics Association Special Volume Series, vol 55. Springer, Dordrecht, pp 333–347
Olesen J, Martin JW, Roessler EW (1996) External morphology of the male of Cyclestheria hislopi (Baird, 1859) (Crustacea, Branchiopoda, Spinicaudata), with a comparison of male claspers among the Conchostraca and Cladocera and its bearing on phylogeny of the ‘bivalved’ Branchiopoda. Zool Scripta 25:291–316
Polanska MA, Yasuda A, Harzsch S (2007) Immunolocalisation of crustacean-SIFamide in the median brain and eyestalk neuropils of the marbled crayfish. Cell Tissue Res 330:331–344
Polanska M, Tuchina O, Agricola H, Hansson B, Harzsch S (2012) Neuropeptide complexity in the crustacean central olfactory pathway: immunolocalization of A-type allatostatins and RFamide-like peptides in the brain of a terrestrial hermit crab. Mol Brain 5:1–17
Rádl E (1912) Neue Lehre vom zentralen Nervensystem. Engelmann, Leipzig
Retzius G (1906) Zur Kenntnis des Nervensystems der Daphniden. Biol Unters N F 13:107–116
Richter S, Loesel R, Purschke G, Schmidt-Rhaesa A, Scholtz G, Stach T, Vogt L, Wanninger A, Brenneis G, Doring C, Faller S, Fritsch M, Grobe P, Heuer C, Kaul S, Moller O, Müller C, Rothe B, Stegner M, Harzsch S (2010) Invertebrate neurophylogeny: suggested terms and definitions for a neuroanatomical glossary. Front Zool 29:1–49
Samassa P (1891) Untersuchungen über das centrale Nervensystem der Cladoceren. Arch Mikrosk Anat 38:100–141
Sandeman D, Mellon D Jr (2002) Olfactory centers in the brain of freshwater crayfish. In: Wiese K (ed) The crustacean nervous system. Springer, Berlin, pp 386–404
Sandeman DC, Scholtz G (1995) Ground plans, evolutionary changes and homologies in crustacean brains. In: Breidbach O, Kutsch W (eds) The nervous systems of invertebrates: an evolutionary and comparative approach. Birkhäuser, Basel, pp 329–347
Sandeman D, Sandeman R, Derby C, Schmidt M (1992) Morphology of the brain of crayfish, crabs, and spiny lobsters - a common nomenclature for homologous structures. Biol Bull 183:304–326
Sandeman DC, Scholtz G, Sandeman RE (1993) Brain evolution in decapod Crustacea. J Exp Zool 265:112–133
Sandeman D, Kenning M, Harzsch S (2014) Adaptive trends in malacostracan brain form and function related to behaviour. In: Derby C, Thiel M (eds) Crustacean nervous system and their control of behaviour. The natural history of the Crustacea, vol 3. Oxford University Press, Oxford, pp 11–48
Schachtner J, Schmidt M, Homberg U (2005) Organization and evolutionary trends of primary olfactory brain centers in Tetraconata (Crustacea + Hexapoda). Arthropod Struct Dev 34:257–299
Schmidt M, Ache BW (1994) Descending neurons with dopamine-like or with substance P/FMRFamide-like immunoreactivity target the somata of olfactory interneurons in the brain of the spiny lobster, Panulirus argus. Cell Tissue Res 278:337–352
Schmidt M, Mellon D Jr (2011) Neuronal processing of chemical information in crustaceans. In: Breithaupt TMT (ed) Chemical communication in crustaceans. Springer, New York, pp 123–147
Schnell B, Joesch M, Forstner F, Raghu SV, Otsuna H, Ito K, Borst A, Reiff DF (2010) Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J Neurophysiol 103:1646–1657
Seda J, Petrusek A (2011) Daphnia as a model organism in limnology and aquatic biology: some aspects of its reproduction and development. J Limnol 70:337–344
Sims SJ, Macagno ER (1985) Computer reconstruction of all the neurons in the optic ganglion of Daphnia magna. J Comp Neurol 233:12–29
Sinakevitch I, Douglass JK, Scholtz G, Loesel R, Strausfeld NJ (2003) Conserved and convergent organization in the optic lobes of insects and isopods, with reference to other crustacean taxa. J Comp Neurol 467:150–172
Smith KC, Macagno ER (1990) UV photoreceptors in the compound eye of Daphnia magna (Crustacea, Branchiopoda). a fourth spectral class in single ommatidia. J Comp Physiol A 166:597–606
Sombke A, Harzsch S (2015) Immunolocalization of histamine in the optic neuropils of Scutigera coleoptrata (Myriapoda: Chilopoda) reveals the basal organization of visual systems in Mandibulata. Neurosci Lett 594:111–116
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Stegner MEJ, Fritsch M, Richter S (2014) The central complex in Crustacea. In: Wägele JW, Bartolomaeus T (eds) Deep metazoan phylogeny: the backbone of the tree of life. De Gruyter, Berlin, pp 361–384
Sterba G (1957) Die neurosekretorischen Zellgruppen einiger Cladoceren (Daphnia pulex und magna, Simocephalus vetulus). Zool Jahrb Anat 76:303–310
Stocker RF, Lienhard MC, Borst A, Fischbach KF (1990) Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res 262:9–34
Stollewerk A (2010) The water flea Daphnia - a ‘new’ model system for ecology and evolution? J Biol 9:21
Strausfeld NJ (2005) The evolution of crustacean and insect optic lobes and the origins of chiasmata. Arthropod Struct Dev 34:235–256
Strausfeld NJ (2009) Brain organization and the origin of insects: an assessment. Proc R Soc Lond B 276:1929–1937
Strausfeld NJ (2012) Arthropod brains - evolution, functional elegance, and historical significance. Belknap Press, Cambridge
Strausfeld NJ, Andrew DR (2011) A new view of insect-crustacean relationships I. Inferences from neural cladistics and comparative neuroanatomy. Arthropod Struct Dev 40:276–288
Strausfeld NJ, Nässel DR (1980) Neuroarchitecture of brain regions that subserve the compound eyes of Crustacea and insects. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6b: comparative physiology and evolution of vision in invertebrates. Springer, Berlin, pp 1–132
Strauß J, Dircksen H (2010) Circadian clocks in crustaceans: identified neuronal and cellular systems. Front Biosci 15:1040–1074
Strauß J, Zhang Q, Verleyen P, Huybrechts J, Neupert S, Predel R, Pauwels K, Dircksen H (2011) Pigment-dispersing hormone in Daphnia interneurons, one type homologous to insect clock neurons displaying circadian rhythmicity. Cell Mol Life Sci 68:3403–3423
Sullivan JM, Beltz BS (2001a) Development and connectivity of olfactory pathways in the brain of the lobster Homarus americanus. J Comp Neurol 441:23–43
Sullivan JM, Beltz BS (2001b) Neural pathways connecting the deutocerebrum and lateral protocerebrum in the brains of decapod crustaceans. J Comp Neurol 441:9–22
Sullivan JM, Genco MC, Marlow ED, Benton JL, Beltz BS, Sandeman DC (2009) Brain photoreceptor pathways contributing to circadian rhythmicity in crayfish. Chronobiol Int 26:1136–1168
Tanaka NK, Awasaki T, Shimada T, Ito K (2004) Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol 14:449–457
Tatarazako N, Oda S (2007) The water flea Daphnia magna (Crustacea, Cladocera) as a test species for screening and evaluation of chemicals with endocrine disrupting effects on crustaceans. Ecotoxicology 16:197–203
Threlkeld ST (1979) Estimating cladoceran birth rates: the importance of egg mortality and the egg age distribution. Limnol Oceanogr 24:601–612
Ungerer P, Eriksson BJ, Stollewerk A (2011) Neurogenesis in the water flea Daphnia magna (Crustacea, Branchiopoda) suggests different mechanisms of neuroblast formation in insects and crustaceans. Dev Biol 357:42–52
Utting M, Agricola HJ, Sandeman R, Sandeman D (2000) Central complex in the brain of crayfish and its possible homology with that of insects. J Comp Neurol 416:245–261
van den Bosch de Aguilar P (1969) New morphological data and hypotheses on the role of the neurosecretory system in Daphnia pulex (Crustacea: Cladocera). Ann Soc R Zool Belg 99:27–44
van den Bosch de Aguilar P (1971) The neurosecretory system of Podon intermedius (Crustacea: Cladocera). Ann Soc R Zool Belg 101:57–63
van den Bosch de Aguilar P (1972) Les caractéristiques tinctoriales des cellules neurosécrétrices chez Daphnia pulex (Crustacea: Cladocera). Gen Comp Endocrinol 18:140–145
van den Bosch de Aguilar P (1979) Neurosecretion in the entomostracan crustaceans. La Cellule 73:1–21
Viallanes H (1893) Etudes histologiques et organologiques sur les organes des sens des animaux articulés. Ann Sci Nat, Série 7 14:405–456
Weiss LC, Tollrian R, Herbert Z, Laforsch C (2012) Morphology of the Daphnia nervous system: a comparative study on Daphnia pulex, Daphnia lumholtzi, and Daphnia longicephala. J Morphol 273:1392–1405
Weiss LC, Laforsch C, Ioannidou I, Herbert Z, Tollrian R (2014) Daphnia longicephala neuropeptides: morphological description of crustacean cardioactive peptide (CCAP) and periviscerokinins in the Ctenodaphnia central nervous system. Neuropeptides 48:287–293
Wildt M, Harzsch S (2002) A new look at an old visual system: structure and development of the compound eyes and optic ganglia of the brine shrimp Artemia salina Linnaeus, 1758 (Branchiopoda, Anostraca). J Neurobiol 52:117–132
Wolff JR, Güldner FH (1970) Über die Ultrastruktur des “Nervus opticus” und des Ganglion opticum I von Daphnia pulex. Z Zellforsch Mikrosk Anat 103:526–543
Wolff G, Harzsch S, Hansson BS, Brown S, Strausfeld N (2012) Neuronal organization of the hemiellipsoid body of the land hermit crab, Coenobita clypeatus: correspondence with the mushroom body ground pattern. J Comp Neurol 520:2824–2846
Yasuda-Kamatani Y, Yasuda A (2006) Characteristic expression patterns of allatostatin-like peptide, FMRFamide-related peptide, orcokinin, tachykinin-related peptide, and SIFamide in the olfactory system of crayfish Procambarus clarkii. J Comp Neurol 496:135–147
Zhang Q, Keller R, Dircksen H (1997) Crustacean hyperglycaemic hormone in the nervous system of the primitive crustacean species Daphnia magna and Artemia salina (Crustacea: Branchiopoda). Cell Tissue Res 287:565–576
Acknowledgments
T.K. gratefully acknowledges receiving a stipend from the Faculty of Natural Sciences, Stockholm University. H.D. is grateful to the Faculty of Natural Sciences, Stockholm University and the Carl-Tryggers Foundation, Stockholm, for support. We thank Prof. Dick R. Nässel, Stockholm University, for providing the Locusta-tachykinin antiserum. The monoclonal antibodies 3C11 (anti SYNORF1)(Klagges et al. 1996) and 5 F10 (anti-Dippu-allatostatin-7, deposited by Barbara Stay) were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Terminology and synonyms of Daphnia and other branchiopodan optic ganglia and brain neuropiles. (DOCX 34.9 kb)
Fig. S1
SYN-ir neuropiles in the central brain of Daphnia magna. The video shows a rotating stack of confocal micrographs depth-coded from the dorsal (red) to ventral (blue) side of the brain. Note especially on the ventral side the inner and outer domains of the protocerebral bridge (PB isd, osd) and the central body and on the dorsal side the anterior and posterior dorsal neuropiles (aDN, pDN) and the lateral and tritocerebral neuropiles (LN, TCN). For more explanations see text and of text Figs. 4–7 (GIF 33370 kb)
Rights and permissions
About this article
Cite this article
Kress, T., Harzsch, S. & Dircksen, H. Neuroanatomy of the optic ganglia and central brain of the water flea Daphnia magna (Crustacea, Cladocera). Cell Tissue Res 363, 649–677 (2016). https://doi.org/10.1007/s00441-015-2279-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2279-4