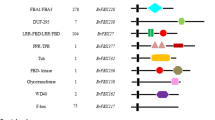
Abstract
Circadian clocks regulate plant growth and development in response to environmental factors. In this function, clocks influence the adaptation of species to changes in location or climate. Circadian-clock genes have been subject of intense study in models such as Arabidopsis thaliana but the results may not necessarily reflect clock functions in species with polyploid genomes, such as Brassica species, that include multiple copies of clock-related genes. The triplicate genome of Brassica rapa retains high sequence-level co-linearity with Arabidopsis genomes. In B. rapa we had previously identified five orthologs of the five known Arabidopsis pseudo-response regulator (PRR) genes that are key regulators of the circadian clock in this species. Three of these B. rapa genes, BrPRR1, BrPPR5, and BrPPR7, are present in two copies each in the B. rapa genome, for a total of eight B. rapa PRR (BrPRR) orthologs. We have now determined sequences and expression characteristics of the eight BrPRR genes and mapped their positions in the B. rapa genome. Although both members of each paralogous pair exhibited the same expression pattern, some variation in their gene structures was apparent. The BrPRR genes are tightly linked to several flowering genes. The knowledge about genome location, copy number variation and structural diversity of these B. rapa clock genes will improve our understanding of clock-related functions in this important crop. This will facilitate the development of Brassica crops for optimal growth in new environments and under changing conditions.






Similar content being viewed by others
References
Acarkan A, Rossberg M, Koch M, Schmidt R (2000) Comparative genome analysis reveals extensive conservation of genome organisation for Arabidopsis thaliana and Capsella rubella. Plant J 23(1):55–62
Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408(6814):796–815
Blanc G, Wolfe KH (2004) Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell 16(7):1679–1691
Blanc G, Barakat A, Guyot R, Cooke R, Delseny M (2000) Extensive duplication and reshuffling in the Arabidopsis genome. Plant Cell 12(7):1093–1101
Bowers JE, Chapman BA, Rong J, Paterson AH (2003) Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422(6930):433–438
Conant GC, Wolfe KH (2008) Turning a hobby into a job: how duplicated genes find new functions. Nat Rev Genet 9:938–950
Conner JA, Conner P, Nasrallah ME, Nasrallah JB (1998) Comparative mapping of the Brassica S locus region and its homeolog in Arabidopsis: implications for the evolution of mating systems in the Brassicaceae. Plant Cell 10(5):801–812
Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AA (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309(5734):630–633
Edger PP, Pires JC (2009) Gene and genome duplications: the impact of dosage-sensitivity on the fate of nuclear genes. Chromosom Res 17(5):699–717
Edwards KD, Anderson PE, Hall A, Salathia NS, Locke JCW, Lynn JR et al (2006) FLOWERING LOCUS C mediates natural variation in the high-temperature response of the Arabidopsis circadian clock. Plant Cell 18(3):639–650
Eriksson ME, Millar AJ (2003) The circadian clock: a plant’s best friend in a spinning world. Plant Physiol 132(2):732–738
Ewing B, Green P (1998) Base-calling of automated sequencer traces using phred: II Error probabilities. Genome Res 8(3):186–194
Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred: I Accuracy assessment. Genome Res 8(3):175–185
Gebhardt C, Walkemeier B, Henselewski H, Barakat A, Delseny M, Stüber K (2003) Comparative mapping between potato (Solanum tuberosum) and Arabidopsis thaliana reveals structurally conserved domains and ancient duplications in the potato genome. Plant J 34(4):529–541
Goldman N, Yang Z (1994) A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol Biol Evol 11(5):725–736
Gómez-Campo C (1999) Biology of Brassica coenospecies. Elsevier, Amsterdam
Gordon D, Abajian C, Green P (1998) Consed: a graphical tool for sequence finishing. Genome Res 8(3):195–202
Grant D, Cregan P, Shoemaker RC (2000) Genome organization in dicots: genome duplication in Arabidopsis and synteny between soybean and Arabidopsis. Proc Natl Acad Sci USA 97(8):4168–4173
Harmer SL (2009) The circadian system in higher plants. Annu Rev Plant Biol 60:357–377
Kim HR, Yang TJ, Kudrna DA, Wing RA (2004) Construction and application of genomic DNA libraries. In: Christou P, Klee H (eds) Handbook of plant biotechnology, vol 1. Wiley, Chichester, pp 71–80
Kim JS, Chung TY, King GJ, Jin M, Yang TJ, Jin YM et al (2006) A sequence-tagged linkage map of Brassica rapa. Genetics 174(1):29–39
Kim JA, Yang TJ, Kim JS, Park JY, Kwon SJ, Lim MH et al (2007) Isolation of circadian-associated genes in Brassica rapa by comparative genomics with Arabidopsis thaliana. Mol Cells 23:145–153
Koch MA, Haubold B, Mitchell-Olds T (2000) Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae). Mol Biol Evol 17(10):1483–1498
Koornneef M, Alonso-Blanco C, Vreugdenhil D (2004) Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol 55:141–172
Kosambi DD (1943) The estimation of map distance from recombination values. Ann Eugen 12:172–175
Kowalski SP, Lan TH, Feldmann KA, Paterson AH (1994) Comparative mapping of Arabidopsis thaliana and Brassica oleracea chromosomes reveals islands of conserved organization. Genetics 138(2):499–510
Ku HM, Vision T, Liu J, Tanksley SD (2000) Comparing sequenced segments of the tomato and Arabidopsis genomes: large-scale duplication followed by selective gene loss creates a network of synteny. Proc Natl Acad Sci USA 97(16):9121–9126
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5(2):150–163
Lagercrantz U, Lydiate DJ (1996) Comparative genome mapping in Brassica. Genetics 144(4):1903–1910
Locke JC, Kozma-Bognár L, Gould PD, Fehér B, Kevei E, Nagy F et al (2006) Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana. Mol Syst Biol 2:59
Lou P, Zhao J, Kim JS, Shen S et al (2007) Quantitative loci for flowering time and morphological traits in multiple populations of Brassica rapa. J Exp Bot 58(14):4005–4016
Lou P, Xie Q, Xu X, Edwards CE, Brock MT, Weinig C, McClung CR (2011) Genetic architecture of the circadian clock and flowering time in Brassica rapa. Theor Appl Genet 123(3):397–409
Lynch M, Conery JS (2000) The evolutionary fate and consequences of duplicate genes. Science 290(5494):1151–1155
Lysak MA, Koch MA, Pecinka A, Schubert I (2005) Chromosome triplication found across the tribe Brassicaceae. Genome Res 15(4):516–525
Maere S, De Bodt S, Raes J, Casneuf T, Van Montagu M, Kuiper M et al (2005) Modeling gene and genome duplications in eukaryotes. Proc Natl Acad Sci USA 102(15):5454–5459
Matsushika A, Makino S, Kojima M, Mizuno T (2000) Circadian waves of expression of the APRR1/TOC1 family of pseudo-response regulators in Arabidopsis thaliana: insight into the plant circadian clock. Plant Cell Physiol 41(9):1002–1012
McClung CR (2010) A modern circadian clock in the common angiosperm ancestor of monocots and eudicots. BMC Biol 8:55
Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM (2005) Integration of flowering signals in winter-annual Arabidopsis. Plant Physiol 137(1):149–156
Millar AJ (2004) Input signals to the plant circadian clock. J Exp Bot 55(395):277–283
Ming R, Hou S, Feng Y, Yu Q, Dionne-Laporte A, Saw JH et al (2008) The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature 452(7190):991–996
Mun JH, Kwon SJ, Yang TJ, Seol YJ, Jin M, Kim JA et al (2009) Genome-wide comparative analysis of the Brassica rapa gene space reveals genome shrinkage and differential loss of duplicated genes after whole genome triplication. Genome Biol 10(10):R111
Mun JH, Kwon SJ, Seol YJ, Kim JA, Jin M, Kim JS et al (2010) Sequence and structure of Brassica rapa chromosome A3. Genome Biol 11(9):R94
Murakami M, Ashikari M, Miura K, Yamashino T, Mizuno T (2003) The evolutionarily conserved OsPRR quintet: rice pseudo-response regulators implicated in circadian rhythm. Plant Cell Physiol 44(11):1229–1236
Murakami M, Matsushika A, Ashikari M, Yamashino T, Mizuno T (2005) Circadian-associated rice pseudo response regulators (OsPRRs): insight into the control of flowering time. Biosci Biotechnol Biochem 69(2):410–414
Murakami M, Tago Y, Yamashino T, Mizuno T (2007) Comparative overviews of clock-associated genes of Arabidopsis thaliana and Oryza sativa. Plant Cell Physiol 48(1):110–121
O’Neill CM, Bancroft I (2000) Comparative physical mapping of segments of the genome of Brassica oleracea var. alboglabra that are homoeologous to sequenced regions of chromosomes 4 and 5 of Arabidopsis thaliana. Plant J 23(2):233–243
Oh DH, Dassanayake M, Haas JS, Kropornika A, Wright C, d’Urzo MP et al (2010) Genome structures and halophyte-specific gene expression of the extremophile Thellungiella parvula in comparison with Thellungiella salsuginea (Thellungiella halophila) and Arabidopsis. Plant Physiol 154(3):1040–1052
Park JY, Koo DH, Park JY, Koo DH, Hong CP, Lee SJ et al (2005) Physical mapping and microsynteny of Brassica rapa ssp. pekinensis genome corresponding to a 222 kbp gene-rich region of Arabidopsis chromosome 4 and partially duplicated on chromosome 5. Mol Genet Genomics 274(6):579–588
Parkin IA, Gulden SM, Sharpe AG, Lukens L, Trick M, Osborn TC (2005) Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana. Genetics 171(2):765–781
Quiros CF, Grellet F, Sadowski J, Suzuki T, Li G, Wroblewski T (2001) Arabidopsis and Brassica comparative genomics: sequence, structure and gene content in the ABI-Rps2-Ck1 chromosomal segment and related regions. Genetics 157(3):1321–1330
Ramírez-Carvajal GA, Morse AM, Davis JM (2008) Transcript profiles of the cytokinin response regulator gene family in Populus imply diverse roles in plant development. New Phytol 177(1):77–89
Sadowski J, Hu J, Delseny M, Quiros CF (1994) Genetical and physical mapping of an Arabidopsis gene complex in Brassica genomes. Cruciferae Newslett 16:47–48
Sadowski J, Gaubier P, Delseny M, Quiros CF (1996) Genetic and physical mapping in Brassica diploid species of a gene cluster defined in Arabidopsis thaliana. Mol Genet Genomics 251(3):298–306
Salathia N, Lynn JR, Millar AJ, King GJ (2007) Detection and resolution of genetic loci affecting circadian period in Brassica oleracea. Theor Appl Genet 114(4):683–692
Salter MG, Franklin KA, Whitelam GC (2003) Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 426(6967):680–683
Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF et al (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288(5471):1613–1616
Schranz ME, Lysak MA, Mitchell-Olds T (2006) The ABC’s of comparative genomics in the Brassicaceae: building blocks of crucifer genomes. Trends Plant Sci 11(11):535–542
Schranz ME, Windsor AJ, Song BH, Lawton-Rauh A, Mitchell-Olds T (2007) Comparative genetic mapping in Boechera stricta, a close relative of Arabidopsis. Plant Physiol 144(1):286–298
Schwartz S, Zhang Z, Frazer KA, Smit A, Riemer C, Bouck J et al (2000) PipMaker—a web server for aligning two genomic DNA sequences. Genome Res 10(4):577–586
Somers DE, Webb AA, Pearson M, Kay SA (1998) The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development 125(3):485–494
Suárez-Lopéz P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410(6832):1116–1120
Takahashi Y, Teshima KM, Yokoi S, Innan H, Shimamoto K (2009) Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc Natl Acad Sci USA 106:4555–4560
Takata N, Saito S, Saito CT, Uemura M (2010) Phylogenetic footprint of the plant clock system in angiosperms: evolutionary processes of pseudo-response regulators. BMC Evol Biol 10:126
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal-W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Tiwari SB, Shen Y, Chang H-C, Hou Y, Harris A, Ma SF, McPartland M, Hymus GJ, Adam L, Marion C et al (2010) The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol 187:57–66
Town CD, Cheung F, Maiti R, Crabtree J, Haas BJ, Wortman JR et al (2006) Comparative genomics of Brassica oleracea and Arabidopsis thaliana reveal gene loss, fragmentation, and dispersal after polyploidy. Plant Cell 18(6):1348–1359
Ueda HR (2006) Systems biology flowering in the plant clock field. Mol Syst Biol 2:60
van Ooijen JW, Voorrips RE (2001) JoinMap Version 3.0: software for the calculation of genetic linkage maps. Plant Research International, Wageningen, The Netherlands
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93(1):77–78
Yang Z (1997) PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13(5):555–556
Yang TJ, Yu Y, Frisch D, Lee S, Kim HR (2004) Construction of various copy number plasmid vectors and their utility for genome sequencing. Genomics Inform 2:174–179
Yang TJ, Kim JS, Lim KB, Kwon SJ, Kim JA, Jin M et al (2005) The Korea Brassica genome project: a glimpse of the Brassica genome based on comparative genome analysis with Arabidopsis. Comp Funct Genomics 6(3):138–146
Yang TJ, Kim JS, Kwon SJ, Lim KB, Choi BS, Kim JA et al (2006) Sequence-level analysis of the diploidization process in the triplicated FLOWERING LOCUS C region of Brassica rapa. Plant Cell 18(6):1339–1347
Zeilinger MN, Farre EM, Taylor SR, Kay SA, Doyle FJ (2006) A novel computational model of the circadian clock in Arabidopsis that incorporates PRR7 and PRR9. Mol Syst Biol 2:58
Zhu HY, Kim DJ, Baek JM, Choi HK, Ellis LC, Kuester H et al (2003) Syntenic relationships between Medicago truncatula and Arabidopsis reveal extensive divergence of genome organization. Plant Physiol 131(3):1018–1026
Acknowledgments
This work was supported by grants from “Research Program for Agricultural Science & Technology Development (Project No. PJ907049)”, National Academy of Agricultural Science and BioGreen 21 Program (project No. PJ008025), the Rural Development Administration, Republic of Korea. We thank Dr. Hans J. Bohnert in University of Illinois for comment about comparison between paralogous genes and editing in English.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by A. Schnittger.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1 The quantitative real-time RT-PCR of BrPRR genes mRNA in B. rapa plants grown under a 16-h day/8-h night photoperiod (A) or for 72 h in the light (B). Plant tissues were collected every 3 h and analyzed for the expression of BrPRR genes by quantitative RT–PCR using the gene-specific probes shown in Table 1. qRT-PCR reaction were performed with 3 technical replication using Bio-Rad system and the SYBR Green I master mix in a volume 20ul.
Supplemental Fig. 2 Comparison of the predicted amino acid sequences of all PRR genes in B. rapa using ClustalW2(http://www.ebi.ac.uk/Tools/msa/clustalw2/) in EMBL-EBI web site. The strictly conserved glutamate (E) and the CCT and PRR domains are indicated.
Supplemental Fig. 3 Comparison of the predicted amino acid sequences of PRR3 and its B. rapa ortholog BrPRR3. The strictly conserved glutamate (E) and the CCT and PRR domains are indicated.
Supplemental Fig. 4 Comparison of the predicted amino acid sequences of PRR5 and its two B. rapa orthologs BrPRR5a and BrPRR5b. The strictly conserved glutamate (E) and the CCT and PRR domains are indicated.
Supplemental Fig. 5 Comparison of the predicted amino acid sequences of PRR7 and its two B. rapa orthologs BrPRR7a and BrPRR7b. The strictly conserved glutamate (E) and the CCT and PRR domains are indicated.
Supplemental Fig. 6 Comparison of the predicted amino acid sequences of PRR9 and its B. rapa ortholog BrPRR9. The strictly conserved glutamate (E) and the CCT and PRR domains are indicated.
Supplemental Fig. 7 Comparison of exon–intron structures of PRR3 (A), PRR5 (B), PRR7 (C), and PRR9 (D) with those of orthologous genes in B. rapa. Lines and boxes indicate introns and exons, respectively. Numbers indicate the start positions of the first exon. Coding regions of PRR genes were respectively aligned with their genomic sequences
Rights and permissions
About this article
Cite this article
Kim, J.A., Kim, J.S., Hong, J.K. et al. Comparative mapping, genomic structure, and expression analysis of eight pseudo-response regulator genes in Brassica rapa . Mol Genet Genomics 287, 373–388 (2012). https://doi.org/10.1007/s00438-012-0682-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-012-0682-z