Abstract
The brain atlas is essential for exploring the anatomical structure and function of the brain. Non-human primates, such as cynomolgus macaque, have received increasing attention due to their genetic similarity to humans. However, current macaque brain atlases only offer coarse sections with intervals along the coronal direction, failing to meet the needs of single-cell resolution studies in functional and multi-omics research of the macaque brain. To address this issue, we utilized fluorescence micro-optical sectioning tomography to obtain sub-micron resolution cytoarchitectonic images of the macaque brain at the sagittal plane. Based on the obtained 8000 image sequences, a reference brain atlas comprising 45 sagittal sections was created, delineating 270 brain regions other than the cortex. Additionally, a website was established to share the reference atlas corresponding image data. This study is expected to provide an essential dataset and tool for scientists studying the macaque brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For a long time, diseases represented by Alzheimer’s disease (AD) and Parkinson’s disease (PD) have imposed substantial medical and caregiving burdens on human society, posing significant challenges in neuroscience research (Atri 2019; Kim et al. 2021; Macchi et al. 2020). Since the genomes are highly similar to those of human beings, non-human primates, particularly macaques, are widely used as model animals to study the disease progression across various brain regions and identify drug target sites (Tarantal et al. 2022; Sato and Sasaki 2018; Qiao et al. 2023). In such studies, the reference brain atlases serve as a vital reference tool, providing crucial spatial localization information of disease lesions and drug targets(Ahmad et al. 2023; Feng et al. 2022).
Numerous macaque brain atlases have been developed, providing valuable spatial references and terminologies (Szabo and Cowan 1984; Martin and Bowden 1996; Paxinos et al. 1999; Saleem and Logothetis 2007; Rushmore et al. 2021;). However, these atlases lack a fine delimitation of the macaque brain on the sagittal plane. Most of them only feature coronal plane images (Bakker et al. 2015). Among the available brain atlases are those illustrated by Martin and Bowden (1996) using traditional histological methods, as well as the rhesus macaque brain atlases by Paxinos and his colleagues (1999), which use AchE immunohistochemical and Nissl staining image data. In contrast, Saleem and Logothetis (2007) utilized a combination of various histological staining methods and magnetic resonance imaging (MRI) techniques to produce a stereotactic brain atlas of rhesus macaques. This atlas consists of images from coronal, horizontal, and sagittal planes. However, its sagittal planes are illustrated based on MRI images, lacking the cytoarchitectonic details required for differentiating fine brain nuclei, thus resulting in rough sagittal annotations.
While atlases consisting of coronal sections have their uses, they are not sufficient for the study of primates’ brains (Calabrese et al. 2015; Reveley et al. 2017; Saleem et al. 2021; Hartig et al. 2021). Lanciego and Vázquez (2012) suggested that the sagittal plane is better suited for studying basal ganglia pathways associated with PD. Additionally, the sagittal plane is crucial for observing and comparing changes in brain structure during development. Thompson et al. (2014) noted that the developing mouse brain atlas uses the sagittal plane, making it easier to compare the longitudinal positions of brain nuclei and their developmental origins. Furthermore, as the axial resolution of the sagittal sections evolved to the single-cell plane, the sagittal reference brain atlases can also complement coronal atlases with equivalent resolution in three-dimensional space, allowing for more precise three-dimensional localization of primate brains.
Here, we utilized the fluorescence Micro-Optical Sectioning Tomography (fMOST) technique along with the propidium iodide (PI) fluorescence staining method to obtain a comprehensive collection of consecutive 8000 sagittal cytoarchitectonic images of the macaque brain with sub-micron resolution(Gong et al. 2016; Zhong et al. 2021; Zhou et al. 2022). By employing these images, we constructed a reference brain atlas for the sagittal plane of the Cynomolgus macaque and created a corresponding interactive website. To the best of our knowledge, this atlas is the first cytoarchitectonic reference atlas of the Cynomolgus macaque’s brain with single-cell resolution on the sagittal plane and is promising to be the cornerstone for further development of micron-resolution three-dimensional macaque brain atlases.
Materials and methods
Sample preparation
A 4-year-old male Cynomolgus Macaque (Macaca fascicularis) living in the Kunming Primate Research Center of the Chinese Academy of Sciences was used for this study. The macaque was raised under standard conditions in an individual cage and fed with standard monkey chow and a daily fruit supplement. The macaque was treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol and animal care protocols were approved by the Ethics Committee of the Kunming Institute of Zoology and the Kunming Primate Research Center (approval No. IACUC18018).
Sample fixation and embedding methods
The macaque was anesthetized with pentobarbital (45 mg/kg, i.m.) and transcardially perfused with 4 L of 4 °C 0.01 mol/L phosphate-buffered saline (PBS) (Sigma Aldrich, St Louis, USA) and 1 L of 4% paraformaldehyde (PFA) powder (Sigma Aldrich). After perfusion, the brain was removed and fixed with 4% PFA. The fixed macaque brain tissue was placed in a prepared hydrogel solution with N-acryloyl Glycinamide (NAGA) and left at 4 °C for 7 days. After complete permeation, the macaque brain was maintained at 40 °C for 4 h for polymerization. In our methods, all sample parameters that we measured were approximately similar after embedding. The relative distances between vascular nodes and neurons did not significantly change after embedding, and the shift between the vascular nodes and neurons was < 5%. All procedures were approved by the Institutional Animal Ethics Committee of Huazhong University of Science and Technology (approval No. S166).
Image acquisition
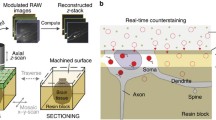
We used consecutive subcellular resolution 3D imaging on the intact macaque brain system (Zhou et al. 2022). As shown in Fig. 1a, a line-scanning confocal microscope was used to scan the surface of the sample soaked in PI solution. With the 10× objective, the system acquired the image with a voxel size of 0.65 × 0.65 × 3 μm. In this imaging mode, the vibratome removes the sample surface with a thickness of 12 μm and acquires the image of four layers with a depth space of 3 μm. This scan-and-remove process were repeated until a set of consecutive sub-micron resolution sagittal sectional images of the macaque half-brain large volume sample was obtained.
Macaque Brain Cytoarchitectonic Atlas methods and website. a illustrates the sample acquisition and imaging acquisition. b shows the original data with three anatomical planes, where the sagittal sections are the original image and the coronal and horizontal sections are re-sliced images. c presents masks of the Putamen(blue) in three orthogonal planes, corresponding to the sagittal plane (red frame), coronal plane (green frame), and horizontal plane (blue frame) depicted in (b). The mask on the sagittal section is manually drawn, while the masks on the coronal and horizontal sections are obtained from re-slicing. d is a schematic of the macaque brain atlas website, featuring a navigation window and a brain structure ontology search bar on the left, a grid-line representing the coordinate behind the atlas and coordinate values in the lower left corner of the page, and the thumbnail image gallery on the right, with the control panel atlas at the bottom of the page, allowing image download, zooming-in, page refresh, atlas switching and users’ feedback. Scale bar: 1 cm (b),100 μm (c)
Image processing
The acquired raw image data was not in the whole section form but in consecutive neighboring strips stored in data packets. Initially, we corrected the lateral illumination of the decompressed strip images by fitting polynomial curves to the average intensity perpendicular to the stripes and determining correction coefficients. Next, we stitched together the stripes of each sagittal section using the known spatial orientation and overlap between adjacent stripes, forming one sagittal image in a 16-bit depth TIFF format using LZW compression. Image preprocessing was implemented using C++, executed in parallel based on the Intel MPI library (v3.2.2.006, Intel, Santa Clara, USA). Image preprocessing for a macaque brain dataset at a voxel size of 0.65 × 0.65 × 3 μm was executed on the computing server (280 cores, 2.4 GHz per core) within 40 d. Image down-sampling and projection were performed in C + + based on the Intel MPI library (v3.2.2.006, Intel, Santa Clara, USA). After image preprocessing, images were transformed into TDat files for further image processing and visualization. As shown in Fig. 1b, down-sampled images could be displayed as three-dimensional data blocks. Coronal and horizontal plane images were obtained by re-slicing, achieving a voxel size of 12 × 12 × 12 μm.
Brain atlasing
We resized the stitched sagittal images to 3.25 × 3.25 μm for creating the atlas. To enhance the visibility of cytoarchitectonic details, we applied techniques such as inverting the gray value, adjusting the image contrast, and adding pseudo-color. We were able to create one image (12 μm-thick) using maximum projection on four consecutive images. For mapping the reference brain atlas, we selected 45 cytoarchitectonic images at every 300 μm interval. As we can see in Fig. 1c, we drew the atlas on the sagittal sections and obtained masks of various nuclei. Furthermore, we resliced the drawn mask into the coronal and horizontal planes for observing and correcting the boundaries of the sagittal plane. Our image enhancement process primarily involved the use of MATLAB 2017a (MathWorks, USA) and Adobe Photoshop 2018 (Adobe, Ireland). The atlas was drawn using Adobe Illustrator 2018 (Adobe Ireland) and saved in SVG format. As depicted in Fig. 1d, the reference brain atlas at the sagittal plane was available for interactive viewing on our website.
Results
High-resolution macaque brain cytoarchitectonic images
We obtained a dataset of consecutive cytoarchitectonic images of the left hemisphere of the cynomolgus macaque brain. This dataset includes a total of 8000 sagittal images, with a planar resolution of 0.65 × 0.65 μm and an axial resolution of 3 μm. Then, we selected 45 sagittal images at 300 μm intervals covering the main subcortical structures. The plates we drew were numbered from Sec-01 to Sec-45.
Macaque Brain Cytoarchitectonic Image Data. a displays original images of Sec-08, Sec-24, and Sec-34. b displays cortical areas 10D, 4 (F1), V2, and EC, cropped from the orange rectangles in (a) and annotated with layer marks. c The orange boxes on the left correspond to the orange rectangles representing MY and AMY in (a), and the right side respectively correspond to the green frames representing V, SUV, LAV, MV and MEA, IMG, BAOT, BMA, and other brain nuclei’s cytoarchitectonic details in the yellow boxes on the left. d blue frames represent the hippocampal complex, which corresponds to the blue dash lines in (a), and the purple frames on the right correspond to the zoom-in images in three anatomical planes, rectangle in (d) and (a). Abbreviations: 10D-area 10 dorsal part, 4(F1)-area 4, V2-visual area 2, EC-entorhinal caudal, MY-medulla, AMY-amygdala, V-Motor nucleus of trigeminal, SUV-Superior vestibular nucleus, LAV-Lateral vestibular nucleus, MV-Medial vestibular nucleus, MEA-Medial amygdalar nucleus, IMG-am intramedul gr, BAOT-bed nucleus of the accessory olfactory tract, BMA-Basomedial amygdalar nucleus. Orientation terms, S-sagittal plane, C-coronal plane, H-horizontal plane. Scale bar:1 cm applies to a and d (blue box), 1 mm applies to b, c (orange box), and d (purple box), and 100 μm applies to c (green box)
We have shown three sagittal section images in Fig. 2a which accurately represent various scales of cytoarchitectonic details. Figure 2b demonstrates the different cytoarchitectonic features for identifying different cortical regions, including 10D, 4 (F1), V2, and EC, which have notable longitudinal distribution differences. Furthermore, the subcortical region contains various structures within the medulla and amygdala regions that feature cells of varying shapes, sizes, and densities, as well as neural fibers and blood vessels that leave traces on the images, serving as bases for dividing brain structures. In cases that identifying brain regions solely by sagittal planes is challenging, we resliced the 12 μm thick isotropic three-dimensional data blocks to obtain coronal or horizontal images, which can help delineate specific brain areas. As illustrated in Fig. 2d, the structures of the Ammon’s horn and dentate gyrus of the hippocampal complex can be observed in all three planes, providing detailed structural features of the hippocampus.
Subcortical structure atlas
We constructed a set of macaque brain atlas with 45 sagittal sections, delineating boundaries of 270 subcortical structures distributed in various brain regions including the basal ganglia, hypothalamus, thalamus, amygdala, midbrain, pons, medulla, and cerebellar nuclei.
Cerebral nuclei
The cerebral nuclei include basal ganglia, basal forebrain, bed nucleus of stria terminalis (BNST), and claustrum, extending from the subcortical region of the frontal lobe, through the medial side of the temporal lobe cortex, and extending laterally to the base of the midbrain. The basal ganglia were differentiated into the striatum, pallidum, subthalamic nucleus (STN), and substantia nigra (SN) based on coherent yet distinguishable fiber bundles indicated by arrows in Fig. 3a–c. The striatum, external and internal pallidum, caudal nucleus and putamen nucleus, lateral and medial pallidum, STN, and SN are separated by fiber tracts, forming quite distinct boundaries between the nuclei. The medial side of the basal forebrain nuclei is relatively dense while the ventral side is sparser and scattered with some larger cells, which we divided as the substantia innominate on the ventral side and the lateral septal nucleus on the medial side. Notably, some small cells’ dense aggregation can be observed in this area, which is referred to as Calleja’s islands (as indicated by arrows in Fig. 3a). The BNST is located between the basal ganglia and basal forebrain. It envelops the anterior commissure and is usually considered an extension of the amygdala. We identified the boundaries of the BNST by the arrangement of cells surrounding the anterior commissure. As shown in Fig. 3f–i, different colors identify the cerebral nuclei, basal forebrain, and BNST.
Cerebral Nuclei and Hypothalamus. a–e depict cytoarchitectonic details of the cerebral nuclei and hypothalamus, corresponding to the regions indicated by the purple boxes in (f–i). Some boundaries are demarcated with black dashed lines, denoting subdivisions within the hypothalamus. f–i display sections of the atlas numbered Sec-01, Sec-03, Sec-05, and Sec-16. Color coding: the background is white, and gray areas represent other regions. Scale bar: 500 μm (a–e), 2 mm (f–i)
Hypothalamus
The hypothalamus is located below the thalamus and is part of the limbic system. It forms the ventral part of the diencephalon. Using the optic chiasm as a landmark, the hypothalamus can be divided into four parts along the anterior-posterior axis: the preoptic area, supraoptic area, tuberal area, and mammillary bodies, as demarcated by dashed lines in Fig. 3d, e. Each of these parts can further be divided along the medial-lateral axis into periventricular, medial, and lateral zones. Within these four sections, the mammillary bodies are primarily identified by the clustering features of cells visible in the images, while other areas are primarily delineated based on their spatial position relative to the optic chiasm. As illustrated in Fig. 3f, g, and h, different colors are used to differentiate the various parts of the hypothalamus.
Thalamus
The thalamus is a large mass of gray matter on the lateral walls of the third ventricle forming the dorsal part of the diencephalon, situating between the cerebral cortex and the midbrain. The thalamus is generally divided into the dorsal thalamus, epithalamus, and geniculate body.
Thalamus. a–e show the cytoarchitectonic details of the thalamus, corresponding to the purple boxes in (f–j). f–j show the thalamic parts of the atlas sections numbered Sec-01, Sec-05, Sec-11, Sec-21, and Sec-25, with black arrows indicating some boundary markers. Colors: white for background, gray for other areas. Scale bar: 500 μm (a–e), 2 mm (f–j)
The dorsal thalamus can be divided into anterior, medial, lateral, posterior, and intralaminar parts. The anterior dorsal thalamus primarily includes the anteroventral nucleus of thalamus (AV), the anteromedial nucleus of thalamus (AM), the anterodorsal nucleus of thalamus (AD), and the lateral dorsal nucleus of thalamus (LD). As shown in Fig. 4a, the boundary between AV and AM is not clear in our images; AD is located dorsally and separated by fiber bundles. LD is located laterally to AD but is not shown in the figure. The medial area of the dorsal thalamus is the mediodorsal nucleus (MD), which can be further divided into dorsal, caudal, and lateral parts based on cellular density differences, as shown in Fig. 4a and b. Additionally, as seen in Fig. 4a and b, there is a band-like area between the medial and lateral areas due to its rich fiber distribution, known as the intralaminar complex, encompassing central lateral nucleus of thalamus, paracentral nucleus, medial division of centromedian nucleus of thalamus, which embraces MD inwardly and is enveloped by the lateral nuclear group. The lateral nucleus group of the dorsal thalamus is more complex and is divided into ventral and dorsal parts, both surrounded by the thalamic reticular nucleus. The ventral part, as shown in Fig. 4c, can be distinguished by its cytoarchitecture from rostral to caudal between ventral anterior-lateral complex of the thalamus (VAL), ventral lateral nucleus of the thalamus (VL), ventral posterior lateral nucleus of the thalamus (VPL), and posterior nuclear complex of the thalamus (PO). Cells are nearly round and relatively dense in VL, markedly different from neighboring areas, with VAL at the rostral, VPL at the caudal, and PO as a transitional area with sparser cells than VPL. The dorsal area is composed of the thalamic pulvinar nucleus (PUL) and lateral complex. Additionally, as shown in Fig. 4d, the internal division of the PUL mainly depends on the direction and number of fiber clusters passing through.
The epithalamus is mainly comprising the internal and external habenula nuclei and the pineal body, extending posteriorly from the top of the thalamus into the third ventricle. The geniculate body is located posteriorly to the lateral area of the dorsal thalamus, below the PUL, mainly divided into medial and lateral geniculate bodies, serving as important relays for auditory and visual pathways, respectively. As shown in Fig. 4e, the medial geniculate body can be further subdivided, with its internal cell arrangement showing a concentric layout around the center, which is not obvious in coronal views. The lateral geniculate body can be observed with a typical layered structure, arranged in a V-shape in six layers in coronal views, but in our sagittal plane images, it appears in a pattern similar to fingerprints. As shown in Fig. 4f–j, we used different colors to identify the various areas of the thalamus.
Brainstem (midbrain, pons, medulla)
Structures of the brainstem are very diverse with respect to functions they participate in, neuroactive elements they contain, and neural pathways they accommodate. As a reflection, the anatomical organization of the brainstem is a complex amalgam of compact neuronal groups and dispersed cell areas with varying cytoarchitecture.
Brainstem and Cerebellum. a–i display cytoarchitectonic details of different parts of the brainstem. a shows the areas around the cerebral aqueduct gray matter and the cap region of the midbrain. b–e respectively showcase the fifth, sixth, and seventh cranial nerve nuclei and the locus coeruleus. f-g display the details of the anterior olivary and vestibular nuclei groups, respectively. h-i respectively show the reticular area details of the midbrain and medulla, located in the purple box shown in (j–m). f–j show the brainstem and berebellum parts of the atlas sections numbered Sec-01, Sec-04, Sec-06, and Sec-08.The backgrounds are white, and gray areas represent other regions. Scale bar: 500 μm (a–i), 2 mm (j–m)
The dorsal brainstem is much more complex than the ventral part. For instance, the midbrain’s dorsal part mainly consists of the cerebral aqueduct gray matter (PAG), superior colliculus (SC), and inferior colliculus (IC), as shown in Fig. 5a, while the ventral part only consists of several nuclei embedded in the reticular formation. The boundary between the PAG and the SC can be recognized based on cell density, the cells in PAG are much denser. The subdivision of layers within the SC can be delineated by the fiber density. The IC can be divided into core and shell parts based on density differences. The dorsal side of the pons from outside to inside contains the parabrachial nucleus, locus coeruleus (LC), and pontine central gray matter. Likewise, the dorsal side of the anterior medulla contains the vestibular nuclei, as shown in Fig. 5b, characterized by giant cells in the lateral vestibular nucleus, with the size of each cell reaching over 50 μm, helping to outline its boundary. The posterior part contains nuclei related to the somatosensory system—the distinctive gracile nucleus, cuneate nucleus, and trigeminal spinal nucleus, which are arranged from the medial to the lateral along the outer edge of the dorsal medulla. In the dorsolateral side of these nuclei, the cochlear nucleus receives auditory signals. These dorsal nuclei groups and the reticular area also contain the visceral input-related solitary nucleus. As shown in Fig. 5c, d, the LC and abducens nucleus(VI) are usually accompanied by fiber bundles and show clear boundaries for their distinctive cell density.
In the brainstem, motor cranial nuclei have a group of projection neurons which are bigger than most cells around, typically located within or at the edges of the reticular formation, as shown in Fig. 5d–f. Similarly, the superior olivary complex (SOC) is also located at the edge of the reticular area. The stratified boundaries of SOC can be clearly observed, as shown in Fig. 5g. The red nucleus, located within the midbrain reticular area, is easily distinguished by typical cell size and density. As shown in Fig. 5h, it can be further divided into the magnocellular part and parvicellular part. These nuclei, along with the imprints of the corresponding cranial nerves, provide important markers for delineating the boundaries of the reticular structures. Additionally, within the reticular area, there are conspicuous clusters of tightly packed neurons in the ambiguous nucleus and linear aggregations of cells in the linear nucleus, which are difficult to observe in coronal sections, as shown in Fig. 5i.
As illustrated in Fig. 5j–m, different colors are used to differentiate the various parts of the midbrain, pons, medulla, and cerebellar nuclei. Bundles of descending nerve fibers serve as a significant feature for identifying the base of the brainstem. For instance, the base of the midbrain is enveloped by the cerebral peduncles surrounding the SN and the ventral tegmental area. The base of the pons primarily consists of expanded pontine gray matter and traversing corticospinal tracts. The base of the medulla features relatively independent structures such as the anterior olivary complex, olivary complex, and lateral reticular nucleus.
Cerebellum
The cerebellum includes the cerebellar cortex and deep cerebellar nuclei. We only simply divide the deep cerebellar nuclei including dentate nucleus, anterior interposed nucleus, posterior interposed nucleus, fastigial nucleus, and basal interstitial nucleus of cerebellum, which are deeply embedded in the cerebellar fiber bundles.
Amygdala
The macaque’s amygdala is located medially within the temporal lobes, enveloped by the piriform cortex and the entorhinal cortex, forming an area known as the semilunar gyrus. The amygdala can be anatomically divided into three main areas, which show significant differences in cellular organization and functional topology connections. As shown in Fig. 6g–j, they are marked in different colors.
The basolateral nucleus group (BLN) in the macaque brain has a cytoarchitectural organization similar to the cerebral cortex, as a region resembling the temporal extension of the claustrum(CLA). Medial to the BLN is the cortical medial nucleus (CMN), dorsal to the BLN is the central and extended amygdala (CEN), and other parts are surrounded by fiber tracts and lateral ventricles. As shown in Fig. 6a–c, BLN primarily includes the lateral nucleus (LA), basolateral nucleus (BLA), accessory basolateral nucleus (PL), and basomedial nucleus(BMA). Generally, LA and BLA as well as BLA and BMA can be well distinguished, as each nucleus in BLN is separated by fiber tracts, and each displays different cytoarchitectonic characteristics. For example, the neurons in LA are smaller and denser, while the neurons in BLA are larger and change from dense at the dorsal side to sparse at the ventral side, and back to dense again at PL. The major part of BMA shows large and sparsely clustered neurons and the accessory nucleus of BMA shows small but dense cells surrounding big cells.
As depicted in Fig. 6a, the CMN includes the anterior cortical nucleus, the posterior ventral cortical nucleus, and the posterior dorsal cortical nucleus, as well as the lateral olfactory tract nucleus. Additionally, the CMN forms three cortical transition zones adjacent to the piriform cortex, entorhinal cortex, and the hippocampus, respectively termed the piriform-amygdala area, and the hippocampal-amygdala transition area. The boundaries between the various regions of the CMN can be distinguished based on the differences in the clustering of densely packed cells in the second layer.
The CEN actually comprises cell groups developed from the same progenitor, including the BNST and the sublentiform nucleus located in the basal forebrain, as well as the central amygdala nucleus (CEA) within the amygdala itself. As shown in Fig. 6a, CEA is separated by fiber tracts and intercalated amygdalar nucleus (IA). This nucleus is characterized by small and dense cells located on the dorsal medial side of the amygdala, which encompasses the CEA. IA also scatter in the interstice between the LA and the BLA, as well as between the BLA and the BMA. The CEA is part of the striatal amygdala, characterized by smaller cells, while the neurons in the medial amygdala nucleus are layered and connected to the cortical amygdala.
Amygdala and Hippocampus Formation. a–f correspond to the areas indicated by the purple boxes in (f–j). a–c illustrate the cytoarchitecture within the amygdala. d–f display the cytoarchitectural details within the hippocampal formation. g–j present sections of the atlas numbered Sec-19, Sec-24, Sec-28, and Sec-32. The background is white, and gray areas represent other regions. Scale bar: 500 μm (a–f), 2 mm (g–j)
Hippocampal formation
The hippocampal formation is located in the temporal lobe region and extends dorsomedially, representing a relatively independent area of the archicortex. As depicted in Fig. 6g–j, the hippocampal formation can be divided into two parts: the hippocampal region and the retrohippocampal region, each displayed in two different colors. The hippocampal structure includes the indusium griseum, fasciola cinerea, dentate gyrus, and Ammon’s horn (field CA1(CA1), field CA2(CA2), field CA3(CA3), field CA4(CA4)). Meanwhile, the retrohippocampal region can be divided into the subiculum (SUB), parasubiculum, presubiculum, and postsubiculum. These retrohippocampal regions are considered transitional zones between the hippocampus and the cortex, as shown in Fig. 6d, with their boundaries primarily defined by variations in the granular cell layer. As shown in Fig. 6e and f, within the hippocampal structure, Ammon’s horn represents a region that changes along the main axis of the hippocampus. The distinction between its internal subregions is based on two criteria: first, the CA3 can be specifically identified by the stratum lucidum, as shown in Fig. 6e, and second, CA2 is adjacent to CA3 and features a thinner pyramidal cell layer compared to CA1. CA1 is typically directly adjacent to SUB, which has a notably expanded pyramidal cell layer different from CA1.
Brain atlas website
To publicly share the drawn atlases and facilitate user feedback, we established the macaque sagittal plane reference brain atlas website. The website link: http://macaque.brains-atlas.org/STAC/index.html. The brain atlas website allows for interactive browsing of different brain structures at the sagittal level. The website includes a navigation window in the upper left corner for switching the sagittal sections. Below the navigation window is the search bar for terminology. The main window shows the macaque brain images and the delineation of brain structures, where users can switch the display mode between showing contours and colored labels. The plane in which the midpoint of the anterior commissure(ac), the midpoint of the posterior commissure(pc), and the bregma point are located is defined as the median sagittal plane. We used the midpoint of the anterior commissure in the median sagittal plane as the origin and the ac-pc line as a horizontal axis to establish a coordinate system for the brain atlas. A grid overlaid behind the atlas is presented to facilitate users in viewing the relative spatial position of anatomical structures of interest.
Discussion and conclusion
The Cynomolgus macaque is an essential non-human primate model for studying brain diseases. However, it lacks a precise sagittal reference atlas. In this study, we used a consecutive subcellular resolution 3D imaging system designed for macaque brains to obtain consecutive cytoarchitectonic images. We manually drew the sagittal reference atlas of the macaque brain based on these images. This atlas consists of 45 sections, including approximately 270 subcortical structures. We have also created an interactive website to browse this atlas.
The consecutive high-resolution cytoarchitectonic images brought significant advantages to creating the brain atlas. Firstly, we can mark almost all cell bodies in brain tissue by PI staining, which allows us to identify the unique cytoarchitectonic characteristics of different brain regions (Hezel et al. 2012). Compared with Nissl staining data (Jones et al. 2011), the PI-staining images could also show similar cytoarchitectonic features, making them a suitable standard for brain atlasing. The original data acquired by the fMOST system has sub-micron resolution, which ensures that every cell can be identified, and different cell groups can be distinguished even after down-sampling. Additionally, the continuity of the image data along the axial direction makes it possible to identify consistent brain region boundaries based on the continuous changes in cytoarchitectonic features. The existing two Cynomolgus macaque brain atlases (Szabo and Cowan 1984; Martin and Bowden 1996) were both illustrated on discrete coronal sections and only a few nuclei of the brainstem were drawn. Lanciego and Vázquez (2012) adopted multiple staining methods to obtain data on three anatomical levels of three macaque brains and drew atlases of the basal ganglia and thalamus, which included only 10 sagittal sections. Compared to existing macaque brain atlases, our brain atlas covers a wider range in the sagittal sections and identifies more brain structures.
The terminology of this atlas is created based on the rules proposed in the Brain Architecture Management System (BAMS) (Bota et al. 2008), which is also widely used in the Allen Reference Atlas(ARA) (Dong H.W. 2008) and the Allen Mouse Brain Common Coordinate Framework (CCFv3) ( Wang et al. 2020). Larry Swanson (2015) aimed to create a Pan-mammalian nomenclature for developing a deep common nomenclature for rodents and humans based on these rules. These rules are also suitable for non-human primates. In this case, we drew our atlas based on the monkey atlas(Szabo and Cowan 1984; Martin and Bowden 1996; Paxinos et al.1999; Saleem and Logothetis 2007; Jones et al. 2011), then named each brain structure based on rules in BAMS. As a result, we find that different atlases take different terminologies, but most of them indicate the same brain structures. Of course there are examples of exceptions. For example, there are different parcellations for the thalamus (García-Cabezas et al. 2023) and the amygdala, so it’s hard to harmonize all criteria. We recognized brain structures by comparing our images with the Nissl-stained sagittal sections (Jones et al. 2011) and coronal sections(Paxinos et al. 1999). In most instances, we used the same criteria as Paxinos (1999). As we can see in the Supplementary Material Table 2, most brain structures are well-matched. The disparities are that we have more parcellations for sublayers such as the cortical amygdala.
The accurate brain structure delimitation is an incredibly time-consuming task. In order to keep up with changes in brain regions, it’s necessary to obtain 8000 consecutive images. However, delineating all brain regions on thousands of images within a limited time is not feasible. As a result, we opted to select 45 sagittal images at 300 μm intervals, with a projection thickness of 12 μm, covering the main subcortical structures. We leveraged the whole three-dimensional data to re-slice masks of brain structures and compare them with corresponding original images during the brain atlas drawing process. This allowed us to fine-tune the results of brain structure delineation at the sagittal level, ensuring that each brain structure’s boundaries are continuous in three anatomical directions. In the future, we aim to develop more sophisticated methods based on the current results to supplement the intermediate sections, rather than manual annotation. However, it’s worth noting that we did not draw cortical brain and white matter boundaries, as we think there are better methods for the precise division of cortical areas(Lu et al. 2024; Chen et al. 2023) and white matter fibers(Feng et al. 2017). Otherwise, we estimated the tissue shrinkage but didn’t correct it. The sample was approximately similar after embedding with a < 5% shift between the vascular nodes and neurons. Moving forward, we will need to combine additional staining and marking techniques to draw a more comprehensive three-dimensional macaque brain atlas.
In summary, this study is the first work to draw the sagittal reference atlas of the Cynomolgus macaque brain using consecutive cytoarchitectonic images. We illustrated an accurate and detailed map of the subcortical structures of the macaque brain. The atlas along with the complete protocol for brain mapping, is also expected to be applied to brain atlasing in other genders, age stages, or even other species in the future.
Data availability
The atlas can be viewed on website: http://macaque.brains-atlas.org/STAC/index.html. The underlying atlas plates and images can be downloaded if necessary. Abbreviations and color coding for the brain structure terminology in this article are provided in Supplementary Material Table 1. The comparation of terminologies is provided in Supplementary Material Table 2.
References
Ahmad S, Wu Y, Wu Z, Thung KH, Liu S, Lin W, Li G, Wang L, Yap PT (2023) Multifaceted atlases of the human brain in its infancy. Nat Methods 20(1):55–64. https://doi.org/10.1038/s41592-022-01703-z
Atri A (2019) The Alzheimer’s Disease Clinical Spectrum: diagnosis and management. Med Clin N Am 103(2):263–293. https://doi.org/10.1016/j.mcna.2018.10.009
Bakker R, Tiesinga P, Kötter R (2015) The scalable brain Atlas: instant web-based Access to Public Brain atlases and related content. Neuroinformatics 13(3):353–366. https://doi.org/10.1007/s12021-014-9258-x
Bota M, Swanson LW (2008) BAMS Neuroanatomical Ontology: Design and Implementation. Front Neuroinformatics. https://doi.org/10.3389/neuro.11.002.2008
Calabrese E, Badea A, Coe CL, Lubach GR, Shi Y, Styner MA, Johnson GA (2015) A diffusion tensor MRI atlas of the postmortem rhesus macaque brain. NeuroImage 117:408–416. https://doi.org/10.1016/j.neuroimage.2015.05.072
Chen A, Sun Y, Lei Y, Li C, Liao S, Meng J, Bai Y, Liu Z, Liang Z, Zhu Z, Yuan N, Yang H, Wu Z, Lin F, Wang K, Li M, Zhang S, Yang M, Fei T, Zhuang Z, Li C (2023) Single-cell spatial transcriptome reveals cell-type organization in the macaque cortex. Cell 186(17):3726–3743e24. https://doi.org/10.1016/j.cell.2023.06.009
Dong HW (2008) Allen reference atlas: a digital color brain atlas of the C57Black/6J male mouse. John Wiley & Sons Inc, New Jersey
Feng L, Jeon T, Yu Q, Ouyang M, Peng Q, Mishra V, Pletikos M, Sestan N, Miller MI, Mori S, Hsiao S, Liu S, Huang H (2017) Population-averaged macaque brain atlas with high-resolution ex vivo DTI integrated into in vivo space. Brain Struct Funct 222(9):4131–4147. https://doi.org/10.1007/s00429-017-1463-6
Feng Z, Li A, Gong H, Luo Q (2022) Constructing the rodent stereotaxic brain atlas: a survey. Sci China Life Sci 65(1):93–106. https://doi.org/10.1007/s11427-020-1911-9
García-Cabezas MÁ, Pérez-Santos I, Cavada C (2023) Mapping the primate thalamus: historical perspective and modern approaches for defining nuclei. Brain Struct Funct 228:1125–1151. https://doi.org/10.1007/s00429-022-02598-4
Gong H, Xu D, Yuan J et al (2016) High-throughput dual-colour precision imaging for brain-wide connectome with cytoarchitectonic landmarks at the cellular level. Nat Commun 7:12142. https://doi.org/10.1038/ncomms12142
Hartig R, Glen D, Jung B, Logothetis NK, Paxinos G, Garza-Villarreal EA, Messinger A, Evrard HC (2021) The Subcortical Atlas of the Rhesus Macaque (SARM) for neuroimaging. NeuroImage 235:117996. https://doi.org/10.1016/j.neuroimage.2021.117996
Hezel M, Ebrahimi F, Koch M, Dehghani F (2012) Propidium iodide staining: a new application in fluorescence microscopy for analysis of cytoarchitecture in adult and developing rodent brain. Micron (Oxford England: 1993) 43(10):1031–1038. https://doi.org/10.1016/j.micron.2012.04.006
Jones EG, Stone JM, Karten HJ (2011) High-resolution digital brain atlases: a Hubble telescope for the brain. Ann New York Acad Sci 1225:E147–E159. https://doi.org/10.1111/j.1749-6632.2011.06009.x
Kim B, Noh GO, Kim K (2021) Behavioural and psychological symptoms of dementia in patients with Alzheimer’s disease and family caregiver burden: a path analysis. BMC Geriatr 21(1):160. https://doi.org/10.1186/s12877-021-02109-w
Lanciego JL, Vázquez A (2012) The basal ganglia and thalamus of the long-tailed macaque in stereotaxic coordinates. A template atlas based on coronal, sagittal and horizontal brain sections. Brain Struct Funct 217(2):613–666. https://doi.org/10.1007/s00429-011-0370-5
Lu Y, Cui Y, Cao L, Dong Z, Cheng L, Wu W, Wang C, Liu X, Liu Y, Zhang B, Li D, Zhao B, Wang H, Li K, Ma L, Shi W, Li W, Ma Y, Du Z, Zhang J, Jiang T (2024) Macaque Brainnetome Atlas: A multifaceted brain map with parcellation, connection, and histology. Sci Bull. https://doi.org/10.1016/j.scib.2024.03.031
Macchi ZA, Koljack CE, Miyasaki JM et al (2020) Patient and caregiver characteristics associated with caregiver burden in Parkinson’s disease: a palliative care approach. Annals Palliat Med 9(Suppl 1):S24–S33. https://doi.org/10.21037/apm.2019.10.01
Martin RF, Bowden DM (1996) A stereotaxic template atlas of the macaque brain for digital imaging and quantitative neuroanatomy. NeuroImage 4(2):119–150. https://doi.org/10.1006/nimg.1996.0036
Paxinos G, Huang X, Toga AW (1999) The rhesus monkey brain in stereotaxic coordinates. Elsevier Academic Press, San Diego, USA
Qiao N, Ma L, Zhang Y, Wang L (2023) Update on Nonhuman Primate models of Brain Disease and Related Research Tools. Biomedicines 11(9):2516. https://doi.org/10.3390/biomedicines11092516
Reveley C, Gruslys A, Ye FQ, Glen D et al (2017) Three-Dimensional Digital Template Atlas of the Macaque Brain. Cereb Cortex 27(9):4463–4477. https://doi.org/10.1093/cercor/bhw248
Rushmore RJ, Bouix S, Kubicki M, Rathi Y, Rosene DL, Yeterian EH, Makris N (2021) MRI-based Parcellation and Morphometry of the individual Rhesus Monkey Brain: the macaque Harvard-Oxford Atlas (mHOA), a translational system referencing a standardized ontology. Brain Imaging Behav 15(3):1589–1621. https://doi.org/10.1007/s11682-020-00357-9
Saleem KS, Logothetis NK (2007) A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Elsevier Academic Press, San Diego, USA
Saleem KS, Avram AV, Glen D, Yen CC, Ye FQ, Komlosh M, Basser PJ (2021) High-resolution mapping and digital atlas of subcortical regions in the macaque monkey based on matched MAP-MRI and histology. NeuroImage 245:118759. https://doi.org/10.1016/j.neuroimage.2021.118759
Sato K, Sasaki E (2018) Genetic engineering in nonhuman primates for human disease modeling. J Hum Genet 63(2):125–131. https://doi.org/10.1038/s10038-017-0351-5
Swanson LW (2015) Brain maps online: toward Open Access atlases and a pan-mammalian nomenclature. J Comp Neurol 523(15):2272–2276. https://doi.org/10.1002/cne.23788
Szabo J, Cowan WM (1984) A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis). J Comp Neurol 222(2):265–300. https://doi.org/10.1002/cne.902220208
Tarantal AF, Noctor SC, Hartigan-O’Connor DJ (2022) Nonhuman Primates in Translational Research. Annu Rev Anim Biosci 10:441–468. https://doi.org/10.1146/annurev-animal-021419-083813
Thompson CL, Ng L, Menon V, Martinez S et al (2014) A high-resolution spatiotemporal atlas of gene expression of the developing mouse brain. Neuron 83(2):309–323. https://doi.org/10.1016/j.neuron.2014.05.033
Wang Q, Ding SL, Li Y, Royall J, Feng D, Lesnar P, Graddis N, Naeemi M, Facer B, Ho A, Dolbeare T, Blanchard B, Dee N, Wakeman W, Hirokawa KE, Szafer A, Sunkin SM, Oh SW, Bernard A, Phillips JW, Ng L (2020) The Allen Mouse Brain Common coordinate Framework: a 3D reference Atlas. Cell 181(4):936–953e20. https://doi.org/10.1016/j.cell.2020.04.007
Zhong Q, Li A, Jin R et al (2021) High-definition imaging using line-illumination modulation microscopy. Nat Methods 18(3):309–315. https://doi.org/10.1038/s41592-021-01074-x
Zhou C, Yang X, Wu S et al (2022) Continuous subcellular resolution three-dimensional imaging on intact macaque brain. Sci Bull 67(1):85–96. https://doi.org/10.1016/j.scib.2021.08.003
Acknowledgements
We would like to thank the MOST group of Britton Chance Center for Biomedical Photonics, Wuhan National Laboratory for Optoelectronics, MoE Key Laboratory for Biomedical Photonics, Huazhong University of Science and Technology. We thank Xintian Hu for providing the brain specimen of macaque. We thank Tao Jiang, Jie Luo and members of HUST-Suzhou Institute for Brainsmatics for help with data acquisition and website building.
Funding
This study was financially supported by STI 2030-Major Projects (2021ZD0200203), National Natural Science Foundation of China.
Author information
Authors and Affiliations
Contributions
Yue Luo made substantial contributions to the conception or design of the work, brain atlas, and drafted the work; Xiangning Li made contributions to the conception or design of the work and the acquisition of a macaque brain and sample preparation; Guangcai Liu made contributions to the acquisition of data; Xueyan Jia made contributions to the analysis of data and website building; Xiaoquan Yang made contributions to the acquisition of data; Anan Li made contributions to the analysis of data and funding acquisition; Zhao Feng and Hui Gong made substantial contributions to the conception or design of the work, and revised it critically for important intellectual content. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, Y., Li, X., Zhou, C. et al. Mapping sagittal-plane reference brain atlas of the cynomolgus macaque (Macaca fascicularis) based on consecutive cytoarchitectonic images. Brain Struct Funct (2024). https://doi.org/10.1007/s00429-024-02851-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00429-024-02851-y