Abstract
The aim of this literature review is to present a summary of the published literature relating the details of the different modifications of specimen preparation for white matter dissection with the Klingler technique. For this review, 3 independent investigators performed an electronic literature search that was carried out in the Pubmed, Scopus and Web of Science databses up to December 2019. Furthermore, we performed citation tracking for the articles missed in the initial search. Studies were eligible for inclusion when they reported details of at least the first 2 main steps of Klingler’s technique: fixation and freezing. A total of 37 full-text articles were included in the analysis. We included original anatomical studies in which human white matter dissection was performed for study purposes. The main three steps of preparation are the same in each laboratory, but the details of each vary between studies. Ten percent formalin is the most commonly used (34 studies) solution for fixation. The freezing time varied between 8 h and a month, and the temperature varied from − 5 to − 80 °C. After thawing and during dissections, the specimens were most often kept in formalin solution (13), and the concentration varied from 4 to 10%. Klingler’s preparation technique involves three main steps: fixation, freezing and thawing. Even though the details of the technique are different in most of the studies, all provide subjectively good quality specimens for anatomical dissections and studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The white matter of the brain is composed of myelinated nerve axons. These axons, when sharing a common origin and destination, form compact structures such as white matter fascicules or tracts. The orientation of axons within the brain can be traced noninvasively through diffusion MRI (dMRI). This technique provides the possibility to study the anatomy of white matter and connections between different cortical regions. Specific fascicules can be isolated from the whole brain based on the anatomical knowledge that comes from classical anatomical white matter dissections. The identification of potentially new tracts based on dMRI findings has also been proven with anatomical dissection. The most common anatomical white matter dissection techniques, currently, are modifications of the initial technique described by (Klingler 1935). According to the initial technical note, the preparation method consists of a systematic approach including three main steps. In the first step, the brain is removed from the body and fixed; in the second step, one specimen is frozen; and in the third step, the specimen is thawed and, finally, white matter tracts are dissected. Klingler performed his dissections mainly with homemade wooden spatulas without any magnification. It is possible to dissect the main white matter tracts within the cerebral hemisphere with the naked eye, but for smaller tracts, such as those within the brain stem, microscopes and microsurgical tools are of great importance.
When following the methods section of manuscripts that include white matter dissection, most authors state that their technique is following Klingler’s original technique (Capilla-Guasch et al. 2019; De Benedictis et al. 2014; Di Carlo et al. 2019; Dini et al. 2013; Fernandez-Miranda et al. 2008; Flores-Justa et al. 2019; Gungor et al. 2018; Martino et al. 2010; Peltier et al. 2006; Peuskens et al. 2004; Serra et al. 2017; Shah et al. 2012; Vergani et al. 2014; Verhaeghe et al. 2018; Wang et al. 2013; Wu et al. 2016a), or that their own modification (Burks et al. 2017; de Castro et al. 2005; Goryainov et al. 2017; Latini et al. 2015; Rigoard et al. 2011; Silva and Andrade 2016; Sincoff et al. 2004) of the Klingler’s technique is applied. This may be confusing for those who want to start their own dissections. Most commonly, the three main steps are the same in each laboratory, but the details of each vary between studies, sometimes significantly. The aim of this manuscript is to present our technique and review data about different techniques, which we hope will give readers a chance to choose the technique that best suits their needs.
Methods
We conducted a literature review regarding the published literature relating the details of the different modifications of specimen preparation for white matter dissection with the Klingler technique. Ethics committee approval was obtained for the part of the study that presents the technique used in our laboratory. The approval number, AKBE/126/2019, was obtained from the Bioethics Committee of our University.
Literature study
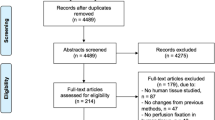
Three independent investigators performed an electronic literature search that was carried out in the Pubmed, Scopus and Web of Science databases up to December 2019. We used a [(Klingler) AND (Technique)] OR (White matter dissection) combination of keyword categories. Furthermore, we performed citation tracking for the articles missed through the initial search. The validity of the studies was assessed by the 3 investigators, and a consensus between them was used to resolve any disagreement. The database search identified 108 articles, and another 12 were found via citation tracking. Therefore, 120 full-text manuscripts were included in the final evaluation (Table 1). The methods sections of these 120 manuscripts were reviewed, and 37 articles eligible for inclusion in the study were identified (Table 2).
Study selection
We included original anatomical studies in which human white matter dissection was performed for research purposes. Studies were eligible for inclusion when they reported details of at least the first 2 main steps of Klingler’s technique: fixation and freezing. We limited our review to studies published in English. When multiple publications from the same laboratory were available and the same technique was described, we used the initial manuscript as a reference describing the technique in this review.
Data extraction
Using a standardized data extraction form, 3 investigators independently extracted the following aspects from the studies included in the review: the number of hemispheres used for the study, type of fixing fluid, concentration of the solution used for fixation, time of fixation, temperature and duration of freezing, and the thawing technique. Additional data about the technique used to avoid brain disfigurement, the type of fluid and its concentration used between dissections, and the magnification technique and type of instruments used for dissection were also extracted from the studies. From all papers dealing with this topic, other features and exceptions from the main technique were also presented in the discussion.
Our technique
The brain is removed from the body on the next working day, except weekends where delay make take up to 72 h. The brain is placed within a container filled with 4% formalin and gauze to protect it from deformation during the fixation process (Fig. 1a). The brain is kept in this solution for at least 4 weeks. After washing out the formalin, the arachnoid and vessels are removed (Fig. 1b, c). The dry brain is placed on the support inside the freezer with the temperature set at − 15 °C and kept there for two weeks (Fig. 1d). For thawing, the specimen is placed in a container with 4% formalin solution at room temperature. Dissection is performed mainly with microsurgical instruments and under microsurgical microscope magnification (Fig. 1e–h). Two- and three-dimensional picture documentation is performed with a digital camera.
The preparation technique used in our laboratory. a The brain is placed within a container filled with 4% formalin and gauze to prevent deformation during the fixation process. b, c After washing out the formalin, the arachnoid and vessels are removed. d The dry brain is placed on the support inside the freezer with the temperature set at − 15 °C and kept there, usually, for 2 weeks. e–h Dissection is performed mainly with microsurgical instruments and under microsurgical microscope magnification
Results
Details of the technique, including fixation, freezing and thawing, are described in detail in Table 2, which summarizes our results (Baran et al. 2019; Bozkurt et al. 2017; Burks et al. 2017; Capilla-Guasch et al. 2019; Costa et al. 2018; De Benedictis et al. 2014; de Castro et al. 2005; Di Carlo et al. 2019; Dini et al. 2013; Fernandez-Miranda et al. 2008; Flores-Justa et al. 2019; Goryainov et al. 2017; Gungor et al. 2018; Ludwig and Klingler 1956; Kadri et al. 2017; Koutsarnakis et al. 2015; Latini 2015; Latini et al. 2015; Mandonnet et al. 2017; Martino et al. 2010; Panesar et al. 2019; Pescatori et al. 2017; Peuskens et al. 2004; Rigoard et al. 2011; Sarubbo et al. 2016; Serra et al. 2017; Shah et al. 2012; Silva and Andrade 2016; Silva et al. 2017; Sincoff et al. 2004; Ture et al. 2000; Vergani et al. 2014; Verhaeghe et al. 2018; Wang et al. 2013; Wu et al. 2016a, b; Yakar et al. 2018).
Fixation
In 6 out of 37 manuscripts, the authors mentioned the time between death, brain removal from the skull and initiation of the fixation process. The authors advised performing this process within the first 12 h in 4 manuscripts, within 8 h in 1 manuscript and in less than 18 h in 1 manuscript. For fixation, specimens were placed in formalin solution in all reviewed manuscripts, but the concentration of solution varied between the studies. Ten percent formalin solution was the most commonly used (34 studies). In 1 study, the concentration was higher and varied between 10 and 15% without mentioning a specific value. In 2 described techniques, the concentration of the solution was lower than 10% with values of 4–5%. The time of fixation varied from 24 h to 3 months. In 2 manuscripts, formalin solution was exchanged during the fixation process after the first 24 h and after 2 weeks. Most of the studies agreed that longer fixation is beneficial rather than harmful. To avoid brain deformity during the fixation process, the brain can be placed on gauze within the formalin solution and float freely (2 manuscripts), or the same effect can be achieved by placing a ligature on the basilar artery and using it to suspend the brain on wooden sticks placed at the top of the container (6 manuscripts). For anatomical studies, the authors used 2–60 hemispheres, with an average of 15 hemispheres per study.
Freezing
In 17 manuscripts, an additional process of washing out formalin was mentioned between the fixation and freezing steps. It was performed with still or running water. This process takes from “several hours” up to 2 days. Twenty of 28 manuscripts reported that the arachnoid and vessels were removed before the freezing process had begun. In 7 of the remaining 8 manuscripts, it was done after freezing and in 1 manuscript, this process was performed between freezing-defreezing episodes. Multiple episodes (from 2 up to 5) of freezing-defreezing were performed as a standard procedure in 3 laboratories. The freezing time varied between 8 h and 1 month. The temperature at which the brain was preserved varied from − 5 to − 80 °C.
Thawing and dissection
Thawing can be completed at room temperature on a tray or the specimen can be placed under running or in still water. After thawing and during dissections, the specimen was kept in formalin (13 manuscripts) or alcohol solution (4 manuscripts). The formalin solution concentration varied from 4 to 10%. In 1 manuscript, the authors stated that the concentration was lower than that used for fixation, which was 10%. In 9 manuscripts, it was lower than 10%. The alcohol solution concentration varied between 70% (2 manuscripts) and 96% (1 manuscript); in 1 manuscript, the author did not mention the concentration of the solution. In 1 manuscript, researchers used both alcohol and formalin but did not mention if there was any subjective difference between these two solutions. In 26 papers, it was mentioned that operative microscopy was beneficial for dissections. In another 2 manuscripts, the authors found the use of loops to be helpful. Thirty-one authors reported the tools that they used for dissection. Eleven used purely homemade wooden spatulas of different sizes, 11 used microsurgical instruments in addition to wooden spatulas, and the rest (9) used purely microsurgical dissectors.
Discussion
White matter dissection was found to be useful not only for anatomical studies but also for simulating neurosurgical approaches to deep-seated intraaxial lesions (Mandonnet et al. 2017). The most commonly adopted brain preparation technique is one described by Klingler in his two manuscripts (Ludwig and Klingler 1956; Klingler and Gloor 1960).
In the first step of the preparation, the brain has to be removed from the skull, but some authors found it useful for anatomical studies to preserve the whole head (Baran et al. 2019; Bozkurt et al. 2017). This allows us to perfuse the cadaver vasculature with a silicon solution to better visualize brain arteries and veins. This also provides an opportunity to study the relationship between craniometric points and cortical and subcortical anatomical points. According to Klingler’s initial description, the brain should be removed from the body within 12 h of death. In 5 manuscripts that were chosen for review, the authors mentioned this step, and the time varied from less than 8–18 h (Di Carlo et al. 2019; Goryainov et al. 2017; Peltier et al. 2006; Rigoard et al. 2011; Ture et al. 2000). The rest of the authors did not mention this part of the preparation, which, as in our case, can be limited due to logistics issues. This part may not be very important as, according to Klingler, the blood content inside the brain is of greater importance than the time between death and removing the brain from the body. Greater amounts of blood inside the skull provide better differentiation between gray and white matter. This can be achieved by placing the head as the lowest point of the body. In none of the reviewed manuscript did the authors mention the importance of proper head positioning before removal of the brain from the skull. With standard techniques that are used during pathomorphological sections, it is easy to damage the lateral surface of the brain (Fig. 1b–d) (de Castro et al. 2005). Silva et al. mentioned the importance of careful removal of the calvaria and described in detail their technique to avoid damage to the underlying dura and the brain (Silva and Andrade 2016). Some authors prefer to start fixation before the brain is removed from the skull, and this is performed with formalin solution, which is injected intraarterially (Latini et al. 2015) (Silva and Andrade 2016; Silva et al. 2017), (Flores-Justa et al. 2019).
Klingler suggested that the brain should be suspended in fixing fluid, which is 5% formalin solution. All authors use formalin for fixation, but various solution concentrations are reported and vary from 4 to 15% (Sincoff et al. 2004; Wu et al. 2016b). Klingler used a concentration of 5% because he believed that a higher concentration of 10%, which is currently the most commonly used concentration, carries the risk of fixing the superficial parts of the hemisphere, yet the penetration of formalin at the 5% concentration to the deeper parts would not be sufficient. This may make the dissection more difficult, often unsatisfactory, and in some cases, even impossible to perform.
When the brain is removed from the body, it has a jelly-like consistency and is vulnerable to deformation. To preserve its shape during fixation, a wooden stick is placed at the top of the container, and the brain is suspended on a ligature placed on the basilar artery, which allows the brain to float freely in formalin solution (Costa et al. 2018; de Castro et al. 2005; Ludwig and Klingler 1956; Kadri et al. 2017; Silva and Andrade 2016; Ture et al. 2000). Other groups achieve the same effect by placing gauze inside the container, enabling free floating, so that the brain does not touch the sides of the container (Goryainov et al. 2017; Peuskens et al. 2004) (Fig. 1a). In the original description and in 2 other studies, the authors recommend exchanging the formalin solution after 24 h and 2 weeks (de Castro et al. 2005; Silva and Andrade 2016). The aim of this part of the preparation was not clarified in the reviewed studies. In total, the fixation process takes 4 weeks, but a longer period is beneficial according to Klingler and most authors (Ludwig and Klingler 1956). In the second manuscript about this technique, Klingler changed his technique and advocated that the brain should be kept in formalin solution for at least 2–3 months (Klingler and Gloor 1960). The reason he changed his practice was not mentioned. According to our review, satisfactory fixation can be achieved even after 24 h, but this may have a negative impact on the quality of the specimen and the ease of dissection (Rigoard et al. 2011).
Before the second step begins, Klingler recommended washing out the formalin from the brain under running water, which should take “several hours” (Kadri et al. 2017; Koutsarnakis et al. 2015; Silva and Andrade 2016; Ture et al. 2000; Wu et al. 2016a). The aim of this step is to remove the formalin solution, which is irritating to the observer during the next step, which includes removing the arachnoid and vessels (Baran et al. 2019; Capilla-Guasch et al. 2019; Costa et al. 2018; Di Carlo et al. 2019; Goryainov et al. 2017; Kadri et al. 2017; Koutsarnakis et al. 2015; Latini 2015; Latini et al. 2015; Martino et al. 2010; Panesar et al. 2019; Pescatori et al. 2017; Serra et al. 2017; Silva and Andrade 2016; Silva et al. 2017; Vergani et al. 2014; Wu et al. 2016a, b). Most authors removed the arachnoid before freezing except for a few who did it after this process is done or between episodes (Burks et al. 2017; De Benedictis et al. 2014; Gungor et al. 2018; Peuskens et al. 2004; Rigoard et al. 2011; Sarubbo et al. 2016; Shah et al. 2012; Sincoff et al. 2004). Adequately washing out formalin can be satisfactorily achieved with the brain placed in a container of still water (de Castro et al. 2005). In the next step, the brain is placed on a flat tray in a freezer for 8 days at a temperature of − 8 to − 10 °C. According to Klingler’s second manuscript, the temperature should be set between − 10° and − 15°, although the author did not define a specific temperature value. Lowering the temperature was combined with extending the freezing time for 2 days, which extended the overall freezing time from 8 to 10 days (Klingler and Gloor 1960). Some authors suggest that the freezing process can be performed when the brain is still in formalin, water or the alcohol solution (de Castro et al. 2005), (Goryainov et al. 2017). In the reviewed manuscripts, the temperature for the freezing step varied from − 5° to − 80° (Costa et al. 2018; Sarubbo et al. 2016). The time of freezing varied from 8 h to 8 weeks and did not correlate with the temperature. The aim of freezing is to spread myelinated nerve fibers apart, as the water solution increases 10% in volume with the formation of ice crystals. The crystals are placed between myelinated fibers as formalin solution does not penetrate the myelin sheath. This concept was proven recently with observations based on electron microscopy (Zemmoura et al. 2016). In 3 laboratories, the freezing–thawing technique is used, which is believed to provide better penetration of formalin solution between myelinated fibers (de Castro et al. 2005; Rigoard et al. 2011; Sincoff et al. 2004). After that time, the brain is thawed under running water (Bozkurt et al. 2017; de Castro et al. 2005; Gungor et al. 2018; Ludwig and Klingler 1956; Martino et al. 2010; Peuskens et al. 2004; Silva and Andrade 2016; Silva et al. 2017) or still water (Dini et al. 2013; Klingler and Gloor 1960; Serra et al. 2017; Shah et al. 2012; Ture et al. 2000) or is placed within formalin solution. This ends the preparation and makes the brain ready for dissection.
According to the initial description, the brain can be kept in 5% formalin solution, but for overnight or longer periods, the specimen should be kept in 2% solution. To minimize the unpleasant formalin odor, the brain in most of the manuscripts is kept in solution with lower formalin concentrations than those used for fixation (Capilla-Guasch et al. 2019; Costa et al. 2018) or in alcohol (Baran et al. 2019; Goryainov et al. 2017; Gungor et al. 2018; Peuskens et al. 2004). Some authors recommend re-freezing for a short period, such as 12 h, when there has to be a longer period without dissection (Goryainov et al. 2017; Ture et al. 2000). Refreezing is also useful for obtaining better definition when a dissection reaches deeper layers of white matter (Sarubbo et al. 2016).
Instruments initially used for dissection were Swiss watchmaker forceps and wooden spatulas, which are 2–4 mm wide, while metal spatulas were not recommended (Ludwig and Klingler 1956; Klingler and Gloor 1960). Most laboratories currently use wooden spatulas (Capilla-Guasch et al. 2019; Costa et al. 2018; De Benedictis et al. 2014; de Castro et al. 2005; Di Carlo et al. 2019; Dini et al. 2013; Flores-Justa et al. 2019; Latini et al. 2015; Panesar et al. 2019; Pescatori et al. 2017; Sarubbo et al. 2016; Serra et al. 2017; Shah et al. 2012; Silva and Andrade 2016; Sincoff et al. 2004; Ture et al. 2000; Vergani et al. 2014), but microsurgical metallic instruments (Capilla-Guasch et al. 2019; Costa et al. 2018; de Castro et al. 2005; Flores-Justa et al. 2019; Gungor et al. 2018; Koutsarnakis et al. 2015; Latini 2015; Latini et al. 2015; Martino et al. 2010; Peltier et al. 2006; Peuskens et al. 2004; Serra et al. 2017; Silva and Andrade 2016; Vergani et al. 2014; Wang et al. 2013; Wu et al. 2016b) have also been found to be useful for precise dissections. For a delicate dissection of low volume tracts, Klingler used snipe feathers, wet hair pencils or wet pieces of cotton wool (Klingler and Gloor 1960).
Most dissections are performed with the unaided eye, which is sufficient when the main cerebral tracts are dissected. For a better visualization of loops(Latini 2015; Martino et al. 2010), a microsurgical microscope can provide higher magnification and illumination (Baran et al. 2019; Bozkurt et al. 2017; de Castro et al. 2005; Fernandez-Miranda et al. 2008; Flores-Justa et al. 2019; Gungor et al. 2018; Koutsarnakis et al. 2015; Latini et al. 2015; Peltier et al. 2006; Peuskens et al. 2004; Rigoard et al. 2011; Serra et al. 2017; Shah et al. 2012; Ture et al. 2000; Verhaeghe et al. 2018; Wang et al. 2013; Wu et al. 2016a, b). In the third step, dissection starts with the removal of the whole hemisphere cortex to expose “U” fibers (Peuskens et al. 2004). This technique can later be confusing due to the loss of cortical anatomical landmarks. Therefore, Martino et al. recently presented a new technique in which the superficial cerebral cortex is preserved and dissection starts at the depth of the sulcus (Martino et al. 2011).
To document the results of dissection, Klingler used an analog camera with four-lamp illumination. Recently, this process has been performed with digital cameras that can potentially improve the quality of images with software dedicated for postprocessing (Bozkurt et al. 2017; Capilla-Guasch et al. 2019; Flores-Justa et al. 2019; Gungor et al. 2018; Kadri et al. 2017; Latini 2015; Martino et al. 2010; Pescatori et al. 2017; Sarubbo et al. 2016; Shah et al. 2012; Vergani et al. 2014; Wu et al. 2016b). Some authors believe that only photographs without any postprocessing are able to present realistic dissection results (Koutsarnakis et al. 2015). The same authors also suggest that taking pictures without the flash may be superficial in terms of fiber tract delineation (Koutsarnakis et al. 2015). Two- or three-dimensional pictures can be taken (Baran et al. 2019; Bozkurt et al. 2017; Capilla-Guasch et al. 2019).
According to our review, some authors changed their preparation technique, but there is lack of information regarding what made them do so and if there was any benefit from a dissection quality point of view (Silva and Andrade 2016; Silva et al. 2017).
For the main white matter tracts high replicability of Klingler methods is observed between the studies. Despite different techniques, the main tracts are easily identifiable, but the ease of dissection is objectively difficult to be assessed. Despite the same anatomical localization of the tracts different definitions across publications lead to the differences in the terminology. Anatomical background about white matter anatomy before performing dissection is mandatory. For better anatomical orientation, it is possible to perform dissections with the aid of MRI-based neuronavigation and custom-made fiducial markers (Skadorwa et al. 2009).
The limitation of this technique, except for being time-consuming and requiring great anatomical knowledge and manual skills, is that it requires good quality specimens and proper technique preparation. This was not the focus of our study, but in the majority of the manuscripts, our subjective assessment of the quality of the dissection based on the available figures was very high.
None of the studies compared different preservation techniques in terms of the quality of dissections or tract visualization in a subjective or objective way.
Conclusion
There is an agreement between studies that the Klingler technique has three main steps: fixing, freezing and thawing with dissection. Even though the details of the technique are different in most of the studies, all provide good quality specimens for anatomical dissections and anatomical studies. This shows that those who wish to start this kind of dissection should choose the technique that fits them the best and follow it. Possible goals for other studies should include a comparison between different techniques in terms of the visualization of tracts and the ease of dissection.
References
Baran O et al (2019) Surgical approaches to the thalamus in relation to the white matter tracts of the cerebrum. World Neurosurg 128:e1048–e1086. https://doi.org/10.1016/j.wneu.2019.05.068
Bozkurt B et al (2017) Fiber connections of the supplementary motor area revisited: methodology of fiber dissection DTI, and three dimensional documentation. J Vis Exp. https://doi.org/10.3791/55681
Burks JD et al (2017) White matter connections of the inferior parietal lobule: a study of surgical anatomy. Brain Behav 7:e00640. https://doi.org/10.1002/brb3.640
Capilla-Guasch P, Quilis-Quesada V, Regin-Neto M, Holanda VM, Gonzalez-Darder JM, de Oliveira E (2019) White matter relationships examined by transillumination technique using a lateral transcortical parietal approach to the atrium: three-dimensional images and surgical considerations. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.08.018
Costa M, Braga VL, Yagmurlu K, Centeno RS, Cavalheiro S, Chaddad-Neto F (2018) A technical guide for fiber tract dissection of the internal capsule. Turk Neurosurg 28:934–939. https://doi.org/10.5137/1019-5149.JTN.20884-17.1
De Benedictis A et al (2014) Anatomo-functional study of the temporo-parieto-occipital region: dissection, tractographic and brain mapping evidence from a neurosurgical perspective. J Anat 225:132–151. https://doi.org/10.1111/joa.12204
de Castro I, Christoph Dde H, dos Santos DP, Landeiro JA (2005) Internal structure of the cerebral hemispheres: an introduction of fiber dissection technique. Arq Neuropsiquiatr 63:252–258. https://doi.org/10.1590/s0004-282x2005000200011
Di Carlo DT et al (2019) Microsurgical anatomy of the sagittal stratum. Acta Neurochir (Wien) 161:2319–2327. https://doi.org/10.1007/s00701-019-04019-8
Dini LI et al (2013) Reproducibility of quantitative fiber tracking measurements in diffusion tensor imaging of frontal lobe tracts: a protocol based on the fiber dissection technique. Surg Neurol Int 4:51. https://doi.org/10.4103/2152-7806.110508
Fernandez-Miranda JC, Rhoton AL Jr, Kakizawa Y, Choi C, Alvarez-Linera J (2008) The claustrum and its projection system in the human brain: a microsurgical and tractographic anatomical study. J Neurosurg 108:764–774. https://doi.org/10.3171/JNS/2008/108/4/0764
Flores-Justa A, Baldoncini M, Perez Cruz JC, Sanchez Gonzalez F, Martinez OA, Gonzalez-Lopez P, Campero A (2019) White matter topographic anatomy applied to temporal lobe surgery. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.08.050
Goryainov SA et al (2017) Long association tracts of the human white matter: an analysis of 18 hemisphere dissections and in vivo HARDI-CSD tractography. Zh Vopr Neirokhir Im N N Burdenko 81:13–25. https://doi.org/10.17116/neiro201780713-25
Gungor A et al (2018) Microsurgical anatomy of the subthalamic nucleus: correlating fiber dissection results with 3-T magnetic resonance imaging using neuronavigation. J Neurosurg 130:716–732. https://doi.org/10.3171/2017.10.JNS171513
Kadri PAS, de Oliveira JG, Krayenbuhl N, Ture U, de Oliveira EPL, Al-Mefty O, Ribas GC (2017) Surgical approaches to the temporal horn: an anatomic analysis of white matter tract interruption. Oper Neurosurg (Hagerstown) 13:258–270. https://doi.org/10.1093/ons/opw011
Klingler J (1935) Erleichterung der makro- skopjschen Praparation des Gehirns durch den Gefrierprozess. Schweiz Arch Neur Psychiat 36:247–256
Klingler J, Gloor P (1960) The connections of the amygdala and of the anterior temporal cortex in the human brain. J Comp Neurol 115:333–369. https://doi.org/10.1002/cne.901150305
Koutsarnakis C, Liakos F, Kalyvas AV, Sakas DE, Stranjalis G (2015) A laboratory manual for stepwise cerebral white matter fiber dissection. World Neurosurg 84:483–493. https://doi.org/10.1016/j.wneu.2015.04.018
Latini F (2015) New insights in the limbic modulation of visual inputs: the role of the inferior longitudinal fasciculus and the Li-Am bundle. Neurosurg Rev 38:179–189. https://doi.org/10.1007/s10143-014-0583-1 (discussion 189-190)
Latini F, Hjortberg M, Aldskogius H, Ryttlefors M (2015) The use of a cerebral perfusion and immersion-fixation process for subsequent white matter dissection. J Neurosci Methods 253:161–169. https://doi.org/10.1016/j.jneumeth.2015.06.019
Ludwig E, Klingler J (1956) Atlas Cerebri Humani. S Karger Basel NY 1956:15–20
Mandonnet E et al (2017) Neuronavigated fiber dissection with pial preservation: laboratory model to simulate opercular approaches to insular tumors. World Neurosurg 98:239–242. https://doi.org/10.1016/j.wneu.2016.10.020
Martino J, Brogna C, Robles SG, Vergani F, Duffau H (2010) Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46:691–699. https://doi.org/10.1016/j.cortex.2009.07.015
Martino J et al (2011) Cortex-sparing fiber dissection: an improved method for the study of white matter anatomy in the human brain. J Anat 219:531–541. https://doi.org/10.1111/j.1469-7580.2011.01414.x
Panesar SS, Belo JTA, Yeh FC, Fernandez-Miranda JC (2019) Structure, asymmetry, and connectivity of the human temporo-parietal aslant and vertical occipital fasciculi. Brain Struct Funct 224:907–923. https://doi.org/10.1007/s00429-018-1812-0
Peltier J, Travers N, Destrieux C, Velut S (2006) Optic radiations: a microsurgical anatomical study. J Neurosurg 105:294–300. https://doi.org/10.3171/jns.2006.105.2.294
Pescatori L, Tropeano MP, Manfreda A, Delfini R, Santoro A (2017) Three-dimensional anatomy of the white matter fibers of the temporal lobe: surgical implications. World Neurosurg 100:144–158. https://doi.org/10.1016/j.wneu.2016.12.120
Peuskens D, van Loon J, Van Calenbergh F, van den Bergh R, Goffin J, Plets C (2004) Anatomy of the anterior temporal lobe and the frontotemporal region demonstrated by fiber dissection. Neurosurgery 55:1174–1184. https://doi.org/10.1227/01.neu.0000140843.62311.24
Rigoard P et al (2011) The accumbofrontal fasciculus in the human brain: a microsurgical anatomical study. Neurosurgery 68:1102–1111. https://doi.org/10.1227/NEU.0b013e3182098e48 (discussion 1111)
Sarubbo S et al (2016) Structural and functional integration between dorsal and ventral language streams as revealed by blunt dissection and direct electrical stimulation. Hum Brain Mapp 37:3858–3872. https://doi.org/10.1002/hbm.23281
Serra C, Ture U, Krayenbuhl N, Sengul G, Yasargil DC, Yasargil MG (2017) Topographic classification of the thalamus surfaces related to microneurosurgery: a white matter fiber microdissection study. World Neurosurg 97:438–452. https://doi.org/10.1016/j.wneu.2016.09.101
Shah A, Jhawar SS, Goel A (2012) Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clin Neurosci 19:289–298. https://doi.org/10.1016/j.jocn.2011.04.039
Silva SM, Andrade JP (2016) Neuroanatomy: the added value of the Klingler method. Ann Anat 208:187–193. https://doi.org/10.1016/j.aanat.2016.06.002
Silva SM, Cunha-Cabral D, Andrade JP (2017) Neurosurgical relevance of the dissection of the diencephalic white matter tracts using the Klingler technique. Clin Neurol Neurosurg 156:35–40. https://doi.org/10.1016/j.clineuro.2017.03.001
Sincoff EH, Tan Y, Abdulrauf SI (2004) White matter fiber dissection of the optic radiations of the temporal lobe and implications for surgical approaches to the temporal horn. J Neurosurg 101:739–746. https://doi.org/10.3171/jns.2004.101.5.0739
Skadorwa T, Kunicki J, Nauman P, Ciszek B (2009) Image-guided dissection of human white matter tracts as a new method of modern neuroanatomical training. Folia Morphol (Warsz) 68:135–139
Ture U, Yasargil MG, Friedman AH, Al-Mefty O (2000) Fiber dissection technique: lateral aspect of the brain. Neurosurgery 47:417–426. https://doi.org/10.1097/00006123-200008000-00028 (discussion 426-417)
Vergani F, Mahmood S, Morris CM, Mitchell P, Forkel SJ (2014) Intralobar fibres of the occipital lobe: a post mortem dissection study. Cortex 56:145–156. https://doi.org/10.1016/j.cortex.2014.03.002
Verhaeghe A, Decramer T, Naets W, Van Paesschen W, van Loon J, Theys T (2018) Posterior quadrant disconnection: a fiber dissection study. Oper Neurosurg (Hagerstown) 14:45–50. https://doi.org/10.1093/ons/opx060
Wang Y, Fernandez-Miranda JC, Verstynen T, Pathak S, Schneider W, Yeh FC (2013) Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cereb Cortex 23:2347–2356. https://doi.org/10.1093/cercor/bhs225
Wu Y, Sun D, Wang Y, Wang Y, Ou S (2016) Segmentation of the cingulum bundle in the human brain: a new perspective based on dsi tractography and fiber dissection study. Front Neuroanat 10:84. https://doi.org/10.3389/fnana.2016.00084
Wu Y, Sun D, Wang Y, Wang Y, Wang Y (2016) Tracing short connections of the temporo-parieto-occipital region in the human brain using diffusion spectrum imaging and fiber dissection. Brain Res 1646:152–159. https://doi.org/10.1016/j.brainres.2016.05.046
Yakar F, Eroglu U, Peker E, Armagan E, Comert A, Ugur HC (2018) Structure of corona radiata and tapetum fibers in ventricular surgery. J Clin Neurosci 57:143–148. https://doi.org/10.1016/j.jocn.2018.08.041
Zemmoura I, Blanchard E, Raynal PI, Rousselot-Denis C, Destrieux C, Velut S (2016) How Klingler’s dissection permits exploration of brain structural connectivity? An electron microscopy study of human white matter. Brain Struct Funct 221:2477–2486. https://doi.org/10.1007/s00429-015-1050-7
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dziedzic, T.A., Balasa, A., Jeżewski, M.P. et al. White matter dissection with the Klingler technique: a literature review. Brain Struct Funct 226, 13–47 (2021). https://doi.org/10.1007/s00429-020-02157-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-020-02157-9