Abstract
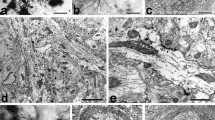
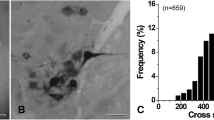
The rostral nucleus of the solitary tract (rNST) receives gustatory input via chorda tympani (CT) afferents from the anterior two-thirds of the tongue and transmits it to higher brain regions. To help understand how the gustatory information is processed at the 1st relay nucleus of the brain stem, we investigated the central connectivity of the CT afferent terminals in the central subdivision of the rat rNST through retrograde labeling with horseradish peroxidase, immunogold staining for GABA, glycine, and glutamate, and quantitative ultrastructural analysis. Most CT afferents were small myelinated fibers (<5 µm2 in cross-sectional area) and made simple synaptic arrangements with 1–2 postsynaptic dendrites. It suggests that the gustatory signal is relayed to a specific group of neurons with a small degree of synaptic divergence. The volume of the identified synaptic boutons was positively correlated with their mitochondrial volume and active zone area, and also with the number of their postsynaptic dendrites. One-fourth of the boutons received synapses from GABA-immunopositive presynaptic profiles, 27 % of which were also glycine-immunopositive. These results suggest that the gustatory information mediated by CT afferents to the rNST is processed in a simple and specific manner. They also suggest that the minority of CT afferents are presynaptically modulated by GABA- and/or glycine-mediated mechanism.








Similar content being viewed by others
Abbreviations
- CT:
-
Chorda tympani
- EM:
-
Electron microscopy
- GG:
-
Geniculate ganglion
- Glut:
-
Glutamate
- Gly:
-
Glycine
- HRP:
-
Horseradish peroxidase
- HTM:
-
High threshold mechanoreceptive
- LDCV:
-
Large dense core vesicle
- LM:
-
Light microscopy
- LTM:
-
Low threshold mechanoreceptive
- PATs:
-
Primary afferent terminals
- rNST:
-
Rostral nucleus of the solitary tract
- SDH:
-
Spinal dorsal horn
- TSN:
-
Trigeminal sensory nuclei
References
Al-Khater KM, Kerr R, Todd AJ (2008) A quantitative study of spinothalamic neurons in laminae I, III, and IV in lumbar and cervical segments of the rat spinal cord. J Comp Neurol 511:1–18
Almeida TF, Roizenblatt S, Tufik S (2004) Afferent pain pathways: a neuroanatomical review. Brain Res 1000:40–56
Alvarez FJ, Kavookjian AM, Light AR (1992) Synaptic interactions between GABA-immunoreactive profiles and the terminals of functionally defined myelinated nociceptors in the monkey and cat spinal cord. J Neurosci 12:2901–2917
Alvarez FJ, Kavookjian AM, Light AR (1993) Ultrastructural morphology, synaptic relationships, and CGRP immunoreactivity of physiologically identified C-fiber terminals in the monkey spinal cord. J Comp Neurol 329:472–490
Bae YC, Nakagawa S, Yoshida A, Nagase Y, Takemura M, Shigenaga Y (1994) Morphology and synaptic connections of slowly adapting periodontal afferent terminals in the trigeminal subnuclei principalis and oralis of the cat. J Comp Neurol 348:121–132
Bae YC, Nakagawa S, Yasuda K, Yabuta NH, Yoshida A, Pil PK, Moritani M, Chen K, Nagase Y, Takemura M, Shigenaga Y (1996) Electron microscopic observation of synaptic connections of jaw-muscle spindle and periodontal afferent terminals in the trigeminal motor and supratrigeminal nuclei in the cat. J Comp Neurol 374:421–435
Bae YC, Ihn HJ, Park MJ, Ottersen OP, Moritani M, Yoshida A, Shigenaga Y (2000) Identification of signal substances in synapses made between primary afferents and their associated axon terminals in the rat trigeminal sensory nuclei. J Comp Neurol 418:299–309
Bae YC, Choi BJ, Lee MG, Lee HJ, Park KP, Zhang LF, Honma S, Fukami H, Yoshida A, Ottersen OP, Shigenaga Y (2002) Quantitative ultrastructural analysis of glycine- and gamma-aminobutyric acid-immunoreactive terminals on trigeminal alpha- and gamma-motoneuron somata in the rat. J Comp Neurol 442:308–319
Bae YC, Ahn HJ, Park KP, Kim HN, Paik SK, Bae JY, Lee HW, Kim KH, Yoshida A, Moritani M, Shigenaga Y (2005a) The synaptic microcircuitry associated with primary afferent terminals in the interpolaris and caudalis of trigeminal sensory nuclear complex. Brain Res 1060:118–125
Bae YC, Park KS, Bae JY, Paik SK, Ahn DK, Moritani M, Shigenaga Y, Yoshida A (2005b) GABA and glycine in synaptic microcircuits associated with physiologically characterized primary afferents of cat trigeminal principal nucleus. Exp Brain Res 162:449–457
Barret KE, Barman SM, Boitano S, Brooks H (2009) Excitable tissue: nerve. In: Barret KE, Barman SM, Boitano S, Brooks H (eds) Ganong’s review of medical physiology, 23rd edn. McGraw-Hill Medical, New York, pp 79–92
Biedenbach MA, Chan KY (1971) Tongue mechanoreceptors: comparison of afferent fibers in the lingual nerve and chorda tympani. Brain Res 35:584–588
Bradley RM, King MS, Wang L, Shu X (1996) Neurotransmitter and neuromodulator activity in the gustatory zone of the nucleus tractus solitarius. Chem Senses 21:377–385
Cho YK, Li CS, Smith DV (2002) Gustatory projections from the nucleus of the solitary tract to the parabrachial nuclei in the hamster. Chem Senses 27:81–90
Corson JA, Bradley RM (2013) Physiological and anatomical properties of intramedullary projection neurons in rat rostral nucleus of the solitary tract. J Neurophysiol 110:1130–1143
Corson J, Aldridge A, Wilmoth K, Erisir A (2012) A survey of oral cavity afferents to the rat nucleus tractus solitarii. J Comp Neurol 520:495–527
Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666
Davis BJ (1993) GABA-like immunoreactivity in the gustatory zone of the nucleus of the solitary tract in the hamster: light and electron microscopic studies. Brain Res Bull 30:69–77
Davis BJ (1998) Synaptic relationships between the chorda tympani and tyrosine hydroxylase-immunoreactive dendritic processes in the gustatory zone of the nucleus of the solitary tract in the hamster. J Comp Neurol 392:78–91
Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G (2011) Axon physiology. Physiol Rev 91:555–602
Farbman AI, Hellekant G (1978) Quantitative analyses of the fiber population in rat chorda tympani nerves and fungiform papillae. Am J Anat 153:509–521
Fyffe RE, Light AR (1984) The ultrastructure of group Ia afferent fiber synapses in the lumbosacral spinal cord of the cat. Brain Res 300:201–209
Grant G (2006) The 1932 and 1944 Nobel Prizes in physiology or medicine: rewards for ground-breaking studies in neurophysiology. J Hist Neurosci 15:341–357
Gray EG (1962) A morphological basis for pre-synaptic inhibition? Nature 193:82–83
Harper AA, Lawson SN (1985) Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 359:31–46
Iriuchijima J, Zotterman Y (1961) Conduction rates of afferent fibres to the anterior tongue of the dog. Acta Physiol Scand 51:283–289
Jonas P, Bischofberger J, Sandkuhler J (1998) Corelease of two fast neurotransmitters at a central synapse. Science 281:419–424
Keay KA, Feil K, Gordon BD, Herbert H, Bandler R (1997) Spinal afferents to functionally distinct periaqueductal gray columns in the rat: an anterograde and retrograde tracing study. J Comp Neurol 385:207–229
Keller AF, Coull JA, Chery N, Poisbeau P, De Koninck Y (2001) Region-specific developmental specialization of GABA-glycine cosynapses in laminas I-II of the rat spinal dorsal horn. J Neurosci 21:7871–7880
King MS (2006) Anatomy of the rostral nucleus of the solitary tract. In: Bradley RM (ed) The role of the nucleus of the solitary tract in gustatory processing. CRC Press, Boca Raton, pp 17–38
Kishimoto H, Bae YC, Yoshida A, Moritani M, Takemura M, Nakagawa S, Nagase Y, Wada T, Sessle BJ, Shigenaga Y (1998) Central distribution of synaptic contacts of primary and secondary jaw muscle spindle afferents in the trigeminal motor nucleus of the cat. J Comp Neurol 391:50–63
Kitamura K, Kimura RS, Schuknecht HF (1982) The ultrastructure of the geniculate ganglion. Acta Otolaryngol 93:175–186
Lasiter PS, Kachele DL (1988) Organization of GABA and GABA-transaminase containing neurons in the gustatory zone of the nucleus of the solitary tract. Brain Res Bull 21:623–636
Lawson SN, Waddell PJ (1991) Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435:41–63
Lawson SN, Crepps BA, Perl ER (1997) Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. J Physiol 505:177–191
Lawson SN, Crepps B, Perl ER (2002) Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol 540:989–1002
Leonard NL, Renehan WE, Schweitzer L (1999) Structure and function of gustatory neurons in the nucleus of the solitary tract. IV. The morphology and synaptology of GABA-immunoreactive terminals. Neuroscience 92:151–162
Li CS, Smith DV (1997) Glutamate receptor antagonists block gustatory afferent input to the nucleus of the solitary tract. J Neurophysiol 77:1514–1525
Li JL, Kaneko T, Shigemoto R, Mizuno N (1997) Distribution of trigeminohypothalamic and spinohypothalamic tract neurons displaying substance P receptor-like immunoreactivity in the rat. J Comp Neurol 378:508–521
Matsuo R, Inoue T, Masuda Y, Nakamura O, Yamauchi Y, Morimoto T (1995) Neural activity of chorda tympani mechanosensitive fibers during licking behavior in rats. Brain Res 689:289–298
May OL, Erisir A, Hill DL (2007) Ultrastructure of primary afferent terminals and synapses in the rat nucleus of the solitary tract: comparison among the greater superficial petrosal, chorda tympani, and glossopharyngeal nerves. J Comp Neurol 502:1066–1078
Moon YS, Paik SK, Seo JH, Yi HW, Cho YS, Moritani M, Yoshida A, Ahn DK, Kim YS, Bae YC (2008) GABA- and glycine-like immunoreactivity in axonal endings presynaptic to the vibrissa afferents in the cat trigeminal interpolar nucleus. Neuroscience 152:138–145
Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H (2004) Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci 7:17–23
Nakagawa S, Kurata S, Yoshida A, Nagase Y, Moritani M, Takemura M, Bae YC, Shigenaga Y (1997) Ultrastructural observations of synaptic connections of vibrissa afferent terminals in cat principal sensory nucleus and morphometry of related synaptic elements. J Comp Neurol 389:12–33
Ogawa H, Sato M, Yamashita S (1968) Multiple sensitivity of chorda tympani fibres of the rat and hamster to gustatory and thermal stimuli. J Physiol 199:223–240
Ogawa H, Imoto T, Hayama T (1984) Responsiveness of solitario-parabrachial relay neurons to taste and mechanical stimulation applied to the oral cavity in rats. Exp Brain Res 54:349–358
Ottersen OP (1987) Postembedding light- and electron microscopic immunocytochemistry of amino acids: description of a new model system allowing identical conditions for specificity testing and tissue processing. Exp Brain Res 69:167–174
Ottersen OP (1989a) Postembedding immunogold labelling of fixed glutamate: an electron microscopic analysis of the relationship between gold particle density and antigen concentration. J Chem Neuroanat 2:57–66
Ottersen OP (1989b) Quantitative electron microscopic immunocytochemistry of neuroactive amino acids. Anat Embryol (Berl) 180:1–15
Ottersen OP, Storm-Mathisen J, Madsen S, Skumlien S, Strømhaug J (1986) Evaluation of the immunocytochemical method for amino acids. Med Biol 64:147–158
Paik SK, Oh SJ, Son YJ, Ma SK, Ahn CH, Kim SK, Chang Z, Moritani M, Yoshida A, Bae YC (2005) Neural mechanisms controlling jaw-jerk reflex in the cat. NeuroReport 16:1565–1568
Paik SK, Bae JY, Park SE, Moritani M, Yoshida A, Yeo EJ, Choi KS, Ahn DK, Moon C, Shigenaga Y, Bae YC (2007) Developmental changes in distribution of gamma-aminobutyric acid- and glycine-immunoreactive boutons on rat trigeminal motoneurons. I. Jaw-closing motoneurons. J Comp Neurol 503:779–789
Paik SK, Park SK, Jin JK, Bae JY, Choi SJ, Yoshida A, Ahn DK, Bae YC (2011) Ultrastructural analysis of glutamate-immunopositive synapses onto the rat jaw-closing motoneurons during postnatal development. J Neurosci Res 89:153–161
Paik SK, Kwak MK, Bae JY, Yi HW, Yoshida A, Ahn DK, Bae YC (2012) γ-aminobutyric acid-, glycine-, and glutamate-immunopositive boutons on mesencephalic trigeminal neurons that innervate jaw-closing muscle spindles in the rat: ultrastructure and development. J Comp Neurol 520:3414–3427
Pierce JP, Mendell LM (1993) Quantitative ultrastructure of Ia boutons in the ventral horn: scaling and positional relationships. J Neurosci 13:4748–4763
Robinson PP (1988) The characteristics and regional distribution of afferent fibres in the chorda tympani of the cat. J Physiol 406:345–357
Russier M, Kopysova IL, Ankri N, Ferrand N, Debanne D (2002) GABA and glycine co-release optimizes functional inhibition in rat brainstem motoneurons in vitro. J Physiol 541:123–137
Shigenaga Y, Moritani M, Oh SJ, Park KP, Paik SK, Bae JY, Kim HN, Ma SK, Park CW, Yoshida A, Ottersen OP, Bae YC (2005) The distribution of inhibitory and excitatory synapses on single, reconstructed jaw-opening motoneurons in the cat. Neuroscience 133:507–518
Smith DV, Liu H, Vogt MB (1994) Neural coding of aversive and appetitive gustatory stimuli: interactions in the hamster brain stem. Physiol Behav 56:1189–1196
Smith DV, Li CS, Davis BJ (1998) Excitatory and inhibitory modulation of taste responses in the hamster brainstem. Ann N Y Acad Sci 855:450–456
Spassova I (1983) Fine structure of the neurons of the geniculate ganglion of the cat. J Hirnforsch 24:123–133
Spike RC, Puskár Z, Andrew D, Todd AJ (2003) A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. Eur J Neurosci 18:2433–2448
Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP (1983) First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature 301:517–520
Streefland C, Jansen K (1999) Intramedullary projections of the rostral nucleus of the solitary tract in the rat: gustatory influences on autonomic output. Chem Senses 24:655–664
Takumi Y, Ramírez-León V, Laake P, Rinvik E, Ottersen OP (1999) Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci 2:618–624
Travers SP, Norgren R (1995) Organization of orosensory responses in the nucleus of the solitary tract of rat. J Neurophysiol 73:2144–2162
Walmsley B, Wieniawa-Narkiewicz E, Nicol MJ (1987) Ultrastructural evidence related to presynaptic inhibition of primary muscle afferents in Clarke’s column of the cat. J Neurosci 7:236–243
Walmsley B, Graham B, Nicol MJ (1995) Serial E-M and simulation study of presynaptic inhibition along a group Ia collateral in the spinal cord. J Neurophysiol 74:616–623
Wang L, Bradley RM (1993) Influence of GABA on neurons of the gustatory zone of the rat nucleus of the solitary tract. Brain Res 616:144–153
Wang L, Bradley RM (1995) In vitro study of afferent synaptic transmission in the rostral gustatory zone of the rat nucleus of the solitary tract. Brain Res 702:188–198
Wang M, Bradley RM (2010) Synaptic characteristics of rostral nucleus of the solitary tract neurons with input from the chorda tympani and glossopharyngeal nerves. Brain Res 1328:71–78
Wang S, Corson J, Hill D, Erisir A (2012) Postnatal development of chorda tympani axons in the rat nucleus of the solitary tract. J Comp Neurol 520:3217–3235
Watson AH (2003) GABA- and glycine-like immunoreactivity in axons and dendrites contacting the central terminals of rapidly adapting glabrous skin afferents in rat spinal cord. J Comp Neurol 464:497–510
Watson AH (2004) Synaptic interactions between the terminals of slow-adapting type II mechanoreceptor afferents and neurones expressing gamma-aminobutyric acid- and glycine-like immunoreactivity in the rat spinal cord. J Comp Neurol 471:168–179
Watson AH, Hughes DI, Bazzaz AA (2002) Synaptic relationships between hair follicle afferents and neurones expressing GABA and glycine-like immunoreactivity in the spinal cord of the rat. J Comp Neurol 452:367–380
Weinberg RJ, van Eyck SL (1991) A tetramethylbenzidine/tungstate reaction for horseradish peroxidase histochemistry. J Histochem Cytochem 39:1143–1148
Wetherton BM, Leonard NL, Renehan WE, Schweitzer L (1998) Structure and function of gustatory neurons in the nucleus of the solitary tract. III. Classification of terminals using cluster analysis. Biotech Histochem 73:164–173
Whitehead MC (1986) Anatomy of the gustatory system in the hamster: synaptology of facial afferent terminals in the solitary nucleus. J Comp Neurol 224:72–85
Whitehead MC (1993) Distribution of synapses on identified cell types in a gustatory subdivision of the nucleus of the solitary tract. J Comp Neurol 332:326–340
Yabuta NH, Yasuda K, Nagase Y, Yoshida A, Fukunishi Y, Shigenaga Y (1996) Light microscopic observations of the contacts made between two spindle afferent types and alpha-motoneurons in the cat trigeminal motor nucleus. J Comp Neurol 374:436–450
Yoshida A, Fukami H, Nagase Y, Appenteng K, Honma S, Zhang LF, Bae YC, Shigenaga Y (2001) Quantitative analysis of synaptic contacts made between functionally identified oralis neurons and trigeminal motoneurons in cats. J Neurosci 21:6298–6307
Zhang LF, Moritani M, Honma S, Yoshida A, Shigenaga Y (2001) Quantitative ultrastructure of slowly adapting lingual afferent terminals in the principal and oral nuclei in the cat. Synapse 41:96–111
Zhang J, Pendlebury WW, Luo P (2003) Synaptic organization of monosynaptic connections from mesencephalic trigeminal nucleus neurons to hypoglossal motoneurons in the rat. Synapse 49:157–169
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP, 2008-0062282). The authors sincerely thank Dr. Juli Valtschanoff for helpful discussion and careful reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. K. Park and D. S. Lee contributed equally to this work.
Rights and permissions
About this article
Cite this article
Park, S.K., Lee, D.S., Bae, J.Y. et al. Central connectivity of the chorda tympani afferent terminals in the rat rostral nucleus of the solitary tract. Brain Struct Funct 221, 1125–1137 (2016). https://doi.org/10.1007/s00429-014-0959-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0959-6