Abstract
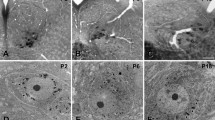
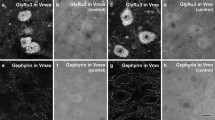
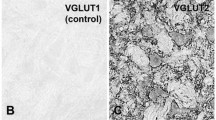
Detailed information about the excitatory and inhibitory synapses on the hypoglossal motoneurons may help understand the neural mechanism for control of the hypoglossal motoneuron excitability and hence the precise and coordinated movements of the tongue during chewing, swallowing and licking. For this, we investigated the distribution of GABA-, glycine (Gly)- and glutamate (Glut)-immunopositive (+) axon terminals on the genioglossal (GG) motoneurons by retrograde tracing, electron microscopic immunohistochemistry, and quantitative analysis. Small GG motoneurons (< 400 μm2 in cross-sectional area) had fewer primary dendrites, significantly higher nuclear/cytoplasmic ratio, and smaller membrane area covered by synaptic boutons than large GG motoneurons (> 400 μm2). The fraction of inhibitory boutons (GABA + only, Gly + only, and mixed GABA +/Gly + boutons) of all boutons was significantly higher for small GG motoneurons than for large ones, whereas the fraction of Glut + boutons was significantly higher for large GG motoneurons than for small ones. Almost all boutons (> 95%) on both small and large GG motoneurons were GABA + , Gly + or Glut + . The frequency of mixed GABA +/Gly + boutons was the highest among inhibitory boutons types for both small and large GG motoneurons. These findings may elucidate the anatomical substrate for precise regulation of the motoneuron firing required for the fine movements of the tongue, and also suggest that the excitability of small and large GG motoneurons may be regulated differently.









Similar content being viewed by others
References
Alvarez FJ, Kavookjian AM, Light AR (1993) Ultrastructural morphology, synaptic relationships, and CGRP immunoreactivity of physiologically identified C-fiber terminals in the monkey spinal cord. J Comp Neurol 329:472–490
Bach-y-Rita P, Lennerstrand G, Alvarado J, Nichols K, McHolm G (1977) Extraocular muscle fibers: ultrastructural identification of iontophoretically labeled fibers contracting in response to succinylcholine. Invest Ophthalmol Vis Sci 16:561–565
Bae YC, Choi BJ, Lee MG, Lee HJ, Park KP, Zhang LF, Honma S, Fukami H, Yoshida A, Ottersen OP, Shigenaga Y (2002) Quantitative ultrastructural analysis of glycine- and gamma-aminobutyric acid-immunoreactive terminals on trigeminal alpha- and gamma-motoneuron somata in the rat. J Comp Neurol 442:308–319
Barret KE, Barman SM, Boitano S, Brooks H (2009) Excitable tissue: nerve. In: Barret KE, Barman SM, Boitano S, Brooks H (eds) Ganong’s review of medical physiology, 23rd edn. McGraw-Hill Medical, New York, pp 79–92
Berkowitz A (2012) Motor Output from the Brain and Spinal Cord. In: eLS. https://doi.org/10.1002/9780470015902.a0000189.pub3
Brull SJ (2014) Physiology of neuromuscular transmission. In: Murray MJ, Harrison BA, Mueller JT, Rose SH, Wass CT, Wedel DJ (eds) Faust’s anesthesiology review, 4th edn. Elsevier Saunders, Philadelphia, pp 98–99
Büttner-Ennever JA (2007) Anatomy of the oculomotor system. Dev Ophthalmol 40:1–14
Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A (2007) Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 579:515–526
Chandler SH, Goldberg LJ (1982) Intracellular analysis of synaptic mechanisms controlling spontaneous and cortically induced rhythmical jaw movements in the guinea pig. J Neurophysiol 48:126–138
Conradi S (1969) Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the adult cat. Acta Physiol Scand Suppl 332:5–48
Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE (2004) Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem 88:1398–1405
Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G (2011) Axon physiology. Physiol Rev 91:555–602
Destombes J, Horcholle-Bossavit G, Thiesson D, Jami L (1992) Alpha and gamma motoneurons in the peroneal nuclei of the cat spinal cord: an ultrastructural study. J Comp Neurol 317:79–90
Engelhardt JK, Silveira V, Morales FR, Pose I, Chase MH (2010) Serotoninergic control of glycinergic inhibitory postsynaptic currents in rat hypoglossal motoneurons. Brain Res 1345:1–8
Fay RA, Norgren R (1997) Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Res Brain Res Rev 25:291–311
Fried G, Terenius L, Hokfelt T, Goldstein M (1985) Evidence for differential localization of noradrenaline and neuropeptide Y in neuronal storage vesicles isolated from rat vas deferens. J Neurosci 5:450–458
Gestreau C, Dutschmann M, Obled S, Bianchi AL (2005) Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol 147:159–176
Goldberg LJ, Chandler SH, Tal M (1982) Relationship between jaw movements and trigeminal motoneuron membrane-potential fluctuations during cortically induced rhythmical jaw movements in the guinea pig. J Neurophysiol 48:110–138
Hall WC (2004) Lower motor neuron circuits and motor control. In: Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia A-S, McNamara JO, Williams SM (eds) Neuroscience, 3rd edn. Sinauer Associates, Sunderland, pp 371–392
Henry JN, Manaker S (1998) Colocalization of substance P or enkephalin in serotonergic neuronal afferents to the hypoglossal nucleus in the rat. J Comp Neurol 391:491–505
Horner RL (2009) Emerging principles and neural substrates underlying tonic sleep-state-dependent influences on respiratory motor activity. Philos Trans R Soc Lond B Biol Sci 364:2553–2564
Ito T, Hioki H, Nakamura K, Kaneko T, Iino S, Nojyo Y (2008) Some gamma-motoneurons contain gamma-aminobutyric acid in the rat cervical spinal cord. Brain Res 1201:78–87
Johnson MD (1994) Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron 12:433–442
Jonas P, Bischofberger J, Sandkuhler J (1998) Corelease of two fast neurotransmitters at a central synapse. Science 281:419–424
Kolston J, Osen KK, Hackney CM, Ottersen OP, Storm-Mathisen J (1992) An atlas of glycine- and GABA-like immunoreactivity and colocalization in the cochlear nuclear complex of the guinea pig. Anat Embryol (Berl) 186:443–465
Krol RC, Knuth SL, Bartlett D Jr (1984) Selective reduction of genioglossal muscle activity by alcohol in normal human subjects. Am Rev Respir Dis 129:247–250
Kubo Y, Enomoto S, Nakamura Y (1981) Synaptic basis of orbital cortically induced rhythmical masticatory activity of trigeminal motoneurons in immobilized cats. Brain Res 230:97–110
Lawson SN, Waddell PJ (1991) Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435:41–63
Li YQ, Takada M, Kaneko T, Mizuno N (1997) Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J Comp Neurol 378:283–294
Liu X, Sood S, Liu H, Nolan P, Morrison JL, Horner RL (2003) Suppression of genioglossus muscle tone and activity during reflex hypercapnic stimulation by GABA(A) mechanisms at the hypoglossal motor nucleus in vivo. Neuroscience 116:249–259
Lu T, Rubio ME, Trussell LO (2008) Glycinergic transmission shaped by the corelease of GABA in a mammalian auditory synapse. Neuron 57:524–535
Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL (2003) GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol 548:569–583
Muller E, Triller A, Legendre P (2004) Glycine receptors and GABA receptor alpha 1 and gamma 2 subunits during the development of mouse hypoglossal nucleus. Eur J Neurosci 20:3286–3300
Muller E, Le Corronc H, Triller A, Legendre P (2006) Developmental dissociation of presynaptic inhibitory neurotransmitter and postsynaptic receptor clustering in the hypoglossal nucleus. Mol Cell Neurosci 32:254–273
Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H (2004) Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci 7:17–23
O’Brien JA, Berger AJ (2001) The nonuniform distribution of the GABA(A) receptor alpha 1 subunit influences inhibitory synaptic transmission to motoneurons within a motor nucleus. J Neurosci 21:8482–8494
O’Reilly PM, FitzGerald MJ (1990) Fibre composition of the hypoglossal nerve in the rat. J Anat 172:227–243
Ornung G, Shupliakov O, Linda H, Ottersen OP, Storm-Mathisen J, Ulfhake B, Cullheim S (1996) Qualitative and quantitative analysis of glycine- and GABA-immunoreactive nerve terminals on motoneuron cell bodies in the cat spinal cord: a postembedding electron microscopic study. J Comp Neurol 365:413–426
Ornung G, Ottersen OP, Cullheim S, Ulfhake B (1998) Distribution of glutamate-, glycine- and GABA-immunoreactive nerve terminals on dendrites in the cat spinal motor nucleus. Exp Brain Res 118:517–532
Ottersen OP (1989) Quantitative electron microscopic immunocytochemistry of neuroactive amino acids. Anat Embryol (Berl) 180:1–15
Ottersen OP, Storm-Mathisen J (1984) Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol 229:374–392
Ottersen OP, Storm-Mathisen J, Madsen S, Skumlien S, Stromhaug J (1986) Evaluation of the immunocytochemical method for amino acids. Med Biol 64:147–158
Paik SK, Bae JY, Park SE, Moritani M, Yoshida A, Yeo EJ, Choi KS, Ahn DK, Moon C, Shigenaga Y, Bae YC (2007) Developmental changes in distribution of gamma-aminobutyric acid- and glycine-immunoreactive boutons on rat trigeminal motoneurons. I. Jaw-closing motoneurons. J Comp Neurol 503:779–789
Paik SK, Kwak WK, Bae JY, Na YK, Park SY, Yi HW, Ahn DK, Ottersen OP, Yoshida A, Bae YC (2012a) Development of gamma-aminobutyric acid-, glycine-, and glutamate-immunopositive boutons on rat jaw-opening motoneurons. J Comp Neurol 520:1212–1226
Paik SK, Kwak MK, Bae JY, Yi HW, Yoshida A, Ahn DK, Bae YC (2012b) gamma-Aminobutyric acid-, glycine-, and glutamate-immunopositive boutons on mesencephalic trigeminal neurons that innervate jaw-closing muscle spindles in the rat: ultrastructure and development. J Comp Neurol 520:3414–3427
Pang YW, Li JL, Nakamura K, Wu S, Kaneko T, Mizuno N (2006) Expression of vesicular glutamate transporter 1 immunoreactivity in peripheral and central endings of trigeminal mesencephalic nucleus neurons in the rat. J Comp Neurol 498:129–141
Park SK, Lee DS, Bae JY, Bae YC (2016) Central connectivity of the chorda tympani afferent terminals in the rat rostral nucleus of the solitary tract. Brain Struct Funct 221:1125–1137
Peters A, Palay SL, Webster Hd (1991) The fine structure of the nervous system: neurons and their supporting cells, 3rd edn. Oxford University Press, New York
Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL (2000) Synaptic control of motoneuronal excitability. Physiol Rev 80:767–852
Remmers JE, deGroot WJ, Sauerland EK, Anch AM (1978) Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol 44:931–938
Richardson KA, Gatti PJ (2004) Genioglossal hypoglossal motoneurons contact substance P-like immunoreactive nerve terminals in the cat: a dual labeling electron microscopic study. Exp Brain Res 154:327–332
Russier M, Kopysova IL, Ankri N, Ferrand N, Debanne D (2002) GABA and glycine co-release optimizes functional inhibition in rat brainstem motoneurons in vitro. J Physiol 541:123–137
Sahara Y, Hashimoto N, Kato M, Nakamura Y (1988) Synaptic bases of cortically-induced rhythmical hypoglossal motoneuronal activity in the cat. Neurosci Res 5:439–452
Sawczuk A, Mosier KM (2001) Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med 12:18–37
Scrima L, Broudy M, Nay KN, Cohn MA (1982) Increased severity of obstructive sleep apnea after bedtime alcohol ingestion: diagnostic potential and proposed mechanism of action. Sleep 5:318–328
Sharifullina E, Ostroumov K, Nistri A (2005) Metabotropic glutamate receptor activity induces a novel oscillatory pattern in neonatal rat hypoglossal motoneurones. J Physiol 563:139–159
Shepherd GM, Koch C (1990) Appendix: Dendritic electrotonus and synaptic integration. In: Shepherd GM (ed) The synaptic organization of the brain, 3rd edn. Oxford University Press, New York, pp 439–473
Shigenaga Y, Moritani M, Oh SJ, Park KP, Paik SK, Bae JY, Kim HN, Ma SK, Park CW, Yoshida A, Ottersen OP, Bae YC (2005) The distribution of inhibitory and excitatory synapses on single, reconstructed jaw-opening motoneurons in the cat. Neuroscience 133:507–518
Simon M, Destombes J, Horcholle-Bossavit G, Thiesson D (1996) Postnatal development of alpha- and gamma-peroneal motoneurons in kittens: an ultrastructural study. Neurosci Res 25:77–89
Singer JH, Berger AJ (2000) Development of inhibitory synaptic transmission to motoneurons. Brain Res Bull 53:553–560
Stanek E 4th, Cheng S, Takatoh J, Han BX, Wang F (2014) Monosynaptic premotor circuit tracing reveals neural substrates for oro-motor coordination. Elife 3:e02511
Steenland HW, Liu H, Horner RL (2008) Endogenous glutamatergic control of rhythmically active mammalian respiratory motoneurons in vivo. J Neurosci 28:6826–6835
Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP (1983) First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature 301:517–520
Sutherland FI, Bannatyne BA, Kerr R, Riddell JS, Maxwell DJ (2002) Inhibitory amino acid transmitters associated with axons in presynaptic apposition to cutaneous primary afferent axons in the cat spinal cord. J Comp Neurol 452:154–162
Travers JB (2015) Oromotor nuclei. In: Paxinos G (ed) The rat nervous system, 4th edn. Academic Press, London, pp 223–245
Travers JB, Yoo JE, Chandran R, Herman K, Travers SP (2005) Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. J Comp Neurol 488:28–47
van Brederode JF, Yanagawa Y, Berger AJ (2011) GAD67-GFP + neurons in the Nucleus of Roller: a possible source of inhibitory input to hypoglossal motoneurons. I. Morphology and firing properties. J Neurophysiol 105:235–248
Weinberg RJ, van Eyck SL (1991) A tetramethylbenzidine/tungstate reaction for horseradish peroxidase histochemistry. J Histochem Cytochem 39:1143–1148
Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH (1999) Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience 94:11–15
Yokota S, Niu JG, Tsumori T, Oka T, Yasui Y (2011) Glutamatergic Kolliker-Fuse nucleus neurons innervate hypoglossal motoneurons whose axons form the medial (protruder) branch of the hypoglossal nerve in the rat. Brain Res 1404:10–20
Zhang J, Pendlebury WW, Luo P (2003) Synaptic organization of monosynaptic connections from mesencephalic trigeminal nucleus neurons to hypoglossal motoneurons in the rat. Synapse 49:157–169
Zhou L, Wang ZY, Lian H, Song HY, Zhang YM, Zhang XL, Fan RF, Zheng LF, Zhu JX (2014) Altered expression of dopamine receptors in cholinergic motoneurons of the hypoglossal nucleus in a 6-OHDA-induced Parkinson’s disease rat model. Biochem Biophys Res Commun 452:560–566
Acknowledgements
The authors sincerely thank Dr. Juli Valtschanoff for helpful discussion and careful reading of the manuscript. We also sincerely thank Dr. O.P. Ottersen for the gift of the glutamate, GABA and glycine antibodies and the sandwich block for the test sections.
Funding
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT, NRF-2017R1A5A2015391, NRF-2017R1A2B2003561).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paik, S.K., Yoo, H.I., Choi, S.K. et al. Distribution of excitatory and inhibitory axon terminals on the rat hypoglossal motoneurons. Brain Struct Funct 224, 1767–1779 (2019). https://doi.org/10.1007/s00429-019-01874-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-019-01874-0